侧脑室微量注射GnIH对OEP大鼠血浆GnRH、LH水平的影响*
苏 玮,魏 萌,冯 超,杨正江,陈心悦,乔跃兵
(承德医学院,河北承德 067000)
侧脑室微量注射GnIH对OEP大鼠血浆GnRH、LH水平的影响*
苏玮,魏萌,冯超,杨正江,陈心悦,乔跃兵△
(承德医学院,河北承德 067000)
目的:观察侧脑室注射促性腺激素抑制激素(GnIH)对OEP大鼠血浆促性腺激素释放激素(GnRH)和黄体生成素(LH)水平的影响。方法:健康成年雌性SD大鼠40只,均建立卵巢摘除兼予雌激素注射(OEP)模型。随机分为GnIH实验组和生理盐水对照组,每组20只,大鼠分别给予侧脑室注射GnIH(5 μ l)和同等剂量生理盐水。采用ELISA法检测注射后1h、2h、4h、6h(n=5)大鼠血浆GnRH和LH的水平。结果:与对照组相比,注射GnIH 1h、6h GnRH、LH水平无明显变化(P>0.05);注射GnIH 2h、4h GnRH、LH水平明显降低(P<0.05)。结论:侧脑室注射GnIH可影响OEP大鼠血浆GnRH和LH的水平,且影响作用与时间有关。
促性腺激素抑制激素(GnIH);促性腺激素释放激素(GnRH);黄体生成素(LH);卵巢摘除兼予雌激素(OEP)大鼠
哺乳动物下丘脑分泌的促性腺激素释放激素(GnRH)可刺激垂体前叶分泌促性腺激素,黄体生成素(LH)和卵泡雌激素(FSH),LH和FSH可作用于性腺,刺激甾体类激素的合成与分泌,从而调节生殖功能[1]。GnRH并不是唯一调节下丘脑-垂体-性腺轴的神经肽。2000年Tsutsui等在鹌鹑脑中发现一种直接作用于垂体抑制促性腺激素分泌的神经肽,被称为促性腺激素抑制激素(GnIH)[2]。本研究给予成年雌性去卵巢兼予雌激素(OEP)大鼠侧脑室微量注射GnIH,探讨注射GnIH不同时间大鼠血浆GnRH、LH水平的变化情况,为GnIH调控生殖的研究提供依据。
1 材料与方法
1.1 仪器与试剂 GnIH,美国Peprotech公司;大鼠GnRH、LH ELISA试剂盒,上海酶连生物有限公司;Multiskan MK3酶标仪,美国Thermo公司。
1.2 实验动物分组与处理 清洁级健康成年雌性SD大鼠40只(北京华阜康生物科技股份有限公司,合格证号:11401300016009)。随机分为GnIH实验组和生理盐水对照组,每组20只大鼠,均建立OEP大鼠模型;GnIH实验组大鼠侧脑室注射微量GnIH(5μl),生理盐水对照组大鼠脑室注射等量生理盐水;GnIH实验组和生理盐水对照组大鼠分别于注射后1h、2h、4h、6h取材,每个时间点5只大鼠。根据《The Rat Brain Stereotaxic Coordinates》(第三版)的方法进行侧脑室定位:前囟点后1.0mm,旁开1.5mm,进针深度4.0mm。
1.3 指标检测 各组大鼠分别于注射后1h、2h、4h、6h麻醉后开胸,自右心房采集静脉血,4℃、3000r/min离心15min后取上清,-80℃储存备用。采用ELISA法检测血浆GnRH和LH的水平。
1.4 统计分析 应用SPSS 19.0统计软件进行统计处理,组间比较采用t检验,组内不同时间点间比较采用单因素方差分析、两两比较采用q检验,P<0.05为差异有统计学意义。
2 结果
与对照组比较:侧脑室注射GnIH 1h,实验组大鼠血浆GnRH、LH水平无明显变化;注射GnIH 2h、4h,实验组大鼠血浆GnRH、LH水平明显降低(P<0.05);注射GnIH 6h,实验组大鼠血浆GnRH、LH水平无明显变化。
实验组内比较:注射GnIH 1h、6h,大鼠血浆GnRH、LH水平比较差异无统计学意义;注射GnIH 2h、4h,大鼠血浆GnRH、LH水平比较差异无统计学意义;注射GnIH 2h、4h大鼠GnRH、LH的水平明显低于注射GnIH 1h、6h(P<0.05)。见附表:
附表 侧脑室注射GnIH对大鼠血浆GnRH和LH水平的影响(±s,n=5)
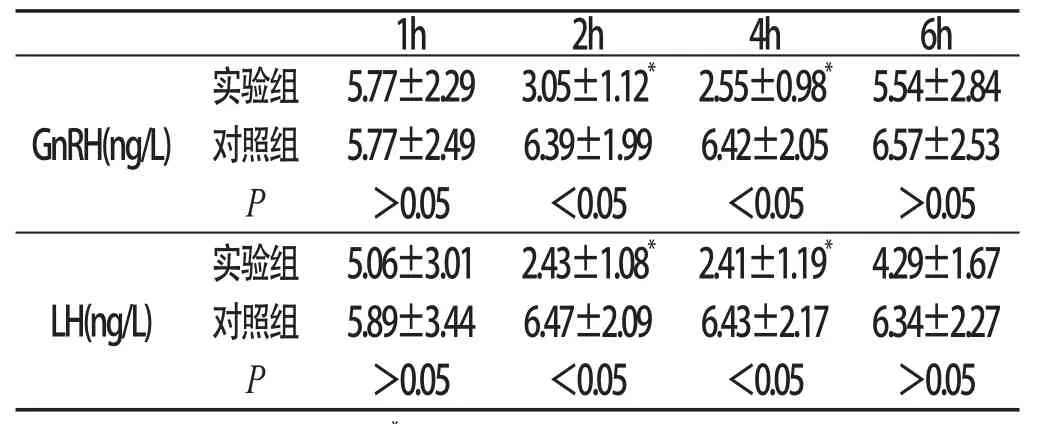
附表 侧脑室注射GnIH对大鼠血浆GnRH和LH水平的影响(±s,n=5)
与本组1h、6h比较:*P<0.05
1h2h4h6h GnRH(ng/L)实验组5.77±2.293.05±1.12*2.55±0.98*5.54±2.8 4对照组5.77±2.496.39±1.996.42±2.056.57±2.53 P>0.05<0.05<0.05>0.05 LH(ng/L)实验组5.06±3.012.43±1.08*2.41±1.19*4.29±1.67对照组5.89±3.446.47±2.096.43±2.176.34±2.27 P>0.05<0.05<0.05>0.05
3 讨论
2000年在日本鹌鹑脑中分离出一种新型精氨酸-苯丙氨酸酰胺肽(RFamide),可呈剂量依赖性的抑制垂体前叶促性腺激素的释放,基于这种功能被命名为促性腺激素抑制激素(GnIH)[2]。因GnIH最早从鸟类中发现,在鸟类中的研究最多,具有抑制鸟类生殖和促性腺激素释放的作用[3-4]。为了证明哺乳动物(包括人类)GnIH是否具有相同作用,Tsutsui等尝试寻找哺乳动物下丘脑中的GnIH[5-6]。哺乳动物的GnIH都具有一个C端LPXRF-酰肽基序列(X=L或Q),这与鸟类的GnIH和GnIH相关肽相似[7-8]。由于分子式结构不同,哺乳动物GnIH也被称为RFRP-1和RFRP-3,如大鼠RFRP-3(GnIH)、叙利亚仓鼠RFRP-1和RFRP-3,鸟类称鹌鹑GnIH、鸡GnIH[9]。哺乳动物研究显示,GnRH神经元存在GnIH的受体,因此推测在哺乳动物GnIH可能通过GnRH神经元发挥作用[10]。Johnson等的研究发现,在雄性大鼠,GnIH(RFRP-3)能抑制GnRH刺激的促性腺激素的释放[11-13];GnIH(RFRP-3)的这种作用在绵羊[14-15]和牛中也得到了证实[16]。上述研究提示,同鸟类GnIH的作用类似,哺乳动物的GnIH同样具有抑制GnRH诱导的促性腺激素释放的作用。
给予切除卵巢(未注射雌激素)的大鼠[17]和性腺完整大鼠(包括雌雄)[18]脑室注射GnIH(RFRP-3),可降低血浆LH的水平,考虑是GnIH(RFRP-3)作用于GnRH神经元的结果。静脉注射RFRP-3对卵巢切除大鼠LH的基础浓度没有影响,却明显降低了由GnRH刺激引起的LH的释放[13]。但Murakami等发现了与之相反的结果:给切除卵巢大鼠脑室注射RFRP-3,LH的浓度和释放频率均没有显著改变[12]。由于上述研究结果不尽相同,因此需要进一步研究验证GnIH对LH和GnRH的作用。、
本研究通过给予OEP大鼠侧脑室微量注射GnIH(RFRP-3),观察血浆GnRH、LH水平的变化。与注射生理盐水的对照组相比:注射GnIH 1h后,GnRH、LH水平无明显变化;注射GnIH 2h后,GnRH、LH的水平明显降低,注射后4h持续降低;注射后6h,GnRH、LH水平升高,恢复至注射后1h的水平。本研究提示,OEP大鼠侧脑室微量注射GnIH后可引起大鼠血浆GnRH和LH的水平发生明显变化,且这种变化与时间有关。因此,GnIH可以在一定时间内抑制LH和GnRH的水平,这为GnIH影响生殖提供了实验依据。
[1]Matsuo H, Baba Y, Nair RM, et al. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence[J]. Biochem Biophys Res Commun, 1971, 43(6):1334-1339.
