基于EPG技术的烟粉虱两个品系取食行为的比较
张文平,刘佰明,张 珊,万方浩,3,褚 栋
基于EPG技术的烟粉虱两个品系取食行为的比较
张文平1,刘佰明2,张 珊1,万方浩1,3,褚 栋1
(1青岛农业大学农学与植物保护学院,山东青岛 266109;2天津市植物保护研究所,天津300384;3中国农业科学院植物保护研究所植物病虫害 生物学国家重点实验室,北京 100193)
【目的】是烟粉虱()体内的一种次生内共生菌。有研究表明Q型烟粉虱品系(C+品系)与未感染品系(C-品系)的寄主适合度有很大差异,但其产生差异的行为机制尚不明确。论文旨在研究两个Q型烟粉虱品系的取食行为差异,以期从行为学角度揭示两个品系适合度差异的原因。【方法】应用刺吸电位技术(electrical penetration graph, EPG)记录Q型烟粉虱C+品系和C-品系在棉花与番茄上6 h的取食波形,然后对取食波形进行分类统计,共选取19个参数(非韧皮部参数11个,韧皮部参数8个)进行数据分析,比较这两个品系在非韧皮部和韧皮部的取食行为差异。【结果】共获得118个有效记录,其中在棉花上58个(C-品系28个,C+品系30个),在番茄上60个(C-品系31个,C+品系29个)。在取食棉花的非韧皮部阶段,烟粉虱C+品系刺探的总持续时间和路径波(C波)总持续时间显著高于C-品系(<0.05),C+品系第一次E波后非刺探时间显著低于C-品系(<0.05);在韧皮部阶段,两个品系分泌唾液(E1波)和吸食汁液(E2波)的相关参数无显著差异(>0.05),6 h能到达韧皮部阶段的烟粉虱比例也无显著差异(>0.05)。在取食番茄的非韧皮部阶段,烟粉虱C+品系的刺探总次数和第一次E波前的刺探次数均显著高于C-品系(<0.05),C+品系从第一次刺探到第一次持续取食的时间显著长于C-品系(<0.05),但刺探的平均持续时间显著短于C-品系;在韧皮部阶段,两个品系在与分泌唾液和吸食汁液相关的参数均无显著差异(>0.05),6 h能到达韧皮部阶段的烟粉虱比例也无显著差异(>0.05)。总体上,烟粉虱C+品系和C-品系在韧皮部的取食行为参数无显著差异;在非韧皮部C+品系较C-品系具有更长的刺探时间和更多的刺探总次数。【结论】烟粉虱C+品系和C-品系在寄主植物韧皮部的取食行为没有差异,在非韧皮部存在显著差异。在非韧皮部的取食行为差异可能与其适合度差异有关。
Q型烟粉虱;适合度;刺吸电位技术;取食行为
0 引言
【研究意义】烟粉虱()是一种世界性农业害虫。该害虫被认为是一个含有许多隐种的物种复合体[1-2],其中Q型烟粉虱(即烟粉虱MED隐种)近十多年来传入许多国家(包括中国)并给农业生产造成严重危害[3]。Q型烟粉虱的入侵灾变机制与其种群的生态适应性等多种因素密切相关[4]。研究表明,内共生菌是许多昆虫种群生态适应性的重要影响因素。内共生菌可以通过调控宿主昆虫的生殖方式或影响宿主昆虫适合度等方式,对昆虫种群生物学与生态学产生深远的影响[5]。烟粉虱体内含有多种内共生菌[6-8],一些内共生菌能对烟粉虱的生物学造成各种影响[9-10]。其中,是烟粉虱体内一种重要的次生内共生菌。对烟粉虱的影响及其机理研究将为烟粉虱的生物防治提供新的思路。【前人研究进展】在许多节肢动物中,可导致宿主的雌性化[11]、孤雌生殖[12]或胞质不亲和[13-14],能影响宿主的适合度[15]和产卵行为[16]。迄今为止,烟粉虱体内的相关研究较少[17]。长期田间监测表明,山东省各地Q型烟粉虱的感染率一直较低(7.6%—17.3%)[18];全国范围内监测结果也发现Q型烟粉虱的感染率较低(<16.3%)[19]。这些调查结果表明,Q型烟粉虱感染种群在田间生态系统中并没有很强的生物学优势。Fang等[20]利用两性生命表和竞争取代的方法研究发现,Q型烟粉虱未感染品系(简称C-品系)比感染品系(简称C+品系)具有更好的适合度和更强的竞争能力。