有机荧光分子2-氰基-3-(3,4-二甲氧基苯基)-2-丙烯酰胺的可逆压致变色和质子刺激响应性能
边高峰 黄 华 占玲玲 吕晓静 曹 枫 张 诚,* 张玉建,*(浙江工业大学化学工程学院,杭州004;杭州师范大学有机硅化学及材料技术重点实验室,杭州00;湖州师范学院材料化学系,浙江湖州000)
有机荧光分子2-氰基-3-(3,4-二甲氧基苯基)-2-丙烯酰胺的可逆压致变色和质子刺激响应性能
边高峰1,2黄华3占玲玲1吕晓静1曹枫3张诚1,*张玉建3,*
(1浙江工业大学化学工程学院,杭州310014;2杭州师范大学有机硅化学及材料技术重点实验室,杭州310012;3湖州师范学院材料化学系,浙江湖州313000)
设计合成了3-芳基-2-腈基丙烯酰胺类有机发光小分子2-氰基-3-(3,4-二甲氧基苯基)-2-丙烯酰胺(CDMPA)。经研究发现,CDMPA化合物具有明显的压致变色和酸致变色现象。在外力刺激下,化合物CDMPA荧光最大发射峰发生20 nm的红移,经过加热或蒸汽处理后可恢复初始状态。对样品研磨前后粉末的X射线衍射图谱及荧光寿命衰减曲线进行测试分析得出,CDMPA压致变色现象归因于分子构型由晶态到无定形态的转化。另外,在酸刺激下CDMPA发光颜色由蓝光红移至黄光,最大发射波长红移33 nm。经过二甲基甲酰胺(DMF)处理后可恢复到初始状态。由测试得到的红外光谱及分子轨道理论计算推测,酸致变色现象是由氨基取代基的质子化影响了CDMPA前线分子轨道引起的。本研究可使人们深入了解这种类型材料的多刺激响应发光机制,且显著的颜色变化性能使CDMPA在传感器和检测装置方面具有潜在的应用前景。
丙烯酰胺衍生物;多刺激响应;荧光转换;前线分子轨道;荧光寿命
doi:10.3866/PKU.WHXB201512083
1 Introduction
Stimuli-responsive organic function materials that demonstrated a emission switch upon external stimuli have gained considerable attention from researchers due to their potential applications in the field of optoelectronics,such as sensors,memory chips,and security inks1-8.Recently,the rapid progress has been achieved in these material systems and understanding the formation mechanism of luminescent materials,which are responsive to external stimuli,such as mechanical force and vapor9-14.Fluorescence arises not only from intramolecular effects,such as the molecular configuration and conjugation length,but also from intermolecular effects,for example,π-π overlap15-18.Piezochromic luminescent materials have been obtained through the manipulation of molecular packing modes by grinding and heating treatments19,20. Thermo-and acid-dependent luminescent materials have also been developed based on the control of their molecular conformations or frontier molecular orbitals21,22.But for all this,it is still essential to further understand the relationship between the acid/alkalidependent luminescent property changes and the molecular structure characteristics at the molecular level.Tian et al.23reported a novel derivative 9,10-bis((E)-2-(pyridin-3-yl)vinyl)anthracene(BP3VA)with remarkable fluorescence change under protonation-deprotonation stimuli.The results indicated that the enhanced excitonic coupling and electron delocalization induced the fluorescence change in crystals.Lu et al.24,25investigated a triphenylamine-based benzoxazole derivative(BVDP)with remarkable fluorescence change under protonation-deprotonation stimuli attributed to the benzoxazole moiety protonated to form a cation.Wang et al.26investigated the vapochromic and piezochromic properties of a donor-acceptor structured compound and demonstrated that the fluorescence emission could be changed from blue to orange using different stimuli.In addition,our group prepared a new compound containing the arylamine,which was different from the above two groups.Clearly,employing slight structural change to investigate the formation mechanism of Mechanochromic fluorescence(MCF)properties is of significant interest27-34.Herein,we reported a combined study based on a novel derivative(Z)-2-cyano-3-(3,4-dimethoxyphenyl)acryamide (CDMPA)(Fig.1(a)),which possesses multi-stimuli responsive fluorescent properties.The CDMPA was found to be a piezochromic fluorescence(PCF)molecule,and the mechanically induced luminescence color could return to the original one by treatment with dichloromethane or ethanol vapors Fig.1(b).Interestingly,we found a larger redshift occurred after acetic acid or hydrochloride vapor treatment Fig.1(b).The fluorescence lifetime experiment data and the frontier molecular orbitals further validated the formation mechanism of PCF behaviors.
