水稻离体叶片抗纹枯病接种方法的研究
马晨燕,袁正杰,杨海河,曲海艳,何海燕,张 玉,瞿绍洪,*
(1.浙江师范大学 化学与生命科学学院, 浙江 金华 321004; 2.浙江省农业科学院 病毒学与生物技术研究所,浙江 杭州 310021)
水稻离体叶片抗纹枯病接种方法的研究
马晨燕1,2,袁正杰2,杨海河1,2,曲海艳1,2,何海燕2,张 玉2,瞿绍洪2,*
(1.浙江师范大学 化学与生命科学学院, 浙江 金华 321004; 2.浙江省农业科学院 病毒学与生物技术研究所,浙江 杭州 310021)
水稻纹枯病是影响水稻生产的重要病害之一。水稻纹枯病抗性属于数量遗传性状,目前尚未发现高抗或免疫的水稻种质材料。为了提高水稻抗纹枯病种质的筛选和研究效率,对水稻离体叶片抗纹枯病接种的实验条件进行了更精确的控制,并采用了量化的病情调查方法。取纹枯菌接种离体水稻叶片组织进行qRT-PCR分析,水稻病程相关基因呈诱导表达,验证了纹枯病菌对水稻的侵染。采用离体叶片接种、大田成株期接种、苗期“微室”接种法,对抗纹枯病水稻品种和敏感品种进行测试。抗性品种和敏感品种纹枯病病级的统计检验呈显著差异,3种方法的结果表现一致。改进的离体叶片接种方法具有易于操作和重复性好的优点,适用于大规模筛选抗纹枯病水稻种质材料。
水稻;纹枯病;Rhizoctoniasolani;离体叶片法;病程相关基因
水稻纹枯病是水稻的重要病害之一[1-2],其病原物为立枯丝核菌(Rhizoctoniasolani),属于寄主范围较广的半腐生性真菌,一般在高温高湿的环境条件下侵染分蘖期水稻,由靠近水面的叶鞘开始侵染和扩展,也可以危害叶片、茎秆甚至稻穗,引起结实率下降,严重的导致植株枯死[3-4]。水稻纹枯病分布于大部分水稻种植区,可以造成高达50%以上的产量损失[3]。随着矮秆品种推广、氮肥用量增加以及气候变化等,水稻纹枯病的危害逐渐加重,成为水稻稳产和高产的严重障碍[1]。
水稻对纹枯病的抗性属于典型的数量性状[5]。迄今为止,还没有发现高抗或免疫的主基因抗病水稻材料,开展纹枯病抗病遗传育种研究难度较大[2]。水稻纹枯病接种和抗性评价方法是筛选抗纹枯病遗传资源和培育抗病品种的重要前提。水稻纹枯病大田成株期接种方法可靠性和重复性较好,但是田间鉴定的环境可控性差,受季节限制,试验周期较长[2]。水稻纹枯病苗期接种具有实验规模小、发病环境可控、鉴定周期短等优点,然而对接种稻苗进行病情调查的时间点不易把握,不同品种的生长势差异等都会影响鉴定结果[2]。Prasad等[6]建立的离体叶片法,具有取材方便、操作快速简便等优点,可以满足大规模水稻材料抗纹枯病鉴定的需求。该方法将水稻离体叶片的正面紧贴湿滤纸,背面接种带纹枯病菌菌丝的马铃薯PDA琼脂块,72 h后目测病斑范围和评定病级。为了提高离体叶片接种方法的稳定性和准确性,本研究利用含有苯并咪唑保鲜剂的水琼脂培养基代替湿滤纸,舍弃纹枯病病级评定的目测调查方式,改用病斑面积积分换算方法,计算水稻叶片的相对病斑面积,从而将发病程度进行量化,取得了较好的效果。
1 材料与方法
1.1 材料
供试水稻材料为纹枯病抗性品种YSBR1[7]、感病品种Lemont[7]和泰粳394[8]。供试纹枯病菌系为YN-7和MH12,由扬州大学潘学彪教授惠赠。