【本研究切入点】C+品系和C-品系的适合度差异产生的行为机制尚不清楚,揭示两个品系的适合度差异产生的原因对理解Q型烟粉虱的入侵、传播和有效防治具有重要意义。刺吸电位技术(electrical penetration graph,EPG)是一种记录植食性刺吸式昆虫取食行为的技术,是研究昆虫寄主适应性[21-23]、植物抗虫性[24-25]、昆虫的传毒机理[26-27]等的重要研究手段。同时,该技术还被用于揭示农药对昆虫取食的影响[28]。【拟解决的关键问题】选取烟粉虱的两种主要寄主植物棉花和番茄,利用EPG技术分析烟粉虱C+品系与C-品系在番茄和棉花上的取食行为,对韧皮部与非韧皮部取食行为参数进行比较,以期从取食行为的角度揭示烟粉虱这两个品系适合度差异产生的原因。
1 材料与方法
室内试验于2014年在青岛农业大学生物入侵实验室进行。
1.1 供试植物与烟粉虱种群
试验用棉花()品种为鲁棉研21号,番茄()品种为浙粉212,将种子分别种在花盆里(1.5 L)的营养土中,然后放置于温度(30±1)℃、相对湿度RH为(60±5)%,光周期L﹕D=16 h﹕8 h的人工气候室内。种子发芽后每隔3 d浇一次水。待棉花长至2—3片真叶期,番茄长至4—5片真叶期,分别选取长势一致的苗用于试验。
试验用Q型烟粉虱种群于2012年3月采自山东省济南市,室内于棉花上长期饲养,参照Khasdan等[29]的方法定期进行纯度检测。在Q型烟粉虱取食番茄植株的EPG试验前,将烟粉虱棉花种群转移至番茄植株上饲养2代以适应寄主。烟粉虱在温度(27±1)℃,相对湿度RH为(60±5)%,光周期L﹕D=16 h﹕8 h的人工气候室内饲养。用于EPG试验的烟粉虱均为羽化不到2 d的未交配雌虫。
1.2 烟粉虱C+与C-品系的建立
在饲养的Q型烟粉虱种群中随机选取1对成虫,放在带有两片真叶棉花苗的养虫杯中,共50个重复。产卵3 d后,将烟粉虱成虫移除,保留叶片上的卵继续发育至羽化。每个重复取后代烟粉虱成虫雌雄分别至少5头用于检测。被检测后代成虫若全部感染,该养虫杯中的烟粉虱被视为C+品系;若全部未感染,该养虫杯中的烟粉虱被视为C-品系。将筛选出的两个烟粉虱品系持续饲养,并定期进行检测。
1.3 共生菌的检测
将烟粉虱成虫放置于0.2 mL PCR管中于-20℃保存,参照Chu等[30]方法提取单头烟粉虱DNA后,以此为模板进行PCR扩增,使用的上下游引物分别为CLOf(5′-GCGGTGTAAAATGAGCGTG-3′)和CLOr1(5′-ACCTMTTCTTAACTCAAGCCT-3′)[31]。反应体系为20 μL,其中1.3 μL的10×PCR缓冲液,0.26 μL(10 mmol·L-1)的dNTP混合物,上下游引物分别为0.26 μL(20 μmol·L-1),Taq酶为0.13 μL,DNA模板为2 μL,无菌纯水9.3 μL。PCR扩增程序为:95℃预变性5 min,35个循环(95℃变性30 s,57℃退火30 s,72℃延伸1 min),最后72℃延伸5 min。PCR产物使用1.0%(g·mL-1)的琼脂糖凝胶电泳进行检测,目的片段长度为450 bp,出现目的条带的为烟粉虱C+品系个体,没有出现目的条带的个体采用实时荧光定量RT-PCR(real-time RT-PCR)进行检测。所用的引物为Card-F(5′-ACGGGAGGCAGCAGTA-3′)和Card-R(5′-CCGCAGGGATTGTTTT-3′)[32],反应体系为10 μL,其中5 μL的SYBR Green Supermix,上下游引物分别为0.1 μL(20 μmol·L-1,DNA模板为1 μL,无菌纯水3.8 μL。扩增程序为:95℃预变性1 min,40个循环(95℃变性10 s,55℃退火15 s,72℃延伸15 s)。
1.4 EPG波形记录