[2]Tsutsui K, Saigoh E, Ukena K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release[J]. Biochem Biophys Res Commun, 2000, 275(2): 661-667.
[3]Tsutsui K, Bentley GE, Bedecarrats GT, et al. Review: gonadotropininhibitory hormone (GnIH) and its control of central and peripheral reproductive function[J]. Front Neuroendocrinol, 2010,31(3): 284-295.
[4]Tsutsui K, Bentley GE, Kriegsfeld LJ, et al. Review: discovery and evolutionary history of gonadotrophin- inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction[J]. J Neuroendocrinol, 2010, 22(7): 716-727.
[5]Ubuka T, Inoue K, Fukuda Y, et al. Identification, expression,and physiological functions of Siberian hamster gonadotropininhibitory hormone[J]. Endocrinology, 2012, 153(1): 373-385.
[6]Ubuka T, Morgan K, Pawson AJ, et al. Identif ication of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor,GPR147 in the human hypothalamic pituitary axis[J]. PLoS One,2009, 4(12): e8400.
[7]Tsutsui K. Review: how to contribute to the progress of neuroendocrinology: new insights from discovering novel neuropeptides and neurosteroids regulating pituitary and brain functions [J]. Gen Comp Endocrinol,2015.
[8]Tsutsui K, Ubuka T, Bentley GE, et al. Review: regulatory mechanisms of gonadotropin-inhibitory hormone (GnIH) synthesis and release in photoperiodic animals[J]. Front Neurosci,2013,7:60.
[9]Tsutsui K, Ubuka T, Son YL, et al. Contribution of GnIH Research to the Progress of Reproductive Neuroendocrinology[J]. Front Endocrinol (Lausanne), 2015, 6:179.
[10]Clarke IJ, Qi Y, Puspita Sari I, et al. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals[J]. Front Neuroendocrinol, 2009, 30(3): 371-378.
[11]Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat[J]. Horm Behav, 2007, 51(1): 171-180.
[12]Murakami M, Matsuzaki T, Iwasa T, et al. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats[J]. J Endocrinol, 2008, 199(1): 105-112.
[13]Rizwan MZ, Porteous R, Herbison AE, et al. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropininhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat[J]. Encocrinology, 2009, 150(3): 1413-1420.
[14]Clarke IJ, Sari IP, Qi Y, et al. Potent action of RFamiderelated peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion[J]. Endocrinology, 2008, 149(11): 5811-5821.
[15]Sari IP, Rao A, Smith JT, et al. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle- stimulating hormone synthesis and secretion in ovine pituitary gonadotropes[J]. Endocrinology, 2009, 150 (12): 5549-5556.
[16]Kadokawa H, Shibata M, Tanaka Y,et al. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines[J]. Domest Anim Endocrinol,2009,36(4): 219-224.
[17]Kriegsfeld LJ, Mei DF, Bentley GE, et al. Identification and characterization of a gonadotropin-inhititory system in the brains of mammals[J]. Pro Natl Acad Sci USA, 2006, 103(7): 2410-2415.
[18]Anderson GM, Relf HL, Rizwan MZ, et al. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats[J]. Endocrinology, 2009, 150(4): 1834-1840.
EFFECTS OF MICROINJECTION OF GNIH INTO LATERAL VENTRICLE ON GNRH AND LH LEVEL IN PLASMA OF OEP RATS
SU Wei, WEI Meng, FENG Chao, et al
(Chengde Medical College, Hebei Chengde 067000, China)
Objective: To observe the effects of microinjection of gonadotropin-inhibitory hormone (GnIH) into lateral ventricle on gonadotrophin releasing hormone(GnRH) and luteinizing hormone (LH) level in plasma of OEP rats. Methods: 40 healthy adult female SD rats were established OEP model and then randomly divided into normal saline control group and GnIH group. ELISA was used to detect the plasma GnRH and LH level 1h, 2h, 4h, 6h (each time point 5 rats)after respectively injected normal saline and GnIH (5μl) into the rats' lateral ventricle. Results: Compared with rats in control group, there was no signifi cant changes in GnRH and LH level of rats in GnIH group 1h and 6h after injection (P>0.05). The GnRH and LH level of rats in GnIH group 2h and 4h after injection were obviously lower than that of rats in control group (P<0.05). Conclusions: Microinjection of GnIH into lateral ventricle can affect the plasma GnRH and LH level of OEP rats; Moreover, these effects are related to time.
GnIH; GnRH; LH; OEP rats
R338.2
A
1004-6879(2016)02-095-03
* 河北省自然科学基金项目(H2013406115),河北省卫生厅医学科学研究项目(20130012),河北省人口计生委科学研究项目(2012-A22),国家卫生计生委计划生育与优生重点实验室开放课题(20150002)
△
(2015-09-11)

