Association between chromosomal aberration of COX8C and tethered spinal cord syndrome: arraybased comparative genomic hybridization analysis
Qiu-jiong Zhao, Shao-cong Bai, Cheng Cheng Ben-zhang Tao Le-kai Wang Shuang Liang Ling Yin, Xing-yi Hang, Ai-jia Shang Department of Neurosurgery, Chinese PLA General Hospital, Beijing, China2 iGeneTech Biotechnology Co., Ltd., Beijing, China Department of Neurology, Chinese PLA General Hospital, Beijing, China
Association between chromosomal aberration of COX8C and tethered spinal cord syndrome: arraybased comparative genomic hybridization analysis
Qiu-jiong Zhao1,#, Shao-cong Bai1,#, Cheng Cheng1, Ben-zhang Tao1, Le-kai Wang1, Shuang Liang1, Ling Yin3, Xing-yi Hang2,*, Ai-jia Shang1,*
1 Department of Neurosurgery, Chinese PLA General Hospital, Beijing, China
2 iGeneTech Biotechnology Co., Ltd., Beijing, China
3 Department of Neurology, Chinese PLA General Hospital, Beijing, China
How to cite this article: Zhao QJ, Bai SC, Cheng C, Tao BZ, Wang LK, Liang S, Yin L, Hang XY, Shang AJ (2016) Association between chromosomal aberration of COX8C and tethered spinal cord syndrome∶ array-based comparative genomic hybridization analysis. Neural Regen Res 11(8)∶1333-1338.
Ai-jia Shang, M.D., Ph.D. or Xing-yi Hang, Ph.D.,
shangaj@163.com or
xingyi.hang@igenetech.com.
#These authors contributed
equally to this study.
orcid:
0000-0002-4895-5442
(Ai-jia Shang)
0000-0002-3736-2203
(Xing-yi Hang)
Accepted: 2016-08-09
Graphical Abstract
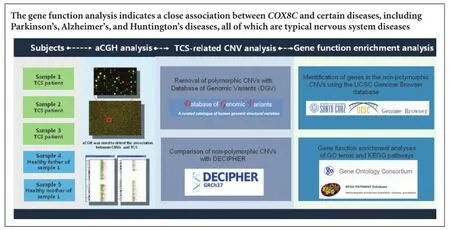
Copy number variations have been found in patients with neural tube abnormalities. In this study, we performed genome-wide screening using high-resolution array-based comparative genomic hybridization in three children with tethered spinal cord syndrome and two healthy parents. Of eight copy number variations, four were non-polymorphic. These non-polymorphic copy number variations were associated with Angelman and Prader-Willi syndromes, and microcephaly. Gene function enrichment analysis revealed that COX8C, a gene associated with metabolic disorders of the nervous system, was located in the copy number variation region of Patient 1. Our results indicate that array-based comparative genomic hybridization can be used to diagnose tethered spinal cord syndrome. Our results may help determine the pathogenesis of tethered spinal cord syndrome and prevent occurrence of this disease.
nerve regeneration; neural tube defects; tethered spinal cord syndrome; comparative genomic hybridization; COX8C; gene function enrichment analysis; database of genomic variants; database of DECIPHER; copy number variations; neural regeneration
Introduction
Tethered spinal cord syndrome (TCS) is a neurodevelopmental disorder that results in spinal cord malformation (Payne, 2007; Cearns et al., 2016). TCS is classified as a neural tube defect, and although the incidence of neural tube defects is approximately 1% worldwide (Feuchtbaum et al., 1999; Tunçbilek et al., 1999; van der Put et al., 2001; Khoshnood et al., 2015; Atta et al., 2016), infants born with neural tube defects account for 20-25% of all congenital malformations (Laharwal et al., 2016). The causes of neural tube defects are multivariate, yet to date there is no convincing mechanistic evidence for their occurrence. Some possible contributing factors include gene mutations, chromosomal abnormalities, and environmental factors (Bassuk and Kibar, 2009; Joó, 2009a, b; Molloy et al., 2009; Wen et al., 2009). Recent studies have revealed novel risk factors for neural tube defects including heterozygous missense mutations in the genes, VANGL1 and FUZZY (Bartsch et al., 2012; Seo et al., 2015), as well as maternal folic acid deficiency (Bartsch et al., 2012; Seo et al., 2015). Altered methylation of MGMT, aDNA repair gene, is also associated with neural tube defects (Tran et al., 2012). Moreover, abnormal expression of genes coding for zinc finger proteins is reported to be risk factors (Grinberg and Millen, 2005; Costa-Lima et al., 2008).