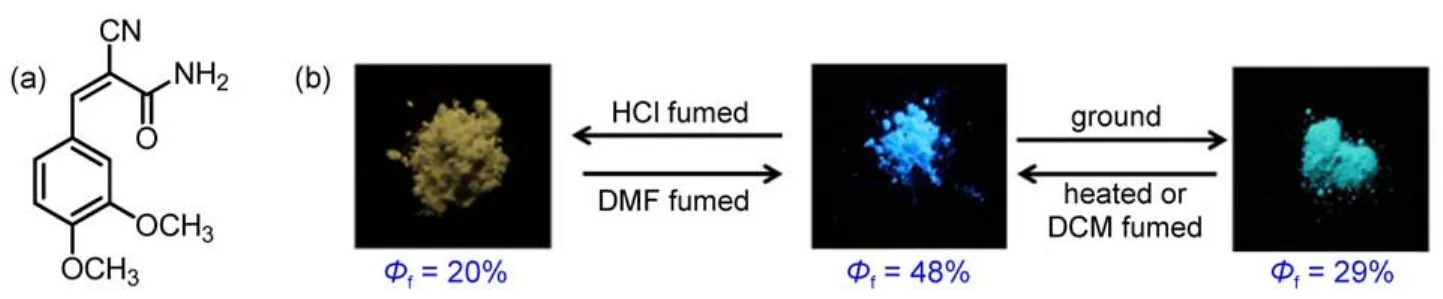
Fig.1 (a)Molecular structure of CDMPAand(b)photographs of the ground powder,heated powder,and the fumed powder under UV light(365 nm)
2 Experimental
2.1Materials and general methods
3,4-Dimethoxybenzaldehyde(Aladdin-reagent,98%).2-Cyanoacetamide(Aladdin-reagent,98%).L-Proline(Aladdin-reagent, 98%).Ethanol was obtained from commercial suppliers and used without further purification unless otherwise indicated.The1H NMR and13C NMR spectra were recorded on a Bruker AVANCE III 500 MHz instrument(Bruker,Switzerland)using chloroformd(CDCl3)as the solvent.High-resolution electrospray ionization mass spectra(HRMS(ESI))were recorded on a Thermo Fisher Exactive mass spectrometer.The UV-Vis absorption spectrum was recorded on a Perkin Elmer Lambda 35 spectrophotometer.Fluorescent measurements were recorded on a Perkin-Elmer LS-55 luminescence spectrophotometer.The fluorescence quantum yield (Φf)was determined using a calibrated integrating sphere.Fluorescence lifetime experiments were performed by using the timecorrelated single-photon counting(PicoHarp 300 TCSPC)system with right-angle sample geometry.Powder X-ray diffraction (PXRD)was performed on a X′Pert PRO,PANalytical,with Cu-Kαradiation operating at 40 kV and 40 mA.
2.2Synthesis of CDMPA
A mixture of 3,4-dimethoxybenzaldehyde(1.66 g,10 mmol),2-cyanoacetamide(0.92 g,11 mmol),and L-proline(0.23 g,2 mmol)in ethanol(20 mL)was heated under reflux for 2 h(Scheme 1).The precipitated solid was filtered and successively washed with ethanol.The product was then purified by recrystallization from ethanol solution to give CDMPA as a white powder(92% yield).1H NMR(500 MHz,DMSO(dimethylsulfoxide))δ 8.11(s, 1H),7.80(s,1H),7.67(d,J=2.0 Hz,2H),7.58(dd,J=8.5,2.0 Hz,1H),7.16(d,J=8.5 Hz,1H),3.86(s,3H),3.81(s,3H).13C NMR(126 MHz,DMSO)δ 163.0,152.5,150.5,148.7,125.4, 124.5,117.1,112.3,111.8,102.9,55.7,55.5.HRMS(ESI)Calcd. for C12H12N2O3([M+H]+):232.0848.Found:232.0859.