使用Giga-8 DC-EPG系统(荷兰瓦赫宁根大学)记录烟粉虱取食植物的刺探电位图谱。为屏蔽外界环境的电磁干扰,烟粉虱C+品系与C-品系的取食试验分别在两个法拉第笼中(50 cm×50 cm×60 cm)进行,每个法拉第笼中记录4个重复。依照汤清波等[33]的方法将约2 cm的金丝(直径12.5 μm)用导电银胶粘在供试烟粉虱的前胸背板上。将粘连好的烟粉虱饥饿20 min,然后连接到EPG昆虫电极的铜钉上。记录前,将粘在金丝上的烟粉虱放置在叶面背面。每头烟粉虱均持续记录6 h。所有试验均在温度(27±1)℃,相对湿度RH为(60±5)%,光周期L﹕D=16 h﹕8 h的人工气候室内进行。
1.5 EPG数据分析
参考Moreno-DELAFUENTE等[34]的标准,将EPG波形划分为非刺探波(np)、路径波(C)[刺吸障碍波(F)和木质部取食波(G)也合并到这类波形里]、韧皮部唾液分泌波(E1)、韧皮部取食波(E2)(E2≥10 min)。使用stylet+d软件(荷兰瓦赫宁根大学)进行波形记录,stylet+a软件(荷兰瓦赫宁根大学)进行波形分析。烟粉虱C-品系和C+品系在棉花上的取食记录分别重复40次,在番茄上分别重复45次。
参照van Helden等[35]和汤清波等[33]共选择19个参数进行统计分析。使用SPSS19.0软件进行显著性分析。首先检测数据方差齐性和正态性,符合正态分布的数据采用独立样本检验(<0.05),不符合正态分布的数据进lg10转换或反正弦转换。转换后的数据仍不符合正态分布的采用Mann-Whitney U-test分析。其中,“能到达韧皮部阶段的烟粉虱比例”使用卡方检测进行分析。
2 结果
EPG试验共获得118个有效记录,其中在棉花上58个(C-品系28个,C+品系30个),在番茄上60个(C-品系31个,C+品系29个)。统计的19个参数中,非韧皮部参数11个,韧皮部参数8个。
2.1 烟粉虱在棉花非韧皮部的EPG参数
在棉花上取食的非韧皮部阶段,统计的11个参数中有3个具有显著性差异(<0.05)(表1)。烟粉虱C+品系刺探的总持续时间和C波总持续时间显著高于C-品系,分别是C-品系的1.51和1.41倍。烟粉虱C+品系的第一次E波后非刺探时间显著低于C-品系。
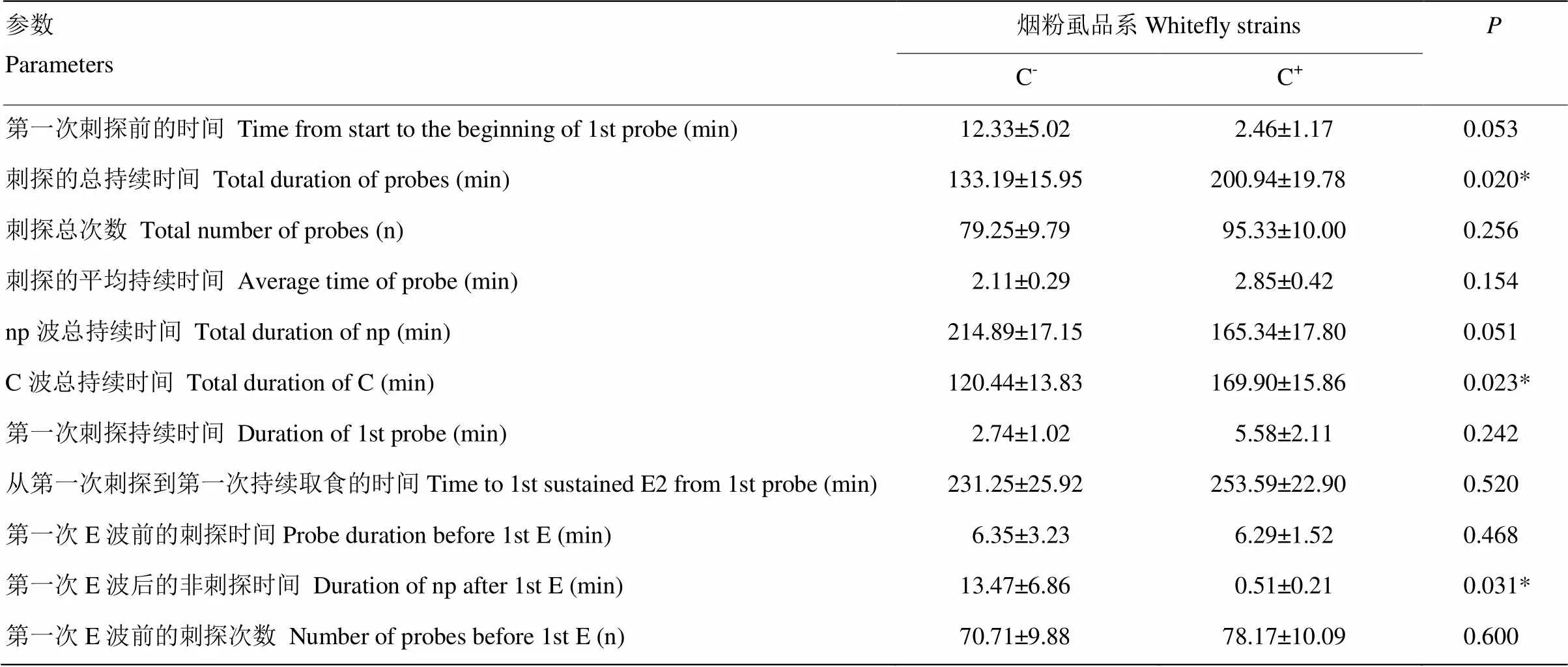
表1 烟粉虱C-品系和C+品系在棉花非韧皮部取食的EPG参数
“*”表示对应参数差异显著(<0.05)。下同“*” indicated significant differences (<0.05). The same as below
2.2 烟粉虱在棉花韧皮部的EPG参数
烟粉虱C-品系和C+品系在棉花上可到达韧皮部并持续取食(E2≥10min)的分别为14和12个。韧皮部参数(包括与唾液分泌和吸食植物汁液的相关参数)均没有显著性差异(>0.05)(表2)。

表2 烟粉虱C-品系和C+品系在棉花韧皮部取食的EPG参数
2.3 烟粉虱在番茄非韧皮部的EPG参数
在番茄上取食的非韧皮部阶段,统计的11个参数中有4个具有显著性差异(<0.05)(表3)。烟粉虱C+品系经历的从第一次刺探到第一次持续取食的时间显著长于C-品系,是C-品系的1.20倍;C+品系的刺探总次数和第一次E波前的刺探次数显著多于烟粉虱C-品系,分别是C-品系的1.54和1.74倍;C+品系刺探的平均持续时间显著短于C-品系,是C-品系的68.45%。

表3 烟粉虱C-品系和C+品系在番茄非韧皮部取食的EPG参数
2.4 烟粉虱在番茄韧皮部的EPG参数
烟粉虱C-品系和C+品系在番茄上可到达韧皮部并持续取食(E2≥10 min)的个体分别为14个和7个。韧皮部参数(包括与唾液分泌和吸食植物汁液的相关参数)均没有显著性差异(>0.05)(表4)。

表4 烟粉虱C+品系和C-品系取食番茄韧皮部的EPG参数
3 讨论