Previous studies have shown that chromosomal imbalances due to genomic instability are closely associated with neural developmental disorders (Au et al., 2010; Zhao et al., 2013). Copy number variations (CNVs) are found in patients with neural tube abnormalities in cerebral and spinal sections (Bassuk et al., 2013; Chen et al., 2013). Array-based comparative genomic hybridization (aCGH) is a modern technique for molecular karyotype analysis that combines conventional comparative genomic hybridization and microarray analysis (Saberi et al., 2014). In contrast to conventional hybridization, aCGH does not detect metaphase chromosomes. Instead, it targets genomic DNA to perform high-throughput screening of the whole genome for CNVs (Vissers et al., 2003). The aCGH approach can accurately locate CNVs on chromosomes, and clearly calculate CNV length and identify genes within variant fragments (Mosse et al., 2005). Nowadays, aCGH is commonly used for cancer and genetic disorder research (Kallioniemi, 2008; Sireteanu et al., 2012). In this study, we used aCGH to detect CNVs in three children with TCS and two healthy parents. In order to examine TCS pathogenesis at the chromosome and gene levels, we determined the relationship between these chromosomal aberrations and TCS, and consequently detected CNVs linked with occurrence and development of TCS.
Subjects and Methods
Subjects
Three children diagnosed with typical TCS based on clinical criteria (Filippidis et al., 2010) by the Department of Neurosurgery at the Chinese PLA General Hospital and the Second Artillery General Hospital, and the healthy parents of Patient 1 were enrolled in the study. Peripheral blood samples were collected from the patients and healthy controls. Before initiation of the study, written consent was obtained from the guardians of all children. The study (Project ID: S2013-117-01) was approved by the ethics committee of the Chinese PLA General Hospital, China.
Case 1 was a 2-year-old girl with a sacrococcygeal mass and right foot deformity. The sacrococcygeal mass was identified at birth. Physical examination revealed spina bifida. Strephenopodia of the right foot and a second enlarging sacrococcygeal mass were first observed at 8 months of age. The patient was diagnosed with TCS with myelomeningocele.
Case 2 was a 12-year-old boy who presented with a lumbosacral mass at the age of 8 months. The patient was diagnosed with TCS with spinal cord lipoma. Surgical treatment was performed. Urinary abnormality occurred 11 years after surgery, along with urinary incontinence, nocturnal enuresis, urinary frequency, and urinary urgency. A further surgery was performed because magnetic resonance imaging showed spinal cord lipoma and recurrence of TCS.
Case 3 was a 5-year-old girl with abnormal hair growth in the lumbosacral region at birth. Physical examination revealed a partial spinal canal defect. Because the hair growth increased, magnetic resonance imaging examination was performed. The results revealed a tethered spinal cord and split cord malformation (Type I). Surgery was performed to correct the malformation.
aCGH analysis
aCGH is a specific array-based genomic hybridization method that uses different fluorescent dyes to label DNA from patients and controls, to identify differences between the two groups (Sealfon and Chu, 2011; Brady and Vermeesch, 2012). By comparing the ratio of two different fluorescence signals at each target spot in the microarray, CNVs are detected in specific sequences or genes between two genomes (Gijsbers et al., 2011; Shoukier et al., 2013).
Total DNA was extracted from peripheral whole blood using a commercially available DNA-isolation kit (BioChain Inc., Beijing, China), according to the manufacturer’s protocol. For each aCGH experiment, purified DNA and normal sex-matched DNA (1 μg each; Promega, Madison, WI, USA) were digested with AluI and RsaI (10 U each; Promega), and differentially labelled with cyanine-5 and cyanine-3 fluorescent dyes using a Genomic DNA Enzymatic Labeling Kit (Agilent, Santa Clara, CA, USA). aCGH analysis was performed using the Agilent 8 × 60K commercial array. This platform contains 60-mer oligonucleotide probes spanning the entire human genome with an overall mean probe spacing of 50 kb. After hybridization, arrays were scanned using a dual-laser scanner (Agilent), and images extracted and analyzed using the Feature Extraction (Agilent) and Workbench genomics software, respectively. Changes in test DNA copy number at specific loci were considered only if they were <-0.38 (deletion) or > 0.38 (amplification) of the log2 ratio values from at least five consecutive probes.
