Does crossover innervation really affect the clinical outcome? A comparison of outcome between unilateral and bilateral digital nerve repair
Melike Oruç, Kadri Ozer, Özlem Çolak, Yüksel Kankaya, Uğur Koçer Ankara Training and Research Hospital, Plastic, Reconstructive and Aesthetic Surgery Clinic, Ankara, Turkey2 Aydin State Hospital, Plastic, Reconstructive and Aesthetic Surgery Clinic, Aydin, Turkey Istanbul Okmeydani Training and Research Hospital, Plastic, Reconstructive and Aesthetic Surgery Clinic, Istanbul, Turkey
Does crossover innervation really affect the clinical outcome? A comparison of outcome between unilateral and bilateral digital nerve repair
Melike Oruç1, Kadri Ozer2,*, Özlem Çolak3, Yüksel Kankaya1, Uğur Koçer1
1 Ankara Training and Research Hospital, Plastic, Reconstructive and Aesthetic Surgery Clinic, Ankara, Turkey
2 Aydin State Hospital, Plastic, Reconstructive and Aesthetic Surgery Clinic, Aydin, Turkey
3 Istanbul Okmeydani Training and Research Hospital, Plastic, Reconstructive and Aesthetic Surgery Clinic, Istanbul, Turkey
How to cite this article: Oruç M, Ozer K, Çolak Ö, Kankaya Y, Koçer U (2016) Does crossover innervation really affect the clinical outcome? A comparison of outcome between unilateral and bilateral digital nerve repair. Neural Regen Res 11(9):1499-1505.
Kadri Ozer, M.D.,
kadriozer@hotmail.com.
orcid:
0000-0003-2966-6618
(Kadri Ozer)
Digital nerve injuries are the mostly detected nerve injury in the upper extremity. However, since the clinical phenomenon of crossover innervation at some degree from uninjured digital nerve to the injured side occurs after digital nerve injuries is sustained, one could argue that this concept might even result in the overestimation of the outcome of the digital nerve repair. With this knowledge in mind, this study aimed to present novel, pure, focused and valuable clinical data by comparing the outcomes of bilateral and unilateral digital nerve repair. A retrospective review of 28 fingers with unilateral or bilateral digital nerve repair using end-to-end technique in 19 patients within 2 years was performed. Weber’s two-point discrimination, sharp/dull discrimination, warm/cold sensation and Visual Analog Scale scoring were measured at final 12-month follow ups in all patients. There was no significant difference in recovery of sensibility after unilateral and bilateral digital nerve repairs. Though there is crossover innervation microscopically, it is not important in the clinical evaluation period. According to clinical findings from this study, crossover innervations appear to be negligible in the estimation of outcomes of digital neurorrhaphy.
nerve regeneration; digital nerve repair; unilateral; bilateral; crossover innervation; sensibility; neurorrhaphy; nerve sprouting; neural regeneration
Introduction
In emergency departments, hand injuries reportedly account for up to 20% of all treated unintended injuries and of those, digital nerve injuries are the mostly seen nerve injury in the upper extremity (Dagum, 1998). Good recovery has been reported after digital nerve repair evaluated using especially Weber’s static two-point discrimination (S2PD) — the choice of the “best” method (Segalman et al., 2001). There is evidence that the clinical phenomenon of crossover innervation to some degree from uninjured digital nerve to the injured side occurs after digital nerve injuries. However, some scholars reported that this concept might even result in the overestimation of the outcome of the digital nerve repair. However, some scholars strongly dispute the existence of this phenomenon (Weinzweig, 2000).