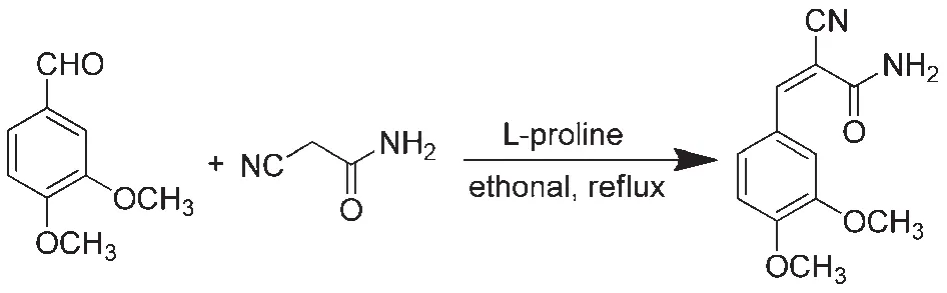
Scheme 1 Synthetic scheme of(Z)-2-cyano-3-(3,4-dimethoxyphenyl)acryamide(CDMPA)
3 Results and discussion
3.1Piezochromic behavior
As shown in Fig.2,the CDMPA sample exhibited rather remarkable PCF properties.The emission spectrum of the CDMPA powder(λmax=487 nm)indicated the blue fluorescence.Once the powder had been ground using a pestle and mortar,a significant red-shift(λmax=503 nm)occurred.After being heated at 50°C for about 10 min,the samples fully recovered their original luminescence.This cycle could be repeated,allowing the powder to switch between the two different emission colors.
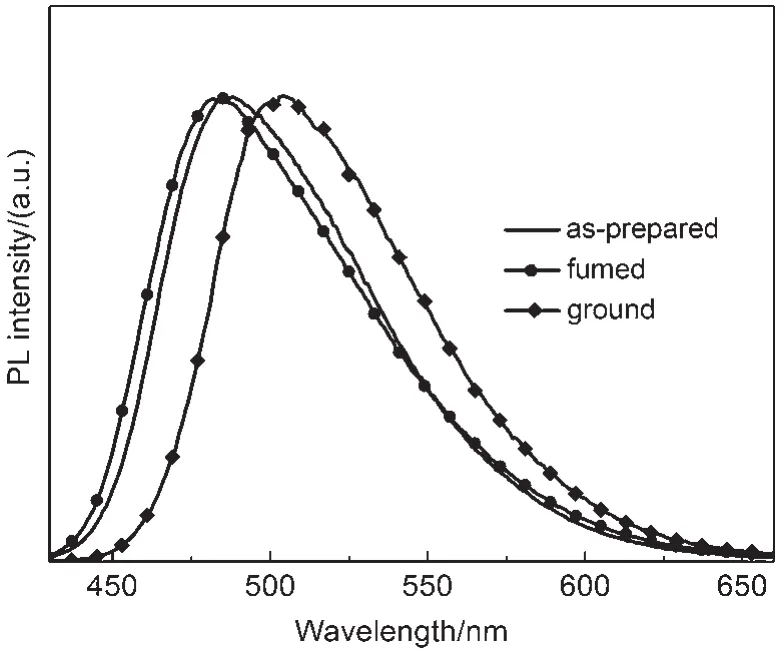
Fig.2 Photoluminescence(PL)spectra of the as-prepared, fumed,and ground powders
In order to gain further insight into the origin of the fluorescence change of the CDMPA powder,powder X-ray diffraction experiments were conducted(Fig.3).The pristine powders of CDMPA clearly exhibited numerous sharp and intense reflection peaks,which indicated a well-defined microcrystalline structure. These peaks of the CDMPA samples almost disappeared and became weak,broad,and diffused peaks upon grinding.The results showed that the crystal lattice was significantly disrupted and a new amorphous phase was formed.The ground samples could be recovered to the original states after solvent or heating treatment.Thus,the piezochromic properties of CDMPA could be attributed to the transformation from the crystalline phase to the amorphous phase,which coincided with previous results24,25.