TCS-related CNV analysis
Removal of polymorphic CNVs using the Database of Genomic Variants
CNV fragments were scanned against the Database of Genomic Variants (Iafrate et al., 2004; Wong et al., 2007). CNVs that completely matched those in the database were removed as they represent common polymorphic variants present in the normal population. Partially overlapping (< 40%) CNVs were considered non-polymorphic and retained for further analysis. In addition, discontinuous polymorphic fragments appearing within CNV sequences (total fragment length was shorter than half-lengths of detected CNVs) were not treated as common polymorphisms and were also retained for further analysis.
Comparison of non-polymorphic CNVs with DECIPHER
The non-polymorphic CNV fragments selected above were searched against the DECIPHER database (Firth et al., 2009). Cases were identified with CNVs similar to those reported in previously tested samples (partial overlap >60%) or containing documented CNVs. Additionally, chromosomal abnormalities, related phenotypes, and syndromes associated with these cases were identified.
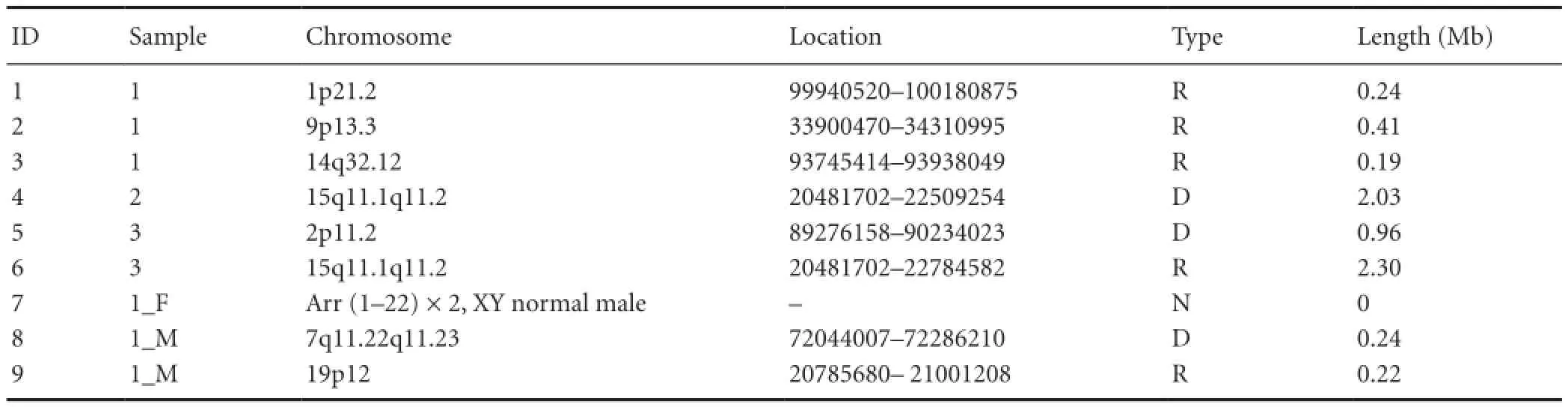
Table 1 Array-comparative genome hybridization analysis of TCS patients and controls
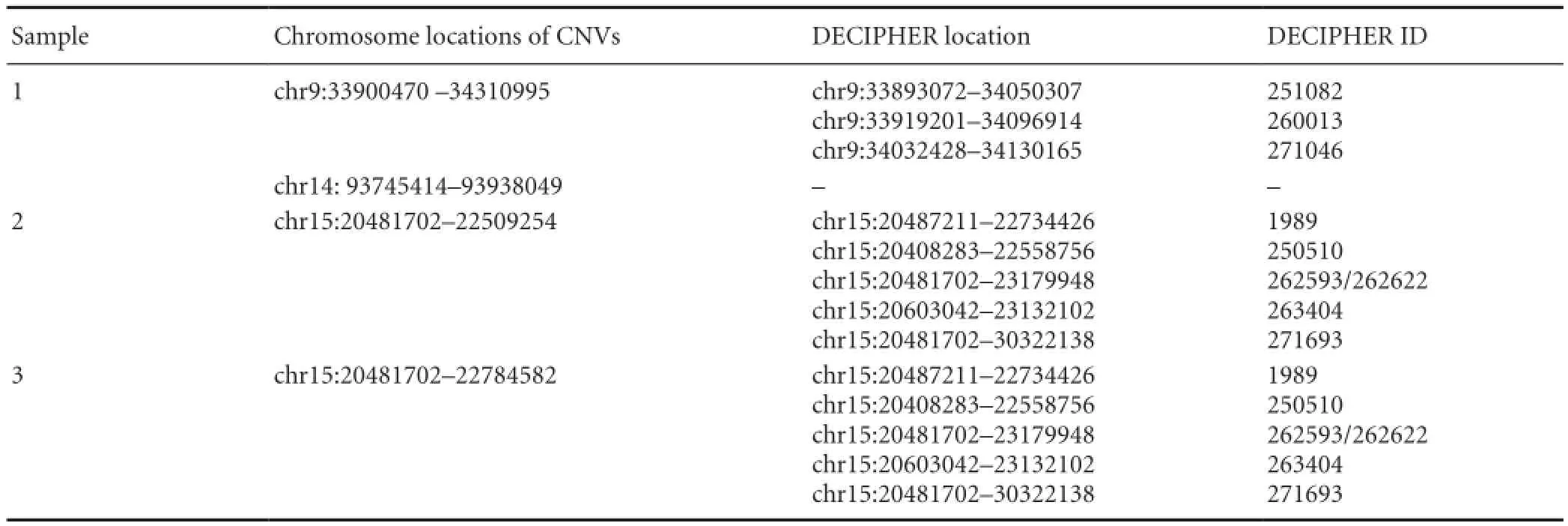
Table 2 DECIPHER search results for non-polymorphic copy number variations (CNVs)
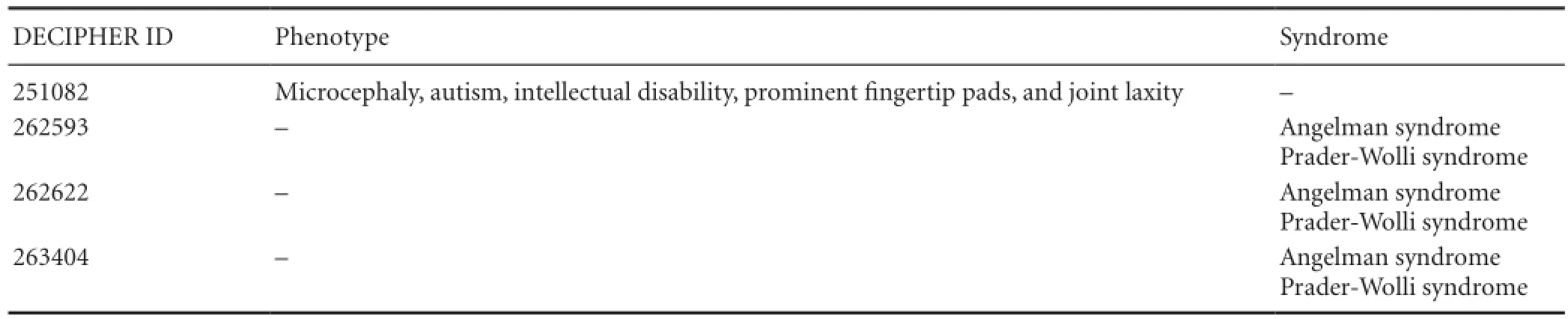
Table 3 Syndromes and clinical phenotypes linked to non-polymorphic copy number variations

Table 4 Genes contained in non-polymorphic copy number variations
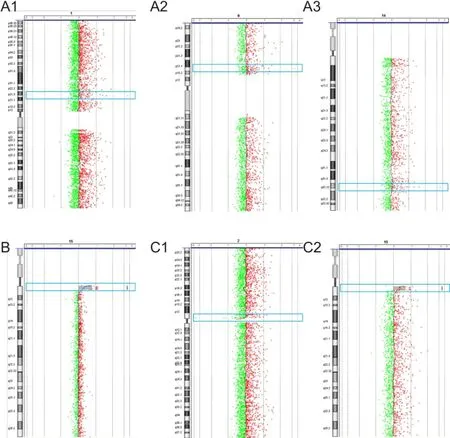
Figure 1 Chromosome maps of the three patients with tethered spinal cord syndrome.