本研究发现,在棉花非韧皮部取食阶段,相对于烟粉虱C-品系,烟粉虱C+品系在路径波上花费更多的时间,同时具有更长的刺探总持续时间(表1);在番茄非韧皮部取食阶段,路径波时间和刺探总持续时间没有显著差异,但C+品系比C-品系进行了更多的刺探,第一次到达韧皮部持续取食之前经过的时间和第一次E波前的刺探次数均比烟粉虱C-品系多(表3);两个品系的韧皮部取食阶段,与吸食植物汁液相关的参数,如E2波的总时间和平均时间等均没有显著性差异(表2、表4)。
相对于烟粉虱C-品系,烟粉虱C+品系在取食过程中需要消耗更多的能量,取食效率较低。首先,两个品系在寄主植物韧皮部的取食行为没有显著差异,表明取食行为可能对二者获取能量没有影响。当烟粉虱到达韧皮部筛管细胞取食时,从韧皮部吸取汁液从而获取氨基酸、糖分等营养[36-37],而糖类是昆虫生长发育的主要能源物质,供给昆虫生长发育所需的能量[38-39],烟粉虱的刺吸式取食是其获取生长发育所需能量的唯一途径。韧皮部取食参数中,韧皮部取食时间是最能直接反映刺吸式口器昆虫寄主适应性的参数。烟粉虱C+品系和C-品系在两种寄主植物上的韧皮部取食时间(即E2波的总时间)均没有差异,这表明两个品系的取食量很接近,营养和能量的获取没有很大差异。因此,两个品系在寄主植物上的取食行为可能没有直接影响二者获取能量。
两个品系在寄主植物非韧皮部的取食行为存在显著差异,表明烟粉虱C+品系可能比C-品系消耗更多的能量。非韧皮部取食阶段是昆虫在口针与植物表面接触后刺入表皮通过叶肉细胞间隙的过程[40],昆虫口针在到达韧皮部之前往往需要经过多次刺探才能找到合适的吸食位点[41-42]。刺探次数越多,刺探时间越长,意味着在寻找合适的吸食位点的过程中消耗能量越多,取食效率较低。本研究中,烟粉虱C+品系比C-品系在棉花非韧皮部具有更长的刺探时间和路径波时间;在番茄上有更多的刺探总次数。这表明C+品系可能比C-品系消耗更多的能量,取食效率相对较低。同时,转录组数据分析也发现(未发表数据),相对于烟粉虱C-品系,C+品系与新陈代谢相关基因(如与ATP酶相关基因)的表达量多呈上升趋势,这也表明烟粉虱C+品系比C-品系可能消耗了更多能量。
研究表明,昆虫能量分配可能会影响其适合度[43-47]。一方面,昆虫用于滞育解除等方面的能量消耗,可能降低其适合度。例如,中华通草蛉()在越冬后,滞育解除过程中可能需要能量消耗,使其生殖可用的能量减少,从而导致产卵量降低[48]。另一方面,昆虫可能通过减少解毒代谢等方面的能量消耗,来增加其适合度。如感染中国番茄黄化曲叶病毒(TYLCCV)的烟粉虱可能通过减少解毒代谢来降低能量消耗,从而来增加自身的适合度[49]。此外,某些昆虫可能存在降低能量代谢消耗进而避免或减缓适合度代价的策略。如橘小实蝇()幼虫在应对杀虫剂的持续胁迫时,解毒代谢酶基因集中在中肠与脂肪体内高度表达,减少在抵御外源杀虫剂伤害时额外能量的消耗[44],这可能避免或减缓了种群的适合度代价。本研究中,烟粉虱C+品系和C-品系的取食行为差异很可能通过影响能量消耗,进而导致两个品系适合度的差异。因此,笔者推测烟粉虱C+品系在取食寄主植物的过程中比C-品系消耗了更多能量,使烟粉虱用于繁殖方面的能量减少,从而导致适合度代价。今后,将结合其他技术(如基因荧光定量、RNAi等技术)进一步验证能量代谢与适合度代价的关系。
4 结论
烟粉虱C+品系和C-品系在寄主植物韧皮部的取食行为没有差异,在非韧皮部存在显著差异。在非韧皮部的取食行为差异可能与其适合度差异有关。烟粉虱C+品系和C-品系的取食行为差异可能与影响能量代谢有关。
致谢:河南农业大学化学生态实验室闫凤鸣教授团队提供了EPG银胶和技术指导,美国肯塔基大学的Jordan Hampton博士和潘慧鹏博士对英文摘要进行了润色与修改,在此一并表示感谢!
References:
[1] De Barro P J, Liu S S, Boykin L M, Dinsdale A B.: a statement of species status., 2011, 56: 1-19.
[2] Firdaus S, Vosman B, Hidayati N, Supena J, Darmo E, Visser R, van Heusden A W. Thespecies complex: additions from different parts of the world., 2013, 20(6): 723-733.
[3] Wang H L, Yang J, Boykin L M, Zhao Q Y, Li Q, Wang X W, Liu S S. The characteristics and expression profiles of the mitochondrial genome for the Mediterranean species of thecomplex., 2013, 14(1): 401.
[4] 褚栋, 潘慧鹏, 国栋, 陶云荔, 刘佰明, 张友军. Q型烟粉虱在中国的入侵生态过程及机制. 昆虫学报, 2012, 55(12): 1399-1405.
Chu D, Pan H P, Guo D, Tao Y L, Liu B M, Zhang Y J. Ecological processes and mechanisms of invasion of the alien whiteflybiotype Q in China., 2012, 55(12): 1399-1405. (in Chinese)
[5] 褚栋, 张友军, 毕玉平, 付海滨.属共生菌及其对节肢动物宿主适合度的影响. 微生物学报, 2005, 45(5): 817-820.
Chu D, Zhang Y J, Bi Y P, Fu H B.endosymbionts and their effects on the fitness of the arthropod hosts., 2005, 45(5): 817-820. (in Chinese)
[6] Chiel E, Inbar M, Mozes-Daube N, White J A, Hunter M S, Zchori-Fein E. Assessments of fitness effects by the facultative symbiontin the sweetpotato whitefly (hemiptera: aleyrodidae)., 2009, 102(3): 413-418.
[7] Su Q, Oliver K M, Pan H P, Jiao X G, Liu B M, Xie W, Wang S L, Wu Q J, Xu B Y, White J A, Zhou X G, Zhang Y J. Facultative symbiontconfers benefits to(Hemiptera: Aleyrodidae), an invasive agricultural pest worldwide., 2013, 42(6): 1265-1271.
[8] Cass B N, Yallouz R, Bondy E C, Mozes-Daube N, Horowitz A R, Kelly S E, Zchori-Fein E, Hunter M S. Dynamics of the endosymbiontin an insect pest., 2015, 70(1): 287-297.
[9] Himler A G, Adachi-Hagimori T, Bergen J E, Kozuch A, Kelly S E, Tabashnik B E, Chiel E, Duckworth V E, Dennehy T J, Zchori-Fein E, Hunter M S. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias., 2011, 332(6026): 254-256.