Based on the presence of crossover innervation on decreased S2PD testing by nerve isolation technique, some advocates administered local anesthetic blocks to all sensory contributions in the exposed digit (Tadjalli et al., 1995a) or demonstrated collateral sprouting in biological studies (Wiesenfeld-Hallin et al., 1989; Matsumoto et al., 1999). Despite that, opposing defenders argued that this phenomenon has no clinically significant effect and meaning (Weinzweig, 2000; Thomas et al., 2014). Although the evaluation of digital nerve repair as described in most observational studies is scored as very good or excellent while using S2PD (Rinkel et al., 2013), this controversy about the role of crossover innervation in assessing the digital nerve lacerations has not still been elucidated exactly. With this knowledge in mind, we believe that this study can provide novel, pure, focused and valuable clinical data by comparing outcomes of bilateral and unilateral digital nerve repair.
Subjects and Methods
Patients
After approval by the ethics committee of our institute (approval number: 2016/0565-4671), patients who underwent direct primary digital nerve repair due to trauma within the last 2 years between January 2013 and December 2014 were contacted by telephone. Those matching the inclusion criteria and willing to participate in a clinical examination were enrolled in this retrospective clinical study (Figure 1). All protocols used in this study were conducted according to the ethical guidelines of the Declaration of Helsinki and international regulations, and all patients gave their written informed consent. Study setting of this study is a large training and research hospital with more than 600 beds, and plastic surgery is the only department responsible for hand injuries.
Inclusion and exclusion criteria
The inclusion criteria for the study were listed as follows: (1) injury of at least one complete proper digital nerve transection; (2) primary end-to-end digital nerve repair performed within 3 days after the injury (Allan, 2004); (3) uninjured matching contralateral digit(s); (4) a minimum follow-up period of 12 months; and (5) with associated tendon injuries. The exclusion criteria were: (1) injury at the level or proximal of one or more common digital nerves; (2) age under 14 or over 65 years at the time of injury; (3) digital nerve injuries in thumb; (4) complex injuries including replantation or revascularization of the same digit; (5) incomplete nerve lesions or nerve repair with any grafts or tubes; (6) nerve injury caused by a crush or avulsion mechanism, (7) patients known to have a disease affecting nerve recovery (e.g., diabetes) and/or a history of peripheral or compression neuropathy.
Surgical repair
The standard method of digital nerve repair in our institution was an epineural end-to-end technique with two 8-0 polypropylene sutures without any tension. Loupes (2.5× magnification) or an operating microscope were used. All surgeries were performed by one of two senior residents.
Assessment and outcome measures
Medical records of patients were identified and evaluated for demographic and clinical features. A complete dataset containing age, gender, hand-dominance, the injured hand, digit and side, pulp distance from the injury, follow-up time, site of the repair, concomitant injuries, time for mobilization of the injured digit and days in physiotherapy were collected and analyzed.
Our primary outcome was the degree of sensory recovery for the repaired proper digital nerve(s) which were recorded by one investigator. Sensation was assessed in the autonomous distribution of the repaired digital nerve(s) in the injured digit(s).
S2PD was determined using the standard protocol of the American Society for Surgery of the Hand and the International Federation of Societies for Surgery of the Hand. Sensory nerve function was graded using the modified Highet classification which was modified by Mackinnon and Dellon (Paprottka et al., 2013; He et al., 2014) (Table 1).
Sharp/dull discrimination was performed by a needle with a dull end of 0.4 cm and a sharp end of 2.7 French gauge (Fakin et al., 2016). Scoring was recorded as (1) both stimuli well recognized, (2) sharp stimuli recognition, recognition of dull stimuli in comparison with the healthy side delayed and/or with less intensity, (3) sharp recognized as dull or either dull or sharp stimuli recognized, and (4) none recognized (Haug et al., 2013).
Warm/cold sensation was tested by two identical plastic tubes, either filled with cold water of 2—5°C and warm water of 38—40°C (Haug et al., 2013). Indicated patients’ current warm/cold discrimination was converted to an ordinal score as (1) both recognized, (2) one recognized immediately, the second with more or less intensity or delayed, (3) one recognized, and (4) none recognized (Haug et al., 2013).