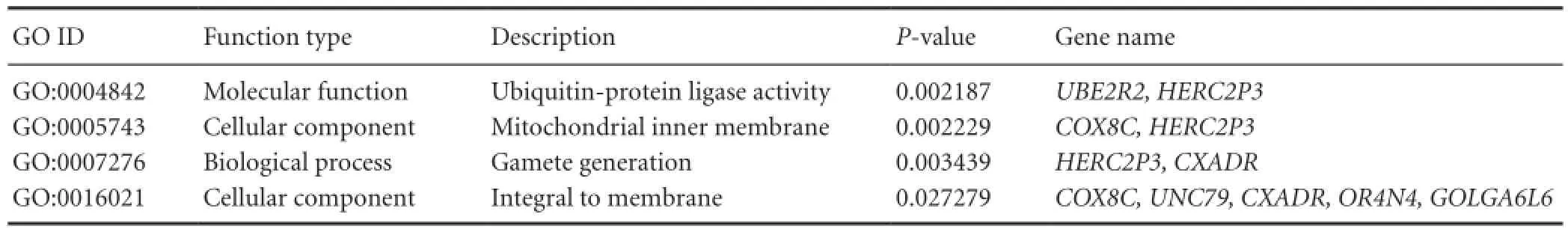
Table 5 Enrichment results for gene ontology (GO) analysis
Gene function enrichment analysis
Entire genes incorporated in non-polymorphic CNVs were identified using the University of California, Santa Cruz (UCSC) Genome Browser database (http://genome.ucsc.edu/). Gene function enrichment analyses were performed for the genes identified, including Gene Ontology (GO) (http://geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway (http://www.genome.jp/kegg/) analyses.
Enrichment P-values for each GO term or KEGG pathway were calculated using the hyper-geometric distribution method. P-values were then corrected for multiple hypotheses testing using the false discovery rate method. A P-value of 0.05 was set as the threshold value for significant gene enrichment for each GO term or KEGG pathway.
Results
Gene micro-repeat fragment location in TCS patients
Results of the aCGH analysis for all three patients and two parents are shown in Table 1. Three micro-repeat fragments were detected in DNA isolated from Patient 1. A micro-deletion fragment was detected in Patient 2, while a micro-deletion and micro-repeat were detected in Patient 3. The father of Patient 1 had a normal karyotype, whereas the mother’s chromosome map showed micro-deletion and micro-repeat fragments. The micro-deletion fragment in Patient 2 and micro-repeat fragment in Patient 3 were located in the same region: 15q11.1q11.2 (Figure 1).
Database searching of CNVs
The eight identified CNVs were searched against the Database of Genomic Variants. The results showed that four CNVs were normal chromosomal polymorphisms, specifically, the 1p21.2 micro-repeat in Patient 1, 2p11.2 micro-deletion in Patient 3, and 7q11.22q11.23 micro-deletion and 19p12 micro-repeat in the mother of Patient 1.
Investigation of the other four non-polymorphic CNVsin DECIPHER revealed eight specific CNVs in these regions (Table 2). Non-polymorphic CNVs in Patients 2 and 3 (ID 4 and 6 in Table 1) shared the same chromosomal initiation site, indicating that multiple CNVs occur in the same location. Further analyses revealed that these CNVs are associated with two syndromes (Angelman and Prader-Willi) and one phenotype (microcephaly) (Table 3).
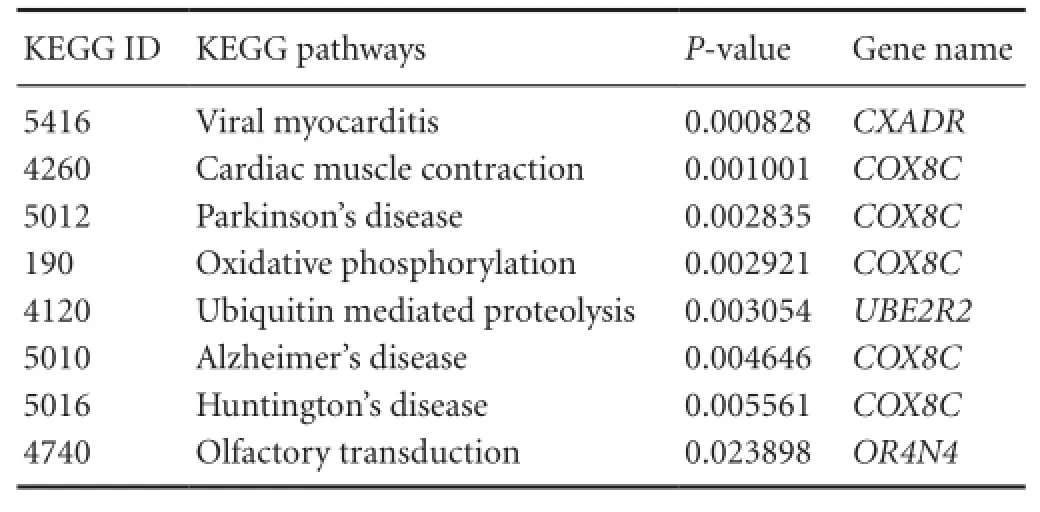
Table 6 Gene enrichment analysis
Gene function enrichment analysis
Within the four non-polymorphic CNVs regions, 13 genes were identified by the UCSC Genome Browser (Table 4). Function enrichment analysis of GO terms and KEGG pathways were performed for these genes. The results included a number of biological functions (e.g., gamete generation), molecular functions (e.g., ubiquitin-protein ligase activity), two cellular components (mitochondrial inner membrane and integral membrane component), as well as eight KEGG pathways, including viral myocarditis, cardiac muscle contraction, Parkinson’s disease, oxidative phosphorylation, ubiquitin-mediated proteolysis, Alzheimer’s disease, Huntington’s disease, and olfactory transduction. From these results, we found that the COX8C gene is closely related to neural system diseases such as Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease (Tables 5, 6).