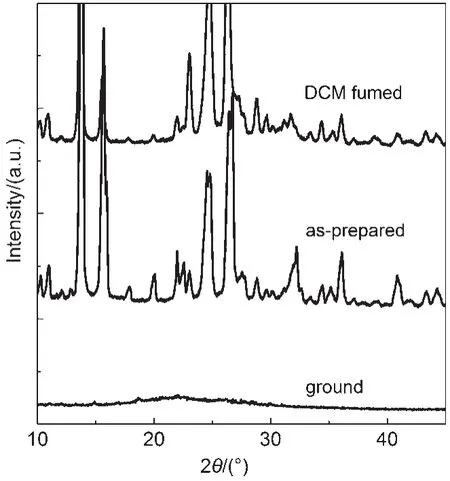
Fig.3 PXRD patterns of CDMPA:the CH2Cl2(DCM)fumed powder,as-prepared powder,and ground powder
Time-resolved fluorescence experiments further verified this hypothesis and the weighted mean lifetimes(τ)are illustrated in Fig.4 and Table 1.The τ values of the CDMPAsamples in different states revealed the significant differences:the value was 5.81 ns for the original samples,and 10.71 ns for the ground samples.The increase in lifetime revealed that the excitonic coupling was obviously enhanced.The as-prepared powders of CDMPA exhibited a fluorescence quantum yield of 48%,which was changed upon grinding(Φf=29%).The non-radiative rate constant[knr=(1-Φf)/τf]of the as-prepared powders was 1.0×108s-1and closed to ground powders,but the radiative rate constants(kF=Φf/τf)was decreased from 7.9×107to 2.1×107s-1.The results indicated that the π-π interaction was further enhanced and blocked the radiative deactivation pathways.The intermolecular interaction between the adjacent aromatic rings was enhanced as a result of the excitonic coupling.Such strong π-π interactions between the neighbouring molecules blocked the decay of the excited speciesthrough radiative pathways,resulting in the altering of the excited state as well as the weak Φfof CDMPA(29%)35,36.
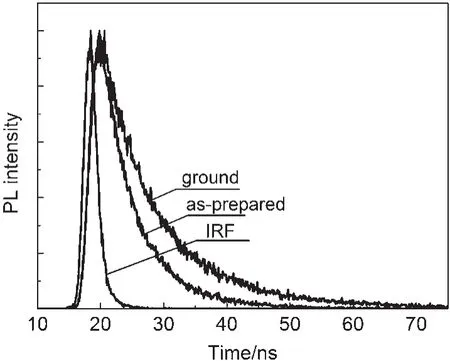
Fig.4 Fluorescence decay profiles of CDMPAat ground and as-prepared states

Table1 Time-resolved emission-decay curves of the compound CDMPAin crystal and ground states
3.2Acidchromic properties
Another notable feature of the compound CDMPA was found that fumigation with acid vapor had a significant effect upon the fluorescence of the CDMPA powder,turning it from blue to yellow,while using N,N-dimethylformamide(DMF)could lead to partial recovery of the original emission.The photoluminescence(PL)spectrum of the CDMPA powder,shown in Fig.5, exhibited a strong blue emission peaking at 487 nm.Interestingly, after being fumed by acetic acid(CH3COOH)or hydrochloride (HCl)vapor,the powder exhibited a yellow emission and the peak was shifted to 520 nm under 365 nm UV illumination.After being fumed with DMF vapor,the color restored to the initial state. Additionally,the deprotonation with the aid of a volatile base could lead to a partially recovered emission.The 33 nm shift in the fluorescence and subsequent recovery towards the initial state were a demonstration of the significant fluorescence switching properties of the CDMPApowder under protonation-deprotonation stimuli.