Additionally, patients were asked to quantify overall subjective estimation of their degree of recovery using a 10-cm“Visual Analog Scale” —defined as the return of the sensibility appreciated by the patient him/herself. A lower score indicated less perceived satisfaction; for instance, a score of‘‘10’’ on the Visual Analog Scale represented a full satisfied recovery in the subject’s opinion; a score of ‘‘0’’ indicated the subject-perceived maximal dissatisfaction.
All patients were carefully examined and questioned as to the manifestation of Tinel’s sign, cold intolerance—defined as“an icy cold feeling rapidly progressing to pain (Vipond et al., 2007)”, hyperesthesia—defined as “when hair or skin on the injured digit is touched, the sensation is unpleasant and excessively sensitive (Vipond et al., 2007)”, or electrical pain (Fakin et al., 2016).
Statistical analysis
Data were analyzed using SPSS 15.0 software (SPSS, Chicago, IL, USA). In addition to using standard descriptive statistical calculations [mean and standard deviation (SD)], when the variables were normally distributed, an unpaired t test was used for comparing intergroup differences. However, when the variables were not normally distributed, the Mann-Whitney U test was utilized. In addition, a chi-square test was used to evaluate the qualitative data. A value of P < 0.05 was considered statistically significant.
Results
Demographic data
A total of 28 fingers in 19 patients who underwent repair of digital nerves following uncomplicated sharp transection of the nerve were included in this study after contact by telephone after at least 12 months of follow up.
Of the 28 fingers, 18 fingers of 12 patients had unilateral digital nerve injury (group 1= one-nerve repair in one finger) and 10 fingers of 7 patients had bilateral digital nerve injury (group 2 = two-nerve repair in one finger). There were no significant differences in follow-up time, mean ages, gender distribution, site of and time required for the repair between two groups (P > 0.05). All demographic results are summarized in Table 2.
Dominant hand, site, level and extension of injury
There were no significant differences in concomitant flexor tendon injuries, days in immobilization and in physiotherapy between groups 1 and 2 (Table 2). No significant difference was found in the dominant hand involved in the fingers and injury level which was recorded as distance from injury site to the tip of the pulp between groups 1 and 2 (P > 0.05; Table 2).
S2PD results
Results of S2PD testing are shown in Figure 2. S2PD averaged 8.67 mm (SEM, 1.16) after unilateral digital nerve repairs compared with 9.21 mm (SEM, 1.25) after bilateralnerve repairs (Table 3). Assessed S2PD revealed the most variability (Figure 2). However, despite a range in the values, neither group showed statistical difference from the other in either unilateral or bilateral nerve injuries (P > 0.05).

Table 1 The modified Highet classification

Table 2 Demographic and some clinical characteristics of the included patients

Table 3 Sensory function and pain between unilateral and bilateral digital nerve repair groups
Recovery of sensation was also stratified into groups according to modified Highet classification (Table 1): excellent, S4; good, S3+; poor, S1—S3; and failure, S0. There was no significant difference in recovery of sensation measured by S2PD after unilateral and bilateral digital nerve repair (P > 0.05; Figure 3).
Warm/cold sensation, sharp/dull discrimination results
While basic sensory functions examined, warm/cold sensation showed faster recovery than sharp/dull sensation in groups 1 and 2 (P < 0.05). However, there were no significant differences in recovery of warm/cold sensation and discrimination of sharp/dull mechanical stimuli between groups 1 and 2 (P > 0.05) (Figure 4).
Overall subjective estimation of recovery results
Patients themselves assessed improvement of their pain after at least 12 months post-injury. The mean average overall subjective estimation of recovery in VAS was 6.67 (range, 2—10) in group 1 whereas it was slightly decreased in group 2 as 6.57 (range, 2—8) (Figure 5). However, statistical analysis did not show any significant difference between groups 1 and 2 (P > 0.05) (Table 3).
Adverse events
No patients exhibited positive Tinel’s sign at or distal to the incision on examination as well as cold intolerance, hyperesthesia or electrical pain.
Discussion
Digital nerves are the most frequently severed peripheral nerves (Thorsén et al., 2012) which may result from simple cuts to severe hand traumas. When digital nerve injuries are not repaired, in addition to sensory loss in the finger, there can be significant functional defects. Since the sensory function of the fingers was studied and described by Moberg (1962), many studies reported the management and the evaluation of the sensibility after repair of nerve injuries (Mermans et al., 2012). In 1990s, some researchers have introduced the argument of the collateral sprouting of nerve fibers from the intact digital nerve to the denervated regions of the injured nerve that could affect the objective measurement of the outcome of the digital nerve injuries. In 1995, Tadjalli et al. (1995a) reported a study to determine how to test nerve repair using a nerve isolation technique consisting of double-gloving, leaving the study finger free, and administering local anesthetic blocks to all other sensory contributions in the exposed digit. They found an excellent result in 77% for S2PD test in 13 single digital nerve lesions and after combination with nerve isolation technique, an excellent result in only 43% for S2PD test in the same digits. They concluded that nerve isolation technique is an important tool to assess the outcome of nerve repair and is the only method of evaluating true end outcomes of nerve regeneration after neurorrhaphy (Tadjalli et al., 1995a). However, another study published at the same year by the same authors which was a retrospective study describing their experiences regarding digital nerve repairs using standard tests without using their proposed nerve isolation technique, i.e., administering a local anesthetic block to the intact digital nerve, may be seen as a question mark (Tadjalli et al., 1995a, b). Thereafter, some researchers also used this isolation technique in their reports regarding outcomes after repair of digital nerve injuries (Wang et al., 1996; Segalman et al., 2001). Some scholars did not chose to anesthetize the uncut digital nerves as digital nerve isolation before assessing sensation because they believed in that the use of local anaesthesia may have inadvertently anaesthetized the digital nerve that they were trying to evaluate (Thomas et al., 2014). Weinzweig (2000) evaluated the role of crossover innervation after digital nerve injury by comparing recovery of sensibility after unilateral and bilateral epineural neurorrhaphies and noted no significant difference in S2PD in the distribution of the injured nerve between 20 digits with repairs of both nerves and 54 digits with repair of a single nerve, again indicating no significant crossover (Thomas et al., 2014). Actually, as Weinzweig (2000) highlighted, bilateral sharp digital nerve laceration could be seen as an excellent model for evaluation of crossover innervation because there is no confusion of anomalous innervation from an intact contralateral nerve. However, although there was no significant difference in recovery of sensibility after unilateral and bilateral digital nerve repairs which supports that crossover innervation did not appear to influence the long-term outcome after digital neurorrhaphy (Weinzweig, 2000), some textbooks are still mentioning the importance of crossover innervation from intact nerves in the long-term result of digital nerve repair (Bindra and Lanzinger, 2013). In contrast to all those articles in the literature proving their proposed theses within a consistency, our study has become an article aiming to clarify this controversy by using the most suitable model (Weinzweig, 2000) with eliminating proven other factors that affect the outcomes (Cheng, 1994) to increase the power of the study.
Cheng (1994) has stated in his article that functional sensory recovery of a repaired nerve is still unpredictable. He has mentioned that good functional sensation — defined as the ability of the hand to engage in full activities of daily living, including those activities in which vision is essentially occluded while the hand manipulates and identifies an object — relies on sufficient innervation density of slowly and quickly adapting fibers and their corresponding mechanical receptors such as Merkel cell-neurite complex, Meissner corpuscle, and Pacinian corpuscle. He has also mentioned that poor functional sensation after surgery is attributed to factors classified as preoperative (age of the patient, associated injuries, level of injury, and mechanism of injury), intraoperative (the surgeon’s experience in choosing a suturing technique and the time of surgery), or postoperative (rehabilitation or the factors that relate to patients themselves, such as their cooperation in rehabilitation programs and habits). In our study, all the factors that can affect the resultswere kept out.