Discussion
Advantages of using aCGH for detection of rare chromosomal micro-variations
Chromosomal sub-microscopic variations are strongly associated with human disease (Feuk et al., 2006). In clinical settings, the definite diagnosis of several diseases cannot be achieved using existing techniques. Consequently, some rare syndromes are labelled idiopathic or unexplained. Most of these syndromes are due to genomic imbalances created by chromosomal micro-variations such as micro-deletions and micro-repeats (D’Angelo et al., 2014). The aCGH approach efficiently detects chromosomal micro-aberrations and aids elucidation of idiopathic or unexplained diseases.
Significance and limitations of aCGH analysis
The main objective of this study was to identify non-random CNVs and evaluate their association with TCS. The main questions regarding the CNVs we identified are: (1) whether the CNVs are inherited; (2) whether they are found in the normal population; (3) whether their lengths are sufficient to contain genes with functional annotations; (4) whether they are linked to diseases in DECIPHER; and (5) whether any are unreported, unidentified, or novel. Although the Database of Genomic Variants and DECIPHER, which are globally representative databases, were used to determine the type of CNVs identified, ethnic differences are inevitable when using international databases.
Diseases similar to TCS that are associated with COX8C
CNVs similar to the ones we detected are found in the DECIPHER database. These CNVs are associated with Angelman and Prader-Willi syndromes, and microcephaly. All of these disorders involve significant neural abnormalities (Mabb et al., 2011; Mahmood et al., 2011; Cassidy et al., 2012). Furthermore, gene function analysis indicated a close association between COX8C and certain diseases including Parkinson’s, Alzheimer’s, and Huntington’s diseases, all of which are typical nervous system diseases (Bassil and Mollaei, 2012; Pogledić and Relja, 2012; Gazewood et al., 2013). By comparing the CNVs from Patient 1 with those identified in her parents, we excluded the possibility of TCS being hereditary. Thus, we propose that the condition may be acquired during neural development.
Conclusion
In this study, we used high-resolution aCGH to identify pathogenic CNVs in samples from patients with typical TCS. Our findings suggest an association between certain CNVs and nervous system disease. Our data may be used in the future as a reference for the integration of available data, or for further studies with larger sample sizes. Ours study demonstrates specific transformation research, and shows that a molecular method can be used to clinically diagnose TCS. Our findings may help to shed new light on the pathogenesis of TCS.
Acknowledgments: We are very grateful to the staffs of iGene-Tech Biotechnology Co., Ltd. in China for some of the experiment operations.
Author contributions: QJZ and SCB performed the experiment. CC, BZT and LKW collected patients, and conducted clinical communication and treatment. SL and LY provided technical and capital supports. QJZ and XYH analyzed and explained data. AJS and XYH served as principle investigators. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, Rajapakse T, Kaplan GG, Metcalfe A (2016) Global birth prevalence of spina bifida by folic acid fortification status: A systematic review and meta-analysis. Am J Public Health 106:e24-34.
Au KS, Ashley-Koch A, Northrup H (2010) Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev 16:6-15.
Bartsch O, Kirmes I, Thiede A, Lechno S, Gocan H, Florian IS, Haaf T, Zechner U, Sabova L, Horn F (2012) Novel VANGL1 gene mutations in 144 Slovakian, Romanian and German patients with neural tube defects. Mol Syndromol 3:76-81.
Bassil N, Mollaei C (2012) Alzheimer’s dementia: a brief review. J Med Liban 60:192-199.
Bassuk AG, Kibar Z (2009) Genetic basis of neural tube defects. Semin Pediatr Neurol 16:101-110.
Bassuk AG, Muthuswamy LB, Boland R, Smith TL, Hulstrand AM, Northrup H, Hakeman M, Dierdorff JM, Yung CK, Long A, Brouillette RB, Au KS, Gurnett C, Houston DW, Cornell RA, Manak JR (2013) Copy number variation analysis implicates the cell polarity gene glypican 5 as a human spina bifida candidate gene. Hum Mol Genet 22:1097-1111.