[10] 杨义婷, 郭建洋, 龙楚云, 刘怀, 万方浩. 昆虫内共生菌及其功能研究进展. 昆虫学报, 2014, 57(1): 111-122.
Yang Y T, Guo J Y, Long C Y, Liu H, Wan F H. Advances in endosymbionts and their functions in insects., 2014, 57(1): 111-122. (in Chinese)
[11] Giorgini M, Monti M M, Caprio E, Stouthamer R, Hunter M S. Feminization and the collapse of haplodiploidy in an asexual parasitoid wasp harboring the bacterial symbiont., 2009, 102(4): 365-371.
[12] Provencher L M, Morse G E, Weeks A R, Normark B B. Parthenogenesis in thecomplex (Hemiptera: Diaspididae): a single origin of a worldwide, polyphagous lineage associated withbacteria., 2005, 98(5): 629-635.
[13] Xie R R, Zhou L L, Zhao Z J, Hong X Y. Male age influences the strength of-induced cytoplasmic incompatibility expression in the carmine spider mite, 2010, 45(3): 417-423.
[14] Harris L, Kelly S, Hunter M, Perlman S. Population dynamics and rapid spread of, a bacterial endosymbiont causing cytoplasmic incompatibility in(Hymenoptera: Aphelinidae)., 2010, 104(3): 239-246.
[15] Wang J J, Dong P, Xiao L S, Dou W. Effects of removal ofinfection on fitness of the stored-product pest(Psocoptera: Liposcelididae)., 2008, 101(5): 1711-1717.
[16] Kenyon S, Hunter M. Manipulation of oviposition choice of the parasitoid wasp,, by the endosymbiotic bacterium., 2007, 20(2): 707-716.
[17] 任素丽, 尹祥杰, 郭秋, 任顺祥, 邱宝利. 利福平与高温对的灭活效果及其对烟粉虱寄主发育与繁殖的影响.环境昆虫学报, 2015, 37(3): 467-474.
Ren S L, Yin X J, Guo Q, Ren S X, Qiu B L. Inactive efficiencies of rifampicin and high temperature toand its effects on the biology ofhost., 2015, 37(3): 467-474. (in Chinese)
[18] Chu D, Gao C S, De Barro P, Zhang Y J, Wan F H, Khan I. Further insights into the strange role of bacterial endosymbionts in whitefly,: comparison of secondary symbionts from biotypes B and Q in China., 2011, 101(4): 477-486.
[19] Pan H P, Li X C, Ge D Q, Wang S L, Wu Q J, Xie W, Jiao X G, Chu D, Liu B M, Xu B Y, Zhang Y J. Factors affecting population dynamics of maternally transmitted endosymbionts in., 2012, 7(2): e30760.
[20] Fang Y W, Liu L Y, Zhang H L, Jiang D F, Chu D. Competitive ability and fitness differences between two introduced populations of the invasive whiteflyQ in China., 2014, 9(6): e100423.
[21] Liu B, Yan F, Chu D, Pan H, Jiao X, Xie W, Wu Q, Wang S, Xu B, Zhou X, Zhang Y. Difference in feeding behaviors of two invasive whiteflies on host plants with different suitability: implication for competitive displacement., 2012, 8(5): 697-706.
[22] 李晓敏, 李静静, 汤清波, 闫凤鸣. 烟碱对B型和Q型烟粉虱取食行为的影响—基于EPG 和液体饲囊技术体系. 中国农业科学, 2013, 46(10): 2041-2049.
Li X M, Li J J, Tang Q B, Yan F M. Effects of nicotine on feeding behavior ofB and Q biotypes based on EPG and liquid diet sac technique., 2013, 46(10): 2041-2049. (in Chinese)
[23] 卢少华, 李静静, 刘明杨, 白润娥, 汤清波, 闫凤鸣. 烟粉虱 B 型和 Q 型竞争能力的室内比较分析. 中国农业科学, 2015, 48(7): 1339-1347.
Lu S H, Li J J, Liu M Y, Bai R E, Tang Q B, Yan F M. Comparative analysis of the competitiveness between B and Q biotypes ofunder laboratory conditions., 2015, 48(7): 1339-1347. (in Chinese)
[24] YinH D, Wang X Y, Xue K, Huang C H, Wang R J, Yan M F, Xu C R. Impacts of transgenic Bt cotton on the stylet penetration behaviors ofbiotype B: evidence from laboratory experiments., 2010, 17(4): 344-352.
[25] 胡想顺, 赵惠燕, 胡祖庆, 李东鸿, 张宇红. 禾谷缢管蚜在三个小麦品种上取食行为的EPG比较. 昆虫学报, 2007, 50(11): 1105-1110.
Hu X S, Zhao H Y, Hu Z Q, Li D H, Zhang Y H. Comparison offeeding behavior on seedlings of three wheat varieties., 2007, 50(11): 1105-1110. (in Chinese)
[26] Liu B M, Preisser E L, Chu D, Pan H P, Xie W, Wang S L, Wu Q J, Zhou X G, Zhang Y J. Multiple forms of vector manipulation by a plant-infecting virus:and., 2013, 87(9): 4929-4937.
[27] 罗晨, 岳梅, 徐洪富, 张芝利. EPG技术在昆虫学研究中的应用及进展. 昆虫学报, 2005, 48(3): 437-443.
Luo C, Yue M, Xu H F, Zhang Z L. Application of electrical penetration graph (EPG) in entomological studies and new findings., 2005, 48(3): 437-443. (in Chinese)
[28] He Y P, Chen L, Chen J M, Zhang J F, Chen L Z, Shen J L, Zhu Y C. Electrical penetration graph evidence that pymetrozine toxicity to the rice brown planthopper is by inhibition of phloem feeding., 2011, 67(4): 483-491.
[29] Khasdan V, Levin, Rosner A, Morin S, Kontsedalov S, Maslenin L, Horowitz A R. DNA marker for identifying biotypes B and Q of(Hemiptera: Aleyrodidae) and studying population dynamics., 2005, 95(6): 605-613.
[30] Chu D, Zhang Y J, Cong B, Xu B Y, Wu Q J, Zhu G R. Sequences analysis of mtDNA COI gene and molecular phylogeny of different geographical populations of(Gennadius)., 2005, 4(7): 533-541.
[31] Weeks A R, Velten R, Stouthamer R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods.:, 2003, 270(1526): 1857-1865.
[32] Pan H P, Chu D, Liu B M, Xie W, Wang S L, Wu Q J, Xu B Y, Zhang Y J. Relative amount of symbionts in insect hosts changes with host-plant adaptation and insecticide resistance., 2013, 42(1): 74-78.
[33] 汤清波, 张大山, 姬琨, 丁识伯, 闫凤鸣. 刺吸电位技术应用中的几个问题. 应用昆虫学报, 2011, 48(5): 1519-1527.
Tang Q B, Zhang D S, Ji K, Ding S B, Yan F M. Some key points in applications of electrical penetration graph technique., 2011, 48(5): 1519-1527. (in Chinese)
[34] Moreno-Delafuente A, Garzo E, Moreno A, Fereres A. A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread., 2013, 8(4): e61543.
[35] van Helden M, Tjallingii W F. Tissue localisation of lettuce resistance to the aphidusing electrical penetration graphs., 1993, 68(3): 269-278.
[36] Lei H, Tjallingii W F, van Lenteren J C, Xu R M. Stylet penetration by larvae of the greenhouse whitefly on cucumber., 1996, 79(1): 77-84.
[37] Jiang Y X, Walker G P. Identification of phloem sieve elements as the site of resistance to silverleaf whitefly in resistant alfalfa genotypes., 2007, 125(3): 307-320.
[38] Nardone E, Dey T, Kevan P G. The effect of sugar solution type, sugar concentration and viscosity on the imbibition and energy intake rate of bumblebees., 2013, 59(9): 919-933.
[39] Detrain C, Prieur J. Sensitivity and feeding efficiency of the black garden antto sugar resources., 2014, 64: 74-80.
[40] Gabrys B, Tjallingii W F, Van Beek T A. Analysis of EPG recorded probing by cabbage aphid on host plant parts with different glucosinolate contents., 1997,23(7): 1661-1673.
[41] Lei H, Tjallingii W F, van Lenteren J C. Effect of tethering during EPG recorded probing by adults of the greenhouse whitefly., 1997, 121(1/5): 211-217.
[42] Lei H, van Lenteren J C, Xu R M. Effects of plant tissue factors on the acceptance of four greenhouse vegetable host plants by the greenhouse whitefly: an electrical penetration graph (EPG) study., 2001, 98(1): 31-36.
[43] 赵静, 王甦, 郭晓军, 高希武, 张帆. 寄生蜂低温贮藏研究进展. 中国农业科学, 2014, 47(3): 482-494.
Zhao J, Wang S, Guo X J, Gao X W, Zhang F. Progress in research of cold storage of insect parasitoids., 2014, 47(3): 482-494. (in Chinese)
[44] 申光茂, 王晓娜, 黄勇, 豆威, 王进军. 橘小实蝇幼虫解毒酶系基因应对高效氯氰菊酯胁迫的组织特异性表达. 中国农业科学, 2015, 48(19): 3857-3865.
Shen G M, Wang X N, Huang Y, Dou W, Wang J J.Tissue specific expression of genes encoding detoxification enzymes in the larvae ofundercypermethrin stress., 2015, 48(19): 3857-3865. (in Chinese)
[45] Carter M J, Simon J C, Nespolo R F. The effects of reproductive specialization on energy costs and fitness genetic variances in cyclical and obligate parthenogenetic aphids., 2012, 2(7): 1414-1425.
[46] Terblanche J S, Klok C J, Chown S L. Metabolic rate variation in(Diptera: Glossinidae): gender, ageing and repeatability., 2004, 50(5): 419-428.
[47] Kliot A, Ghanim M. Fitness costs associated with insecticide resistance., 2012, 68(11): 1431-1437.
[48] 陈珍珍, 李明贵, 郭亚楠, 印象初, 张帆, 许永玉. 光周期和温度对中华通草蛉不同越冬时期成虫滞育后生物学特性的影响. 中国农业科学, 2013, 46(8): 1610-1618.
Chen Z Z, Li M G, Guo Y N, Yin X C, Zhang F, Xu Y Y.Effects of photoperiod and temperature on the post-diapause biology of(Tjeder) adults in different overwintering periods., 2013, 46(8): 1610-1618. (in Chinese)
[49] Luan J B, Wang Y L, Wang J, Wang X W, Liu S S. Detoxification activity and energy cost is attenuated in whiteflies feeding on-infected tobacco plants., 2013, 22(5): 597-607.
(责任编辑 岳梅)
Comparison of Feeding Behavior Between TwoStrains Using EPG Technique
ZHANG Wen-ping1, LIU Bai-ming2, ZHANG Shan1, WAN Fang-hao1,3, CHU Dong1
(1College of Agronomy and Plant Protection, Qingdao Agricultural University, Qingdao 266109, Shandong;2Tianjin Institute of Plant Protection, Tianjin 300384;3State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193)
【Objective】is one of the endosymbiont species infects in sweetpotato whiteflyGennadius (Hemiptera: Aleyrodidae). Previous studies have shown that there was a significantdifference in the fitness between- infected (C+) and -uninfected (C-) strains ofQ biotype. However, the behavioral mechanism causing the difference in fitness between the two strains is not clear. This study investigated the difference in the feeding behavior of these two strains to reveal the underlying mechanism responsible for the difference in fitness. 【Method】The feeding behavior of C+strain and C-strain on cotton and tomato during 6 h was recorded using an electrical penetration graph (EPG). The waveform types of feeding behavior were then identified and analyzed. A total of 19 parameters (11 parameters associated with non-phloem phase and 8 parameters associated with phloem phase) were calculated and analyzed to compare the two strains’ feeding behavior at phloem stage and non-phloem stage.【Result】Of the 118successful recordings obtained in this experiment, 58 recordings (C-strain=28 replicates and C+strain=30 replicates) were on cottons and 60 recordings (C-strain=31 replicates and C+strain=29 replicates) were on tomatoes. The results showed that at the non-phloem phase on cottonC+strain had a significantly longer total duration of probes than C-strain. C+strain had a significantly shorter time than C-strain in terms of in duration of np after the first E.The parameters associated with salivation into a sieve element and ingestion of a sieve element sap had no significant difference between the two strains on cotton at phloem phase, and the percentage of whiteflies reaching phloem phase within 6 hours had no significant difference. Meanwhile, at the non-phloem phase, C+strain had a greater number of total probes and probes before the 1st E than C-strain on tomato. C+strain also had a longer time from the 1st probe to the 1st sustained E2 and a shorter average probe time than C-strain. The parameters regarding salivation into a sieve element and ingestion of sieve element sap also had no significant difference between the two strains on tomato at phloem phase. Also, the percentage of whiteflies reaching phloem phase within 6 hours had no significant difference. On the whole, the parameters associated phloem phase had no significant difference between the two strains. Compared with C-strain, C+strain has a longer probing time and requires more probes at non-phloem phase.【Conclusion】The feeding behavior of C+strain and C-strain has no significant difference at phloem phase, but does have a significant difference at non-phloem phase. The results indicate that the feeding behavior difference of the two strains at non-phloem phase is most likely related to the difference in fitness.
Q biotype; fitness; electrical penetration graph (EPG); feeding behavior
2016-01-27;接受日期:2016-03-22
国家自然科学基金(31272105,31572064)、泰山学者建设工程专项经费
张文平,Tel:0532-88030319;E-mail:wenpingzhang@126.com。通信作者褚栋,Tel:0532-88030319;E-mail:chinachudong@qau.edu.cn