1.2 水稻离体叶片纹枯病接种
1.2.1 水稻种植
将YSBR1、Lemont和泰粳394的种子封装于羊皮纸袋中进行浸种发芽,发芽第6天挑选长势基本一致的稻芽点播于育秧田中,25 d后将生长良好的秧苗移栽大田。在水稻分蘖时期(大约移栽后30 d),选取叶色浓绿、无病虫害、生长健壮的分蘖,剪取完全展开的倒1叶或倒2叶,进行纹枯菌接种。
1.2.2 纹枯菌培养
用无菌手术刀将带有纹枯菌菌丝的琼脂块切成0.4~0.5 cm小块,接种到含0.005%四环素(wt·voL)和20 mL马铃薯葡萄糖琼脂(potato dextrose agar,PDA)培养基的平板上,封口膜封口,倒置放在28 ℃培养箱中,活化培养2~3 d,培养基表面长满白色菌丝后拿出。将来自活化培养的菌块接种到新的PDA培养基,相同条件下进行扩大培养。
1.2.3 纹枯病菌接种
剪取叶片中间4~5 cm长的部分,平铺于含有苯并咪唑(0.000 1 g·mL-1)的水琼脂培养基(0.005 g·mL-1agar)上。叶片正面紧贴培养基表面。在含纹枯菌的培养皿相同半径处,用表面灭菌的打孔器取直径7 mm带菌丝的PDA琼脂块(图1-A),将菌块置于离体叶片中间部位,带菌丝的一面紧贴叶片背轴面(图1-B)。封口膜封口,置28 ℃培养。每个含有水琼脂的培养皿作为1个接种重复,其中,每个水稻品种取1个离体叶片组织,接种1个带菌琼脂块。用无菌琼脂块接种相同水稻材料,作为对照。
1.2.4 水稻离体叶片接种后病情调查及统计
接种纹枯病菌后72 h,利用数字扫描仪,将同一培养皿中不同水稻品种的离体叶片同时进行扫描。利用Photoshop软件,对扫描照片上的病斑面积进行像素换算,计算相对病斑面积(relative lesion area, RLA),即单位叶面积的病斑面积,作为衡量病害严重程度的评价指标[9]。RLA的计算公式为:RLA=病斑面积/叶片面积=病斑区域像素/叶片区域像素[9]。通过Tukey法进行品种间病级的多重比较,确定不同品种的纹枯病抗性。
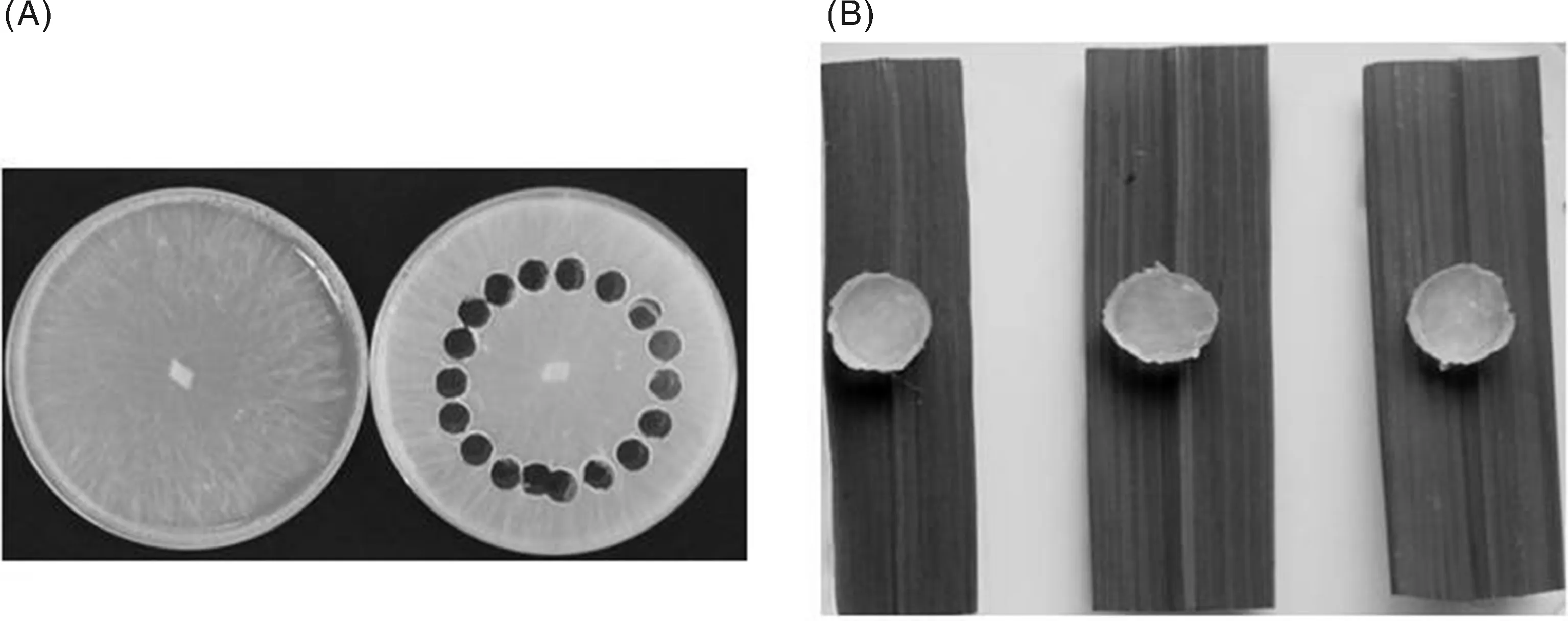
A,纹枯菌生长3 d的PDA培养基(左),掏空圆形部分的菌块已切出,供纹枯病接种(右);B,接种纹枯病菌的水稻离体叶片A, Potato dextrose agar (PDA) medium with R. solani growing for 3 d (left), PDA discs carrying R. solani were excised for inoculation (right); B, Detached rice leaves inoculated with R. solani图1 利用水稻离体叶片进行抗纹枯病接种鉴定Fig.1 Inoculation of the sheath blight pathogen (R. solani) to rice using detached-leaf method
1.2.5 接种纹枯病菌水稻材料的取样
在接种24和48 h,于无菌操作台中打开培养皿,分别从YN-7菌株和无菌对照的水稻材料取样。剪取以琼脂块为中心的菌丝扩展区域(叶片径向约2 cm长),5~6块叶组织混合,迅速置于液氮冷冻,然后于-80 ℃保存备用。
1.2.6 水稻RNA提取及RT-PCR鉴定
用TRIzol试剂(Invitrogen, USA)提取水稻RNA[10]。1%琼脂糖凝胶电泳检测RNA质量,用NanoDrop紫外—可见光分光光度计(NanoDrop 8000 UV-Vis Spectrophotometer)检测RNA浓度和纯度。RNA反转录采用PrimeScriptTM RT reagent Kit试剂盒(TaKaRa公司)并按说明书进行操作。以反转录的cDNA为模板,利用水稻病程相关基因的特异引物(表1)进行实时定量RT-PCR分析。设置3次重复,采用SYBR Premix ExTaqⅡ(2x)实时定量试剂盒(TaKaRa公司)的反应体系,以EF-1α为内参基因,采用2-⊿⊿Ct法进行RT-PCR数据分析。
表1 水稻病程相关基因的RT-PCR引物
Table 1 Primers for RT-PCR analysis of rice pathogenesis-related genes
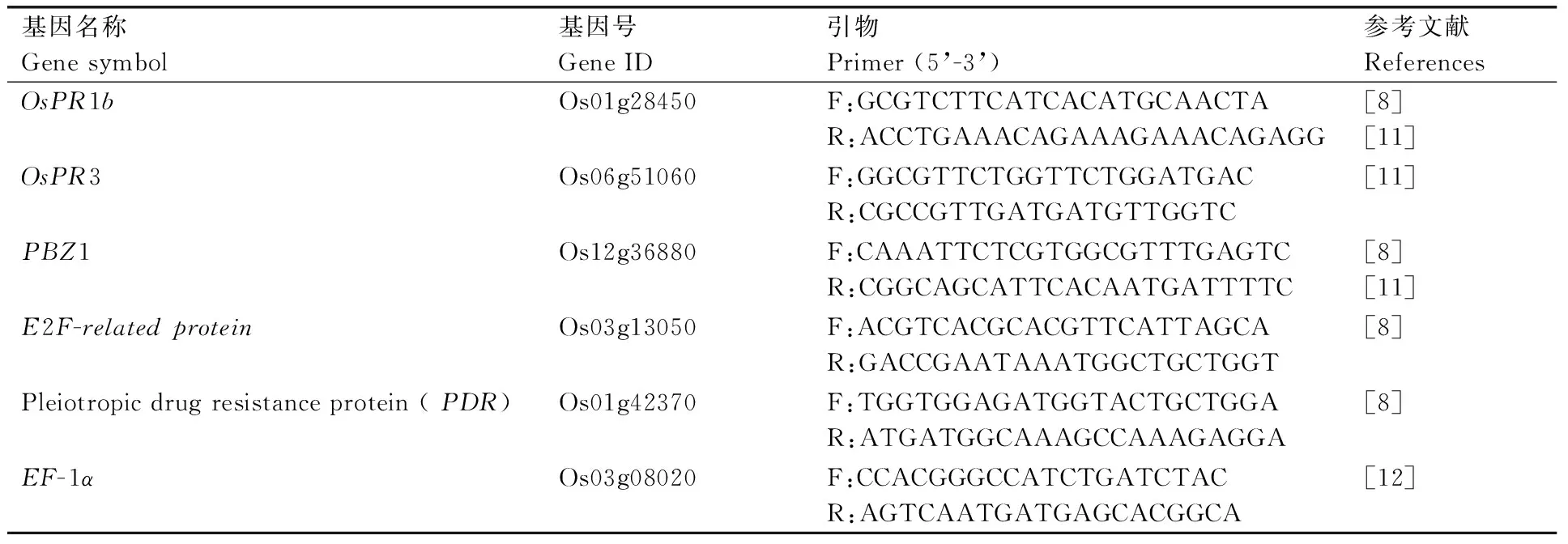
基因名称Genesymbol基因号GeneID引物Primer(5’-3’)参考文献ReferencesOsPR1bOs01g28450F:GCGTCTTCATCACATGCAACTAR:ACCTGAAACAGAAAGAAACAGAGG[8][11]OsPR3Os06g51060F:GGCGTTCTGGTTCTGGATGACR:CGCCGTTGATGATGTTGGTC[11]PBZ1Os12g36880F:CAAATTCTCGTGGCGTTTGAGTCR:CGGCAGCATTCACAATGATTTTC[8][11]E2F-relatedproteinOs03g13050F:ACGTCACGCACGTTCATTAGCAR:GACCGAATAAATGGCTGCTGGT[8]Pleiotropicdrugresistanceprotein(PDR)Os01g42370F:TGGTGGAGATGGTACTGCTGGAR:ATGATGGCAAAGCCAAAGAGGA[8]EF-1αOs03g08020F:CCACGGGCCATCTGATCTACR:AGTCAATGATGAGCACGGCA[12]
1.3 水稻大田成株期纹枯病接种鉴定
2014年4—10月在扬州大学进行水稻品种YSBR1、Lemont和泰粳394的大田成株期纹枯病接种试验。水稻种子于5月3~7日发芽,5月9日播种。秧苗于6月9日移栽大田,每个小区栽插3行,每行12个单株。每个品种种植3次重复,随机区组设计。在水稻分蘖末期(7月27~29日),取小区第2行(即中间行)的10个植株,每株取3个分蘖,采用嵌入法[13-14]接种纹枯病菌系YN-7。在抽穗后30 d(10月9~12日),采用Rush等[15]提出的0~9级病情评价体系,评定个体病级,具体方法参照相关文献[8,14]。
1.4 水稻苗期纹枯病接种鉴定
采用本实验室优化的纹枯病“微室”接种法[8],进行水稻苗期抗纹枯病接种鉴定。接种后7~9 d,测量水稻植株病斑高度和叶枕高度,计算单株病级,利用DPS生物统计软件进行病级数据的方差分析。通过Tukey法进行品种间病级的多重比较。
2 结果与分析
2.1 水稻离体叶片纹枯病菌接种的病级分析
为了检测水稻品种YSBR1、Lemont、泰粳394对纹枯病菌的抗感程度,剪取分蘖期的水稻植株生理状态一致的叶片组织,背面朝上紧贴在水琼脂上,分别接种含有纹枯病菌株YN-7和MH12的PDA琼脂块,于28 ℃恒温箱中培养。在接种第3天时观察,不同品种的叶片出现面积大小不等的纹枯病病斑(图2-A,B)。YSBR1叶片大部分保持青绿色,病斑面积较小;Lemont和泰粳394的叶色发黄,病斑蔓延叶片2/3以上。
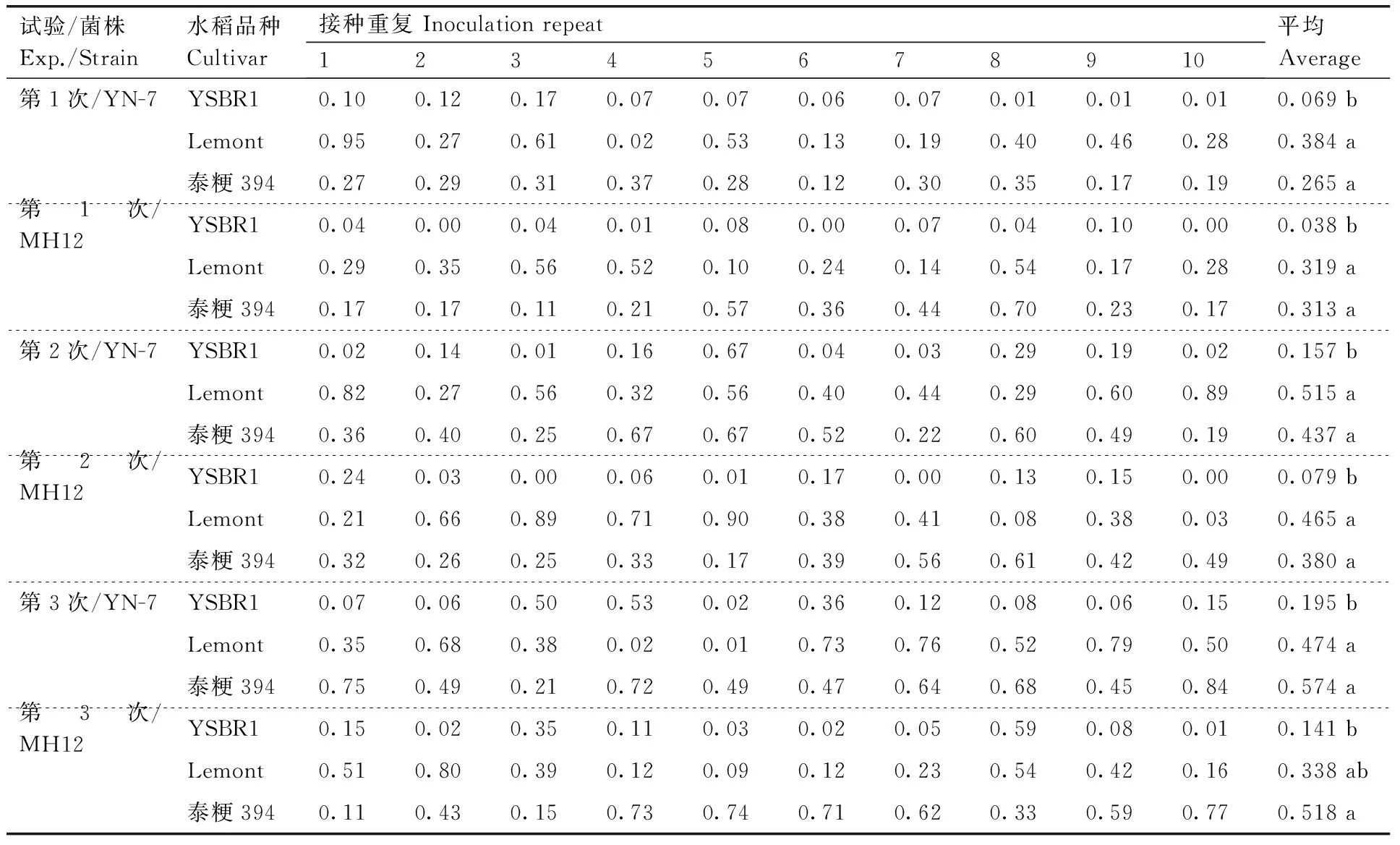
利用数字扫描仪,对接种3 d的离体叶片进行扫描。用Photoshop软件将扫描照片上的病斑面积进行像素换算,计算相对病斑面积(RLA),然后对YSBR1、Lemont和泰粳394的RLA数据进行方差分析(表2)。在YN-7菌株和MH12菌株的接种试验中,YSBR1和泰粳394的RLA均呈显著差异(P<0.05),YSBR1表现抗病,泰粳394感病。Lemont的离体叶片材料,用以上2个菌株进行了6次接种试验,除了用MH12菌株接种的第3次试验,在其他5次试验中,Lemont和YSBR1的RLA数据均表现显著差异(P<0.05)。
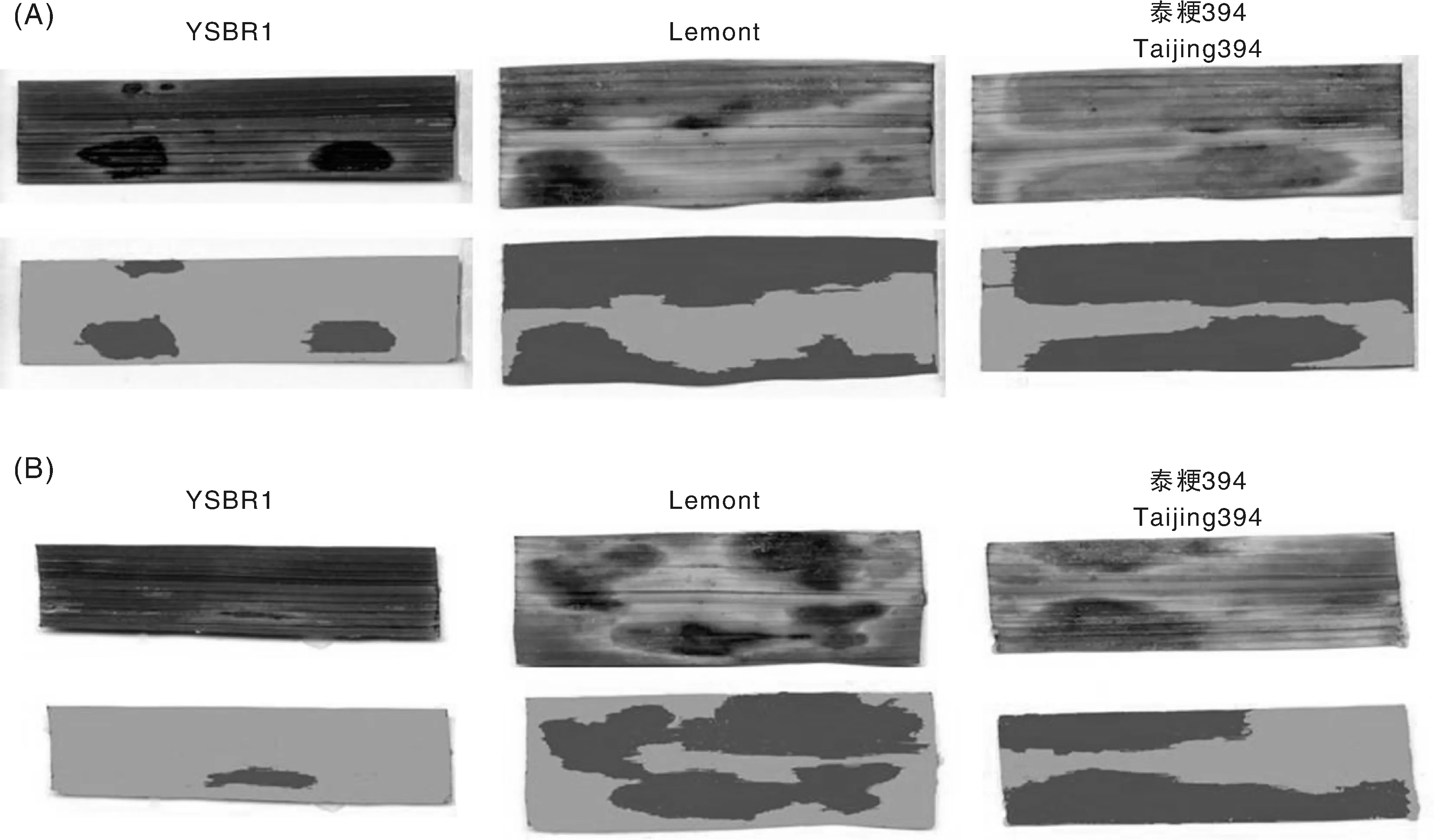
纹枯病菌株YN-7(A)和MH12(B)侵染水稻品种YSBR1、Lemont、泰粳394离体叶片第3天Leaf tissues of rice cultivars YSBR1, Lemont, and Taijing 394 infected with R. solani strains YN-7 (A) and MH12 (B) for 3 days图2 水稻离体叶片接种纹枯病菌的病斑扫描Fig.2 Digital scanning of the sheath blight lesions on detached rice leaves infected with R. solani
表2 纹枯菌侵染水稻离体叶片相对病斑面积的统计分析
Table 2 Statistical analysis of the relative lesion area of detached rice leaves infected withR.solani
试验/菌株Exp./Strain水稻品种Cultivar接种重复Inoculationrepeat12345678910平均Average第1次/YN-7YSBR10.100.120.170.070.070.060.070.010.010.010.069bLemont0.950.270.610.020.530.130.190.400.460.280.384a泰粳3940.270.290.310.370.280.120.300.350.170.190.265a第1次/MH12YSBR10.040.000.040.010.080.000.070.040.100.000.038bLemont0.290.350.560.520.100.240.140.540.170.280.319a泰粳3940.170.170.110.210.570.360.440.700.230.170.313a第2次/YN-7YSBR10.020.140.010.160.670.040.030.290.190.020.157bLemont0.820.270.560.320.560.400.440.290.600.890.515a泰粳3940.360.400.250.670.670.520.220.600.490.190.437a第2次/MH12YSBR10.240.030.000.060.010.170.000.130.150.000.079bLemont0.210.660.890.710.900.380.410.080.380.030.465a泰粳3940.320.260.250.330.170.390.560.610.420.490.380a第3次/YN-7YSBR10.070.060.500.530.020.360.120.080.060.150.195bLemont0.350.680.380.020.010.730.760.520.790.500.474a泰粳3940.750.490.210.720.490.470.640.680.450.840.574a第3次/MH12YSBR10.150.020.350.110.030.020.050.590.080.010.141bLemont0.510.800.390.120.090.120.230.540.420.160.338ab泰粳3940.110.430.150.730.740.710.620.330.590.770.518a
分别用纹枯病菌株YN-7和MH12对每个水稻品种接种3次,每次10个重复(10皿);扫描纹枯病病斑,通过积分换算方法,计算相对病斑面积。对3个水稻品种的平均相对病斑面积进行方差分析和多重比较,同列数据后无相同小写字母表明品种平均相对病斑面积具有显著差异(P<0.05)。
R.solanistrains YN-7 and MH12 were inoculated to each rice cultivar for three times (10 repeats each time). The sheath blight lesions were scanned electronically. The relative lesion area was calculated using the integration method. The average lesion areas of the cultivars were analyzed by ANOVA and multiple comparisons. The average lesion areas labeled with different letters showed significant difference (P<0.05).