Brady PD, Vermeesch JR (2012) Genomic microarrays: a technology overview. Prenat Diagn 32:336-343.
Cassidy SB, Schwartz S, Miller JL, Driscoll DJ (2012) Prader-Willi syndrome. Genet Med 14:10-26.
Cearns MD, Escuin S, Alexandre P, Greene ND, Copp AJ (2016) Microtubules, polarity and vertebrate neural tube morphogenesis. J Anat 229:63-74.
Chen X, Shen Y, Gao Y, Zhao H, Sheng X, Zou J, Lip V, Xie H, Guo J, Shao H, Bao Y, Shen J, Niu B, Gusella JF, Wu BL, Zhang T (2013) Detection of copy number variants reveals association of cilia genes with neural tube defects. PLoS One 8:e54492.
Costa-Lima MA, Meneses HN, El-Jaick KB, Amorim MR, Castilla EE, Orioli IM (2008) No association of the polyhistidine tract polymorphism of the ZIC2 gene with neural tube defects in a South American (ECLAMC) population. Mol Med Rep 1:443-446.
D’Angelo CS, Varela MC, de Castro CI, Kim CA, Bertola DR, Lourenço CM, Perez ABA, Koiffmann CP (2014) Investigation of selected genomic deletions and duplications in a cohort of 338 patients presenting with syndromic obesity by multiplex ligation-dependent probe amplification using synthetic probes. Mol Cytogenet 7:75.
Feuchtbaum LB, Currier RJ, Riggle S, Roberson M, Lorey FW, Cunningham GC (1999) Neural tube defect prevalence in California (1990-1994): eliciting patterns by type of defect and maternal race/ ethnicity. Genet Test 3:265-272.
Feuk L, Marshall CR, Wintle RF, Scherer SW (2006) Structural variants: changing the landscape of chromosomes and design of disease studies. Hum Mol Genet 15:R57-66.
Filippidis AS, Kalani MY, Theodore N, Rekate HL (2010) Spinal cord traction, vascular compromise, hypoxia, and metabolic derangements in the pathophysiology of tethered cord syndrome. Neurosurg Focus 29:E9.
Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, Rajan D, Vooren SV, Moreau Y, Pettett RM, Carter NP (2009) DECIPHER: Database of chromosomal imbalance and phenotype in humans using ensembl resources. Am J Hum Genet 84:524-533.
Gazewood JD, Richards DR, Clebak K (2013) Parkinson disease: an update. Am Fam Physician 87:267-273.
Gijsbers AC, Schoumans J, Ruivenkamp CA (2011) Interpretation of array comparative genome hybridization data: a major challenge. Cytogenet Genome Res 135:222-227.
Grinberg I, Millen KJ (2005) The ZIC gene family in development and disease. Clin Genet 67:290-296.
Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y, Scherer SW, Lee C (2004) Detection of large-scale variation in the human genome. Nat Genet 36:949-951.
Joó JG (2009a) Recent perspectives on the development of the central nervous system and the genetic background of neural tube defects. Orv Hetil 150:873-882.
Joó JG (2009b) Recent perspectives on the genetic background of neural tube defects with special regard to iniencephaly. Expert Rev Mol Diagn 9:281-293.
Kallioniemi A (2008) CGH microarrays and cancer. Curr Opin Biotechnol 19:36-40.
Khoshnood B, Loane M, de Walle H, Arriola L, Addor MC, Barisic I, Beres J, Bianchi F, Dias C, Draper E, Garne E, Gatt M, Haeusler M, Klungsoyr K, Latos-Bielenska A, Lynch C, McDonnell B, Nelen V, Neville AJ, O’Mahony MT, et al. (2015) Long term trends in prevalence of neural tube defects in Europe: population based study. BMJ 351:h5949.
Laharwal MA, Sarmast AH, Ramzan AU, Wani AA, Malik NK, Arif SH, Rizvi M (2016) Epidemiology of the neural tube defects in Kashmir Valley. Surg Neurol Int 7:35.
Mabb AM, Judson MC, Zylka MJ, Philpot BD (2011) Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci 34:293-303.
Mahmood S, Ahmad W, Hassan MJ (2011) Autosomal recessive primary microcephaly (MCPH): clinical manifestations, genetic heterogeneity and mutation continuum. Orphanet J Rare Dis 6:39-39.