Figure 1 A diagram illustrating study procedure.
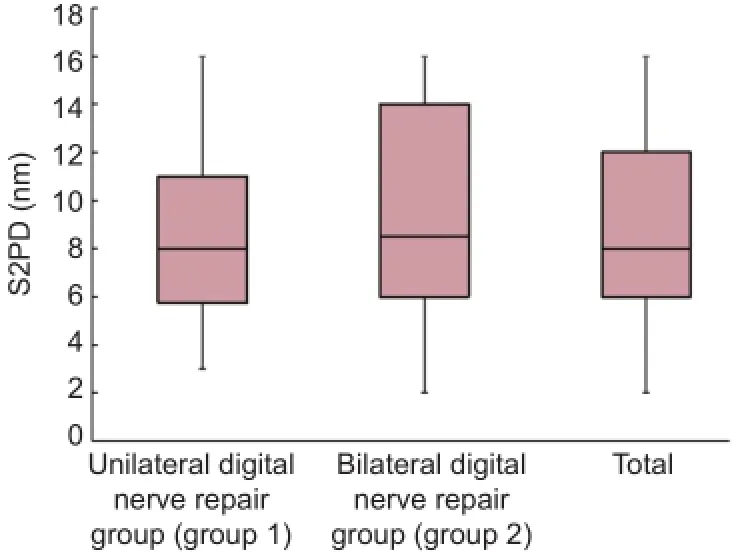
Figure 2 Static two-point discrimination (S2PD) results.

Figure 3 The modified Highet classification results.
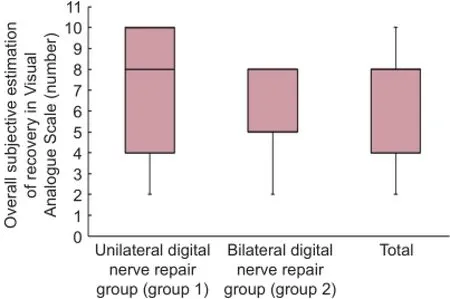
Figure 5 Assessment outcome of the Visual Analog Scale.
A meta-analysis and systematic review on outcome after digital nerve repair pointed out that patient’s age seems to play an important role in sensory recovery (Paprottka et al., 2013). The best results are seen in children and furthermore, nerve regeneration seems to deteriorate after the fifth- to sixth decade of life (Ruijs et al., 2005; Lohmeyer et al., 2009). Despite the study of Weinzweig (2000) which includes a minimum age of 6-year-old and 8-year-old patients, in our study age lower than 15 years old and greater than 65 years old were excluded. Additionally, in his retrospective study, Weinzweig (2000) reported the time interval from injury to surgical intervention as from less than 1 day to 300 days with a follow-up ranging from 6 to 77 months. However, despite the controversial studies that advocate no effect of delay(Mermans et al., 2012), the timing of surgical repair could be outlined to be a significant predictor for clinical outcome in digital nerve repair (Lohmeyer et al., 2009) and, from a general standpoint, there is a growing evidence showing that delaying peripheral nerve repair adversely affects outcome (Ozyurek and Atik, 2015). This can also make end-to-end repair difficult due to retraction and scarring of the nerve ends (Bindra and Lanzinger, 2013). Apart from that, there is an unfavorable prognosis for waiting more than 6 months or 1year after performing a nerve repair (Ruijs et al., 2005; Paprottka et al., 2013). Besides that, a very wide range of follow-up time within 6 to 77 months was reported in the study of Weinzweig (2000). Currently, the practice is that the peripheral nerves regenerate slowly, functional improvement may continue for a long time and recovery generally improves with an increased duration of follow-up (He et al., 2014). The timing of outcome evaluation after the repair of peripheral nerve injuries is therefore extremely important because if the duration of follow-up is too short, the final recovery of function cannot be assessed properly. Therefore, a minimum follow-up time should be at least 1 year, giving the nerve enough time for regeneration (Paprottka et al., 2013; He et al., 2014). Our study differs from most others cited in the literature in particular from Weinzweig’s (2000), because we narrowed the follow-up time and included-not late repair-only standardized time repair for eliminating negative estimates on the outcome.
Uninjured peripheral nerve axons have also been shown to be able to grow and reinnervate denervated territories in response to injury of neighboring axons, a process termed collateral sprouting (Wiesenfeld-Hallin et al., 1989). It is a fact that there is an evidence showing that collateral sprouting of at least some types of primary afferent fibers may occur in the skin of adult mammals (Snow and Wilson, 1991). The phenomenon of collateral sprouting has been shown to occur from sensory axons to denervated skin in the adult sensory system (Weddell et al., 1941). In Weddell et al.’s (1941) original study, they followed the progress of collateralization by testing the response of awake rabbits to pricking of the dorsal skin of ear which is supplied by two nerve trunks, the greater auricular nerve supplying the lateral part of the ear, and the occipital nerve supplying the medial part. They cut the greater auricular nerve and failed to evoke a characteristic withdrawal response to pin-prick test along the lateral part of the ear and, after 2—3 weeks, they observed that the medial area of the skin which remained sensitive to pin-prick testing expanded to include the area which had been insensitive previously (Weddell et al., 1941; Snow and Wilson, 1991). However, the point should be noticed is that those area is not containing all the insensitive area. So, it is not possible to know whether the spread of sensitivity is due to collateral innervation, or whether it is due to a strengthening within the central nervous system of normally ineffective afferent projections from pre-existing innervation (Snow and Wilson, 1991). In recent years, there is a growing interest regarding a new nerve repair technique named “end-to-side neurorrhaphy” described as the distal stump of a transected nerve is coapted to the side of an uninjured donor nerve for using when the proximal part of nerve stump is not available to classical end-to-end repair. End-to-side nerve repair can be seen a well-documented model for the phenomenon of collateral sprouting of axons. In an experimental study, it was concluded that invasion of the Schwann cells into the epineural layer of the donor nerve is the crucial step for the initiation of collateral axonal sprouting from the intact axons (Matsumoto et al., 1999). So, it is noticed that the most important theoretical and technical point is the necessity to create an epineural window when performing an end-to-side repair (Battiston et al., 2007). Unless one of the sprouts of an axon enters the distal segment of the axon’s own former Schwann tube, it will be unable to regain its original cutaneous receptive field (Snow and Wilson, 1991; Matsumoto et al., 1999).
In the literature, it is also commented as protective sensation does not return in affected skin if nerves are left unrepaired (Thorsén et al., 2012). In one prospective study, 108 unilateral digital nerve injuries were assessed by analyzing the outcomes of those patients who underwent digital nerve repair (72 digital nerves) compared with those nerves left unrepaired (36 digital nerves). In that study, it was shown that if no repair was carried out there would be some improvement in sensation for a period of 3—6 months only, and the end result would be inferior to repaired nerves (Chow and Ng, 1993; Clarke, 2016). In the repaired group, the result continued to improve for up to 2 years, 90% reached S3+or above whereas in the unrepaired group, improvement plateaued after 6 months, and at 2 years only 6% reached S3+or above. Also 94% of patients with unrepaired nerves regained some protective sensibility (Chow and Ng, 1993). It is clear that in a digital nerve transection, the result of recovery will remain poor if the axons not passed or not sprouted through a tube formed by Schwann cells. When injured axons fail to regenerate, collateral sprouting could be useful by, for example, providing protective pain sensation to a denervated cutaneous area (Wiesenfeld-Hallin et al., 1989). However, as in our results, it may be commented as if the transected nerves repaired properly, the collateral sprouting and innervation could be in a negligible level.
There are significant limitations of this study including mainly our relatively reduced number of injured nerves due to our extended exclusion criteria to compare only the clinically importance of the crossover innervation. The retrospective nature of the study is the other limitation which may introduce selection bias related to such type of study. Furthermore, one additional limitation is that a standardized rehabilitation program after surgery was not applied which was shown that it may improve the clinical outcomes.
As a conclusion, after eliminating all the factors that may affect the outcome of digital nerve repairs, our results suggest that there may be no significant difference in sensibility recovery at least 1 year after nerve repair of complete digital nerve laceration between unilateral and bilateral digital nerve repairs. In our clinical and retrospective study, it is impossible to deny the crossover innervation from uninjureddigital nerve to injured side; but, if any, we could say it has no importance in the evaluation and/or measurement of the outcomes of digital nerve repair outcomes. Within the observations, clinically, the effect of crossover innervation appeared negligible in the estimation of digital neurorrhaphy results. Despite some limitations, we believe that this study can provide valuable data with significant clinical perspectives. As it is said: “It is not so important to know everything as to know the exact value of everything, to appreciate what we learn, and to arrange what we know.”
Author contributions: MO and KO designed the study, wrote and revised the paper. ÖÇ and YK analyzed the data and revised the paper. UK designed the postoperative evaluation of the patients and revised the paper. All authors contributed to the surgical operations of the patients and approved the final version of the paper.
Conflicts of interest: None declared.
Author statements: This study is one part of a designed study and the other part was presented as oral presentation at the 9thCongress of the Balkan Association of Plastic Reconstructive and Aesthetic Surgeons (B.A.P.R.A.S) in Thessaloniki, Greece on 17 and 20 September 2015.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Allan CH (2004) Primary nerve repair: Indications and results. J Am Soc Surg Hand 4:195-199.
Battiston B, Tos P, Conforti LG, Geuna S (2007) Alternative techniques for peripheral nerve repair: conduits and end-to-side neurorrhaphy. Millesi H, Schmidhammer R, eds. Acta Neurochir Suppl 100:43-50.
Bindra RR, Lanzinger WD (2013) Digital nerve repair. In: The Art of Microsurgical Hand Reconstruction (Slutsky DJ, ed), pp 83-88. Thieme.
Cheng AS (1994) Use of early tactile stimulation in rehabilitation of digital nerve injuries. Am J Occup Ther 54:159-165.
Chow SP, Ng C (1993) Can a divided digital nerve on one side of the finger be left unrepaired? J Hand Surg Br 18:629-630.
Clarke AM (2016) Commentary on long-term clinical outcome after epineural coaptation of digital nerves. R. M. Fakin, M. Calcagni, H. J. Klein and P. Giovanoli. J Hand Surg. 2016, 41: 148-154. J Hand Surg Eur Vol 41:155-156.
Dagum AB (1998) Peripheral nerve regeneration, repair, and grafting. J Hand Ther 11:111-117.
Fakin RM, Calcagni M, Klein HJ, Giovanoli P (2016) Long-term clinical outcome after epineural coaptation of digital nerves. J Hand Surg Eur Vol 41:148-154.
Haug A, Bartels A, Kotas J, Kunesch E (2013) Sensory recovery 1 year after bridging digital nerve defects with collagen tubes. J Hand Surg Am 38:90-97.
He B, Zhu Z, Zhu Q, Zhou X, Zheng C, Li P, Zhu S, Liu X, Zhu J (2014) Factors predicting sensory and motor recovery after the repair of upper limb peripheral nerve injuries. Neural Regen Res 9:661-672.
Lohmeyer JA, Sommer B, Siemers F, Mailänder P (2009) Nerve injuries of the upper extremity-expected outcome and clinical examination. Plast Surg Nurs 29:88-93.
Matsumoto M, Hirata H, Nishiyama M, Morita A, Sasaki H, Uchida A (1999) Schwann cells can induce collateral sprouting from intact axons: experimental study of end-to-side neurorrhaphy using a Y-chamber model. J Reconstr Microsurg 15:281-286.
Mermans JF, Franssen BB, Serroyen J, Van der Hulst RR (2012) Digital nerve injuries: a review of predictors of sensory recovery after microsurgical digital nerve repair. Hand (N Y) 7:233-241.
Moberg E (1962) Criticism and study of methods for examining sensibility in the hand. Neurology 12:8-19.
Ozyurek S, Atik A (2015) Re: Imao K, Tsubokawa N, Maki Y. Trans-scaphoid-perilunate dislocation with an ulnar nerve injury. J Hand Surg Eur. Epub ahead of print 12 May 2015. DOI: 10.1177/1753193415583951. J Hand Surg Eur Vol 40:876-877.
Paprottka FJ, Wolf P, Harder Y, Kern Y, Paprottka PM, Machens HG, Lohmeyer JA (2013) Sensory recovery outcome after digital nerve repair in relation to different reconstructive techniques: meta-analysis and systematic review. Plast Surg Int 2013:704589.
Rinkel WD, Huisstede BM, van der Avoort DJ, Coert JH, Hovius SE (2013) What is evidence based in the reconstruction of digital nerves? A systematic review. J Plast Reconstr Aesthet Surg 66:151-164.
Ruijs AC, Jaquet JB, Kalmijn S, Giele H, Hovius SE (2005) Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg 116:484-494.
Segalman KA, Cook PA, Wang BH, Theisen L (2001) Digital neurorrhaphy after the age of 60 years. J Reconstr Microsurg 17:85-88.
Snow PJ, Wilson P (1991) Plasticity in the Somatosensory System of Developing and Mature Mammals — The Effects of Injury to the Central and Peripheral Nervous System. Berlin, Heidelberg: Springer Berlin Heidelberg.
Tadjalli HE, McIntyre FH, Dolynchuk KN, Murray KA (1995a) Importance of crossover innervation in digital nerve repair demonstrated by nerve isolation technique. Ann Plast Surg 35:32-35.
Tadjalli HE, McIntyre FH, Dolynchuk KN, Murray KA (1995b) Digital nerve repair: relationship between severity of injury and sensibility recovery. Ann Plast Surg 35:36-40.
Thomas PR, Saunders RJ, Means KR (2015) Comparison of digital nerve sensory recovery after repair using loupe or operating microscope magnification. J Hand Surg Eur Vol 40:608-613.
Thorsén F, Rosberg HE, Steen Carlsson K, Dahlin LB (2012) Digital nerve injuries: epidemiology, results, costs, and impact on daily life. J Plast Surg Hand Surg 46:184-190.
Vipond N, Taylor W, Rider M (2007) Postoperative splinting for isolated digital nerve injuries in the hand. J Hand Ther 20:222-231.
Wang WZ, Crain GM, Baylis W, Tsai TM (1996) Outcome of digital nerve injuries in adults. J Hand Surg Am 21:138-143.
Weddell G, Guttmann L, Guttmann E (1941) The local extension of nerve fibers into denervated areas of skin. J Neurol Psychiat 4:206-225.
Weinzweig N (2000) Crossover innervation after digital nerve injury: myth or reality? Ann Plast Surg 45:509-514.
Wiesenfeld-Hallin Z, Kinnman E, Aldskogius H (1989) Expansion of innervation territory by afferents involved in plasma extravasation after nerve regeneration in adult and neonatal rats. Exp Brain Res 76:88-96.
Copyedited by Li CH, Song LP, Zhao M
10.4103/1673-5374.191226 Accepted: 2016-09-07
*Correspondence to:
- 中国神经再生研究(英文版)的其它文章
- Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Sigma-1 receptor and neuroprotection: current outlook and potential therapeutic effects
- Intra-axonal protein synthesis - a new target for neural repair?
- Nanobiomaterials for neural regeneration
- Cell transplantation for the treatment of spinal cord injury — bone marrow stromal cells and choroid plexus epithelial cells