在以上离体叶片的接种试验中,感病品种Lemont和泰粳394的相对病斑面积均无显著差异(P>0.05)。
2.2 离体叶片接种后水稻病程相关基因的qRT-PCR分析
受到病原菌侵染的植物会启动一系列复杂高效的保护机制,包括病程相关基因的激活,以抵抗病原菌的侵袭[16]。在接种24和48 h,取纹枯病菌株YN-7侵染和无菌对照的离体叶片材料,对其中水稻病程相关基因OsPR1b[8,11]、OsPR3[11]、PBZ1[8,11]、转录因子E2F[8]以及PDR[8]进行实时定量RT-PCR分析。比较接种处理和无菌对照的基因相对表达量,结果表明,以上5个基因在YSBR1和Lemont的24或48 h接种材料中均表现表达上调(接种处理的基因相对表达量显著或极显著高于对照的相应表达量,图3)。OsPR1b、PBZ1和PDR在YSBR1中相对表达量的峰值高于Lemont中的相应峰值。YSBR1中E2F相对表达量的峰值则低于Lemont的相应峰值。OsPR3在YSBR1和Lemont中的表达水平相差不大。根据上述结果,离体叶片材料中水稻病程相关基因呈典型诱导表达,说明纹枯病菌已侵染水稻叶片组织。
2.3 供试水稻品种田间成株期和苗期“微室”法接种验证
对YSBR1、Lemont和泰粳394进行了大田成株期纹枯病接种试验。根据Rush等[15]的0~9级病情评价体系,YSBR1、Lemont和泰粳394的平均病级分别为2.85(抗病)、7.60(感病)、4.31(中等感病),相互之间呈显著差异(表3)。此外,根据水稻苗期“微室”接种试验结果,YSBR1、Lemont和泰粳394的苗期平均病级分别为2.98(抗病)、7.65(感病)和6.19(感病),相互呈显著差异(表4)。因此,上述结果验证了抗病和感病品种YSBR1和Lemont之间,以及YSBR1和泰粳394之间的纹枯病病级差异。
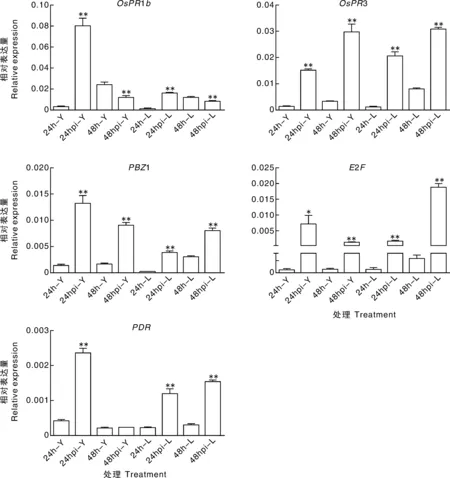
Y和L分别表示水稻品种YSBR1和Lemont;24 hpi和48 hpi分别表示接种纹枯病菌之后24和48 h的取样材料;24h和48h表示未接种水稻材料,作为对照。*和**分别表示处理组与对照组基因相对表达量存在显著差异(P<0.05)和极显著差异(P<0.01)Y, the rice cultivar YSBR1; L, the cultivar Lemont; 24 hpi and 48 hpi represent the rice samples at the time points of 24 and 48 hours post inoculation (hpi) of R. solani, respectively; 24h and 48h, the uninoculated rice samples at the 24 hpi and 48 hpi, as negative controls. * and ** indicate significant difference (P<0.05) and extremely significant difference (P<0.01), respectively图3 离体水稻叶片接种纹枯病菌后病程相关基因的表达量变化Fig.3 Changes of the relative expression levels of rice pathogenesis-related genes in the R.solani-infected detached rice leaves
3 讨论
本研究对Prasad等[6]建立的水稻离体叶片纹枯病接种方法进行了改进,对接种条件进行更精确的控制,并采用了量化的病情调查方法。在2个纹枯病菌株的6次重复接种试验中,有5次试验取得了抗病和感病品种的相对病斑面积表现显著差异的结果。大田接种试验以及苗期“微室”接种试验进一步验证了上述结果。改进的离体叶片接种方法具有快速简便和易于重复的优点,适用于大规模水稻种质材料抗纹枯病性状的筛选。
根据水稻离体叶片病程相关基因的RT-PCR分析结果,在纹枯病接种条件下,OsPR1b、OsPR3、PBZ1、E2F和PDR呈典型的诱导表达,分每个水稻植株接种3个分蘖,计算分蘖的纹枯病平均病级,作为水稻单株病级。对3个水稻品种的单株病级进行方差分析,平均病级后无相同小写字母的品种的纹枯病病级具有显著差异(P<0.05)。下同。
表3 水稻大田成株期纹枯病接种的病级
Table 3 The sheath blight scores of rice adult plants inoculated withR.solanion field
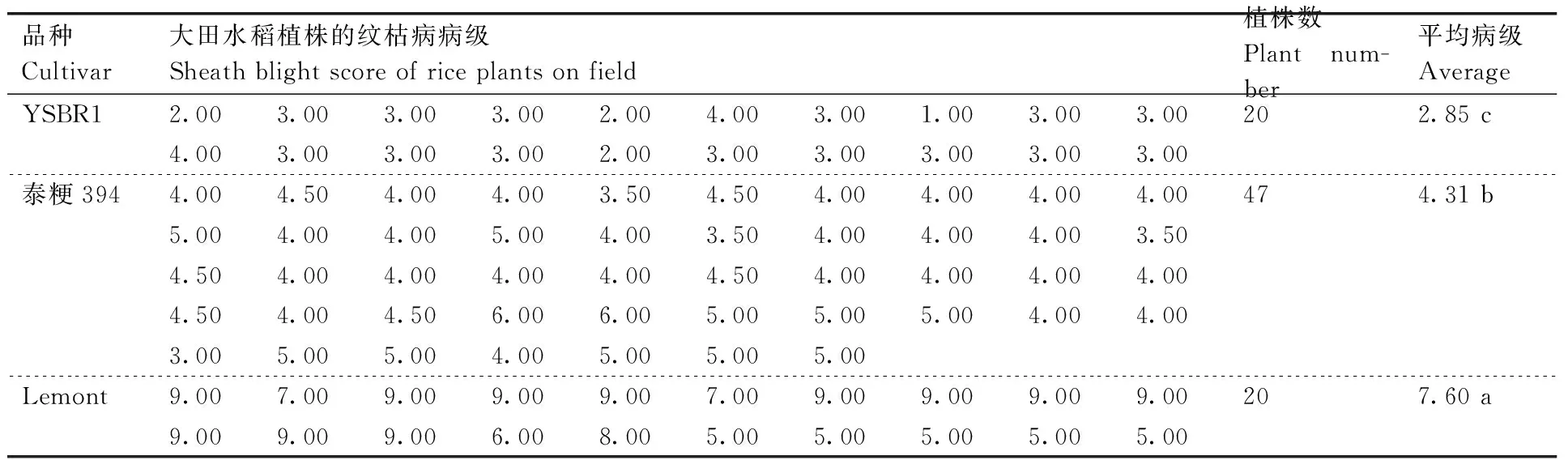
品种Cultivar大田水稻植株的纹枯病病级Sheathblightscoreofriceplantsonfield植株数Plantnum-ber平均病级AverageYSBR12.003.003.003.002.004.003.001.003.003.00202.85c4.003.003.003.002.003.003.003.003.003.00泰粳3944.004.504.004.003.504.504.004.004.004.00474.31b5.004.004.005.004.003.504.004.004.003.504.504.004.004.004.004.504.004.004.004.004.504.004.506.006.005.005.005.004.004.003.005.005.004.005.005.005.00Lemont9.007.009.009.009.007.009.009.009.009.00207.60a9.009.009.006.008.005.005.005.005.005.00
Three tillers of each rice plant were inoculated withR.solani. The disease score of a single plant were rated by calculating the average of the scores of tillers.The disease scores of three rice cultivars were analyzed by ANOVA. The average scores labeled without the same lower letters showed significant difference (P<0.05). The same as the table below.
表4 水稻苗期纹枯病菌“微室”接种的病级
Table 4 The sheath blight scores of rice seedlings inoculated withR.solaniusing micro-chamber method

品种Cultivar苗期水稻植株“微室”接种的纹枯病病级Sheathblightscoreofriceplantsinoculationwiththemicro-chambermethods植株数Plantnumber平均病级AverageYSBR12.062.893.002.274.492.183.323.602.212.342.385.03122.98c泰粳3947.146.024.486.425.179.006.495.606.319.008.257.08246.19b4.763.269.004.996.544.533.308.072.946.934.399.00Lemont7.889.007.548.259.008.249.005.423.848.268.606.72127.65a
每个微室接种4~5个水稻幼苗,作为1次接种重复,然后计算全部植株的平均病级。
For a single experimental repeat, 4-5 rice seedlings were inoculated withR.solaniin a micro-chamber, and the average disease score of the plants was calculated.
别与徐国娟等[8]和Wang等[11]的结果一致。并且,抗病品种OsPR1b、PBZ1和PDR诱导表达的水平显著高于感病品种。Wang等[11]对抗纹枯病的OsWRKY4过表达转基因水稻和未转化品种进行RT-PCR分析,OsPR1b和PBZ1在纹枯病菌侵染条件下呈诱导表达,并且抗病株系诱导表达的峰值显著高于未转化感病品种。因此,水稻病程相关基因表达量的变化,验证了纹枯病菌对水稻离体叶片组织的侵染。这种在精确控制的环境条件下检测水稻基因表达的实验体系,可以运用于水稻和纹枯病菌分子互作的研究。
本研究对水稻离体叶片抗纹枯病接种方法、大田成株期以及苗期接种方法进行了比较。在抗病品种和感病品种的差异显著性方面,3种方法取得了一致的试验结果。对于抗感差异较小的中感品种和感病品种,离体叶片接种的相对病斑面积差异性不显著。因此,在水稻抗纹枯病种质筛选过程中,建议利用离体叶片方法快速简便的优势,先用该方法筛选抗性水平较高的水稻材料,再结合其他接种方法,对筛选材料进行验证,从而满足大规模水稻抗性种质资源筛选的需求。
[1] 孟庆忠, 刘志恒, 王鹤影, 等. 水稻纹枯病研究进展[J]. 沈阳农业大学学报, 2001, 32(5): 376-381.
MENG Q Z, LIU Z H, WANG H Y, et al. Research progress in rice sheath blight [J].JournalofShenyangAgriculturalUniversity, 2001, 32(5): 376-381. (in Chinese with English abstract)
[2] 左示敏, 张亚芳, 陈宗祥, 等. 水稻抗纹枯病遗传育种研究进展[J]. 中国科学:生命科学, 2010, 40(11): 1014-1023.
ZUO S M, ZHANG Y F, CHEN Z X, et al. Current progress on genetics and breeding in resistance to rice sheath blight[J].ScientiaSinicaVitae, 2010, 40(11): 1014-1023. (in Chinese)
[3] OKUBARA P A, DICKMAN M B, BLECHIl A E. Molecular and genetic aspects of controlling the soilborne necrotrophic pathogensRhizoctoniaandPythium[J].PlantScience, 2014, 228: 61-70.
[4] TAHERI P, TARIGHI S. Cytomolecular aspects of rice sheath blight caused byRhizoctoniasolani[J].EuropeanJournalofPlantPathology, 2011, 129(4): 511-528.
[5] PINSON S R M, CAPDEVIELLE F M, OARD J H. Confirming QTLs and finding additional loci conditioning sheath blight resistance in rice using recombinant inbred lines [J].CropScience, 2005, 45(2): 503-510.
[6] PRASAD B, EIZENGA G C. Rice sheath blight disease resistance identified inOryzaspp. Accessions [J].PlantDisease, 2008, 92(11): 1503-1509.
[7] 陈夕军, 王玲, 左示敏, 等. 水稻纹枯病寄主—病原物互作鉴别品种与菌株的筛选[J]. 植物病理学报, 2009, 39(5): 514-520.
CHEN X J, WANG L, ZUO S M, et al. Screening of cultivars and isolates for identifying interaction between host and pathogen of rice sheath blight [J].ActaPhytopathologicaSinica, 2009, 39(5): 514-520. (in Chinese with English abstract)
[8] 徐国娟, 袁正杰, 左示敏, 等. 水稻苗期纹枯病抗性鉴定微室接种技术的改良[J]. 中国水稻科学, 2015, 29(1): 97-105.
XU G J, YUAN Z J, ZUO S M, et al. Improvement of the micro-chamber inoculation method for determination of rice seedling resistance to sheath blight (Rhizoctoniasolani) [J].ChineseJournalofRiceScience, 2015, 29(1): 97-105. (in Chinese with English abstract)
[9] 崔华威, 杨艳丽, 黎敬涛, 等. 一种基于Photoshop的叶片相对病斑面积快速测定方法[J]. 安徽农业科学, 2009, 37(22): 10760-10762.
CUI H W, YANG Y L, LI J T, et al. A faster method for measuring relative lesion area on leaves based on software Photoshop [J].JournalofAnhuiAgriculturalSciences, 2009, 37(22): 10760-10762. (in Chinese with English abstract)
[10] SINGH G, KUMAR S, SINGH P. A quick method to isolate RNA from wheat and other carbohydrate-rich seeds [J].PlantMolecularBiologyReporter, 2003, 21(1): 93a-93f.
[11] WANG H, MENG J, PENG X, et al. RiceWRKY4 acts as a transcriptional activator mediating defense responses towardRhizoctoniasolani, the causing agent of rice sheath blight [J].PlantMolecularBiology, 2015, 89(1/2): 157-171.
[12] MUKHOPADHYAY P, TYAGI A K.OsTCP19 influences developmental and abiotic stress signaling by modulating ABI4-mediated pathways[J].ScientificReports, 2015, 5: 9998. doi: 10.1038/srep09998.
[13] 王子斌, 左示敏, 李刚, 等. 水稻成株期对纹枯病的抗性表现研究[J]. 吉林农业大学学报, 2011, 33(2): 144-150.
WANG Z B, ZUO S M, LI G, et al. Study of resistance of rice to sheath blight at adult plant stage [J].JournalofJilinAgriculturalUniversity, 2011, 33(2): 144-150. (in Chinese with English abstract)
[14] 潘学彪, 陈宗祥, 徐敬友, 等. 不同接种调查方法对抗水稻纹枯病遗传研究的影响[J]. 江苏农学院学报, 1997, 18(3): 27-32.
PAN X B, CHEN Z X, XU J Y, et al. The effects of different methods of inoculation and investigation on genetic research of resistance to rice sheath blight [J].JournalofJiangsuAgriculturalCollege, 1997, 18 (3): 27-32. (in Chinese with English abstract)
[15] RUSH M C, HOFF B J, MELLRATH W O. A uniform disease rating system for rice disease in the United States [C]// Proceedings of the 16th Rice Technical Working Group, Lake Charles, Louisiana, USA, 1976: 64.
[16] CROUZET J, TROMBIK T, FRAYSSE A S, et al. Organization and function of the plant pleiotropic drug resistance ABC transporter family [J].FebsLetters, 2006, 580(4): 1123-1130.
(责任编辑 侯春晓)
Studies on the detached-leaf inoculation method for determination of rice resistance to sheath blight (Rhizoctoniasolani)
MA Chen-yan1,2,YUAN Zheng-jie2,YANG Hai-he1,2,QU Hai-yan1,2,HE Hai-yan2,ZHANG Yu2,QU Shao-hong2,*
(1.CollegeofChemistryandLifeSciences,ZhejiangNormalUniversity,Jinhua321004,China; 2.InstituteofVirologyandBiotechnology,ZhejiangAcademyofAgriculturalSciences,Hangzhou310021,China)
Rice sheath blight (Rhizoctoniasolani) is one of the important diseases causing large loss to rice production. Rice resistance toR.solanibelongs to quantitative genetic trait. Rice germplasm with high resistance or immunity to sheath blight has not yet been identified. In order to improve the efficiency of screening and characterizing sheath blight-resistant rice germplasm, we made precise control of the experimental conditions for the detached-leaf inoculation method, and developed a quantitative protocol for the evaluation of disease severity. The detached leaf tissues inoculated withR.solaniwere subjected to quantitative reverse-transcription qRT-PCR analysis. The induced expression of rice pathogenesis-related genes verified the infection of the pathogen into rice. A sheath blight-resistant rice cultivar and a sensitive cultivar were tested using the detached-leaf method, the field inoculation method and the micro-chamber inoculation method, respectively. The three methods showed similar results, and disease scores of the resistant and sensitive cultivars were significantly different based on statistical tests. Taken together, the improved detached-leaf inoculation method had the advantages of easy operation and good repeatability, and could be used for large-scale screening of sheath blight-resistant rice germplasm.
rice (Oryzasativa); sheath blight;Rhizoctoniasolani; detached-leaf inoculation; pathogenesis-related gene
http://www.zjnyxb.cn
10.3969/j.issn.1004-1524.2016.10.15
2016-03-15
转基因生物新品种培育重大专项(2012ZX08009001);浙江省农业科学院科技创新能力提升工程项目(2015CX07);浙江省农业科学院省部共建国家重点实验室培育基地开放基金(2010DS700124-KF1210,2010DS700124-KF1406)
马晨燕(1988—),女,山东菏泽人,硕士研究生,主要从事水稻生物技术研究。E-mail: mcy1025181205@163.com
*通信作者,瞿绍洪,E-mail: squ@mail.zaas.ac.cn
S511;S41-30
A
1004-1524(2016)10-1730-08
浙江农业学报ActaAgriculturaeZhejiangensis, 2016,28(10): 1730-1737
马晨燕, 袁正杰, 杨海河, 等. 水稻离体叶片抗纹枯病接种方法的研究[J]. 浙江农业学报, 2016, 28(10): 1730-1737.