Molloy AM, Brody LC, Mills JL, Scott JM, Kirke PN (2009) The search for genetic polymorphisms in the homocysteine/folate pathway that contribute to the etiology of human neural tube defects. Birth Defects Res A Clin Mol Teratol 85:285-294.
Mosse YP, Greshock J, Weber BL, Maris JM (2005) Measurement and relevance of neuroblastoma DNA copy number changes in the post-genome era. Cancer Lett 228:83-90.
Payne J (2007) Tethered spinal cord syndrome. BMJ 335:42-43.
Pogledić I, Relja M (2012) Huntington’s disease. Lijec Vjesn 134:346-350.
Saberi A, Shariati G, Hamid M, Galehdari H, Abdorasouli N (2014) Wolf-Hirschhorn syndrome: a case with normal karyotype, demonstrated by array CGH (aCGH). Arch Iran Med 17:642-644.
Sealfon SC, Chu TT (2011) RNA and DNA Microarrays. Methods Mol Biol 671:3-34.
Seo JH, Zilber Y, Babayeva S, Liu J, Kyriakopoulos P, De Marco P, Merello E, Capra V, Gros P, Torban E (2015) Mutations in the planar cell polarity gene, Fuzzy, are associated with neural tube defects in humans. Hum Mol Genet 24:3893.
Shoukier M, Klein N, Auber B, Wickert J, Schröder J, Zoll B, Burfeind P, Bartels I, Alsat EA, Lingen M, Grzmil P, Schulze S, Keyser J, Weise D, Borchers M, Hobbiebrunken E, Röbl M, Gärtner J, Brockmann K, Zirn B (2013) Array CGH in patients with developmental delay or intellectual disability: are there phenotypic clues to pathogenic copy number variants? Clin Genet 83:53-65.
Sireteanu A, Covic M, Gorduza EV (2012) Array CGH: technical considerations and applications. Rev Med Chir Soc Med Nat Iasi 116:545-551.
Tran S, Wang L, Le J, Guan J, Wu L, Zou J, Wang Z, Wang J, Wang F, Chen X, Cai L, Lu X, Zhao H, Guo J, Bao Y, Zheng X, Zhang T (2012) Altered methylation of the DNA repair gene MGMT is associated with neural tube defects. J Mol Neurosci 47:42-51.
Tunçbilek E, Boduro lu K, Alika ifo lu M (1999) Neural tube defects in Turkey: prevalence, distribution and risk factors. Turk J Pediatr 41:299-305.
van der Put NM, van Straaten HW, Trijbels FJ, Blom HJ (2001) Folate, homocysteine and neural tube defects: an overview. Exp Biol Med (Maywood) 226:243-270.
Vissers Lisenka E, de Vries Bert B, Osoegawa K, Janssen Irene M, Feuth T, Choy Chik O, Straatman H, van der Vliet W, Huys Erik H, van Rijk A, Smeets D, van Ravenswaaij-Arts Conny M, Knoers Nine V, van der Burgt I, de Jong Pieter J, Brunner Han G, van Kessel Ad G, Schoenmakers Eric F, Veltman Joris A (2003) Array-based comparative genomic hybridization for the genomewide detection of submicroscopic chromosomal abnormalities. Am J Hum Genet 73:1261-1270.
Wen S, Lu W, Zhu H, Yang W, Shaw GM, Lammer EJ, Islam A, Finnell RH (2009) Genetic polymorphisms in the thioredoxin 2 (TXN2) gene and risk for spina bifida. Am J Med Genet A 149A:155-160.
Wong Kendy K, deLeeuw Ronald J, Dosanjh Nirpjit S, Kimm Lindsey R, Cheng Z, Horsman Douglas E, MacAulay C, Ng Raymond T, Brown Carolyn J, Eichler Evan E, Lam Wan L (2007) A comprehensive analysis of common copy-number variations in the human genome. Am J Hum Genet 80:91-104.
Zhao J, Guan T, Wang J, Xiang Q, Wang M, Wang X, Guan Z, Xie Q, Niu B, Zhang T (2013) Influence of the antifolate drug Methotrexate on the development of murine neural tube defects and genomic instability. J Appl Toxicol 33:915-923.
Copyedited by James R, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.189200
*Correspondence to:
- 中国神经再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials
- No synergism between bis(propyl)-cognitin and rasagiline on protecting dopaminergic neurons in Parkinson's disease mice
- Rebuilding motor function of the spinal cord based on functional electrical stimulation
- Acellular allogeneic nerve grafting combined with bone marrow mesenchymal stem cell transplantation for the repair of long-segment sciatic nerve defects: biomechanics and validation of mathematical models
- Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury