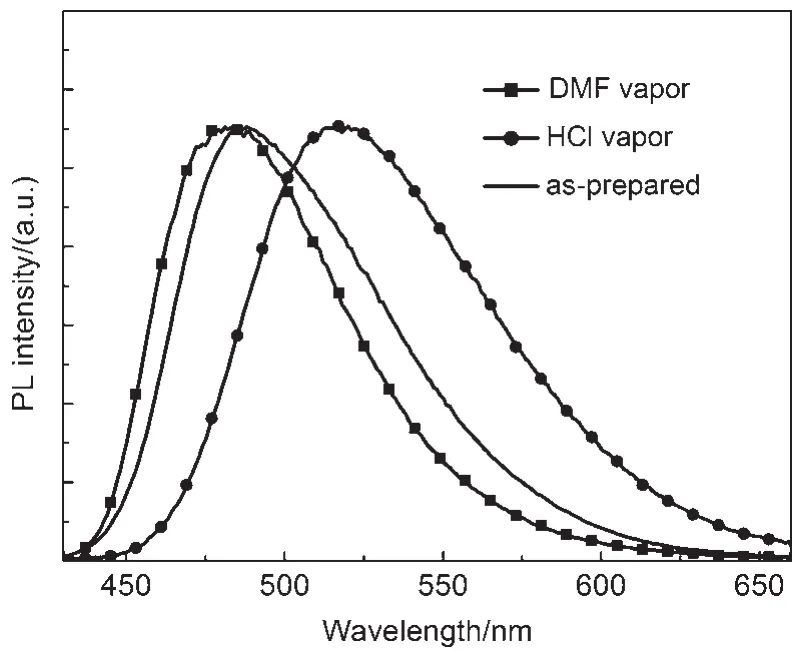
Fig.5 PLspectra of the DMF fumed,HCl fumed, and as-prepared powders
To gain more insights into the origin of the fluorescence change of the CDMPA powder,the phase characteristics of the CDMPA powder were investigated by PXRD analysis and the patterns were shown in Fig.6.As the PXRD patterns showed,the as-prepared powders of CDMPA exhibited numerous sharp and intense reflection peaks,which indicated a well-defined microcrystalline structure.After being fumed by HCl vapor,the obtained PXRD pattern was obviously different from that of the initial powder. Some new peaks appeared and the resolvable peaks drifted.As a result,the initial microcrystals of CDMPAwere broken down and other microcrystals were formed under acid stimuli.In addition, the PXRD pattern of the DMF fumed powders were consistent with those of the as-prepared powders.
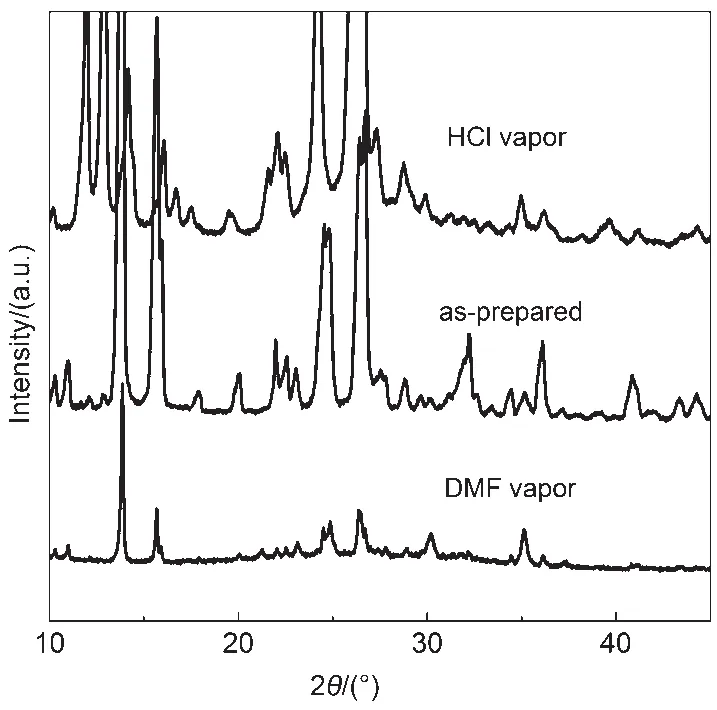
Fig.6 PXRD patterns of the HCl fumed,as-prepared, and DMF fumed powders
IR spectra of CDMPA powders were inconsistent in crystalline and its protonated form(Fig.7).IR peaks at 3199 cm-1(―NH2) and 1720 cm-1(C=O)exhibited blue-shift upon fumigation of acid vapor.The results indicated that proton binding of the CDMPA was ascribed to the protonation of the nitrogen atom in the amino acid unit which reduced the electron density aroundthese protons.Thus,IR peak of the carboxide attaching to the amino acid moiety appeared deviation37,38.It was suspected that the CDMPA may function as a sensory material to detect H+because the amino moiety can be protonated.
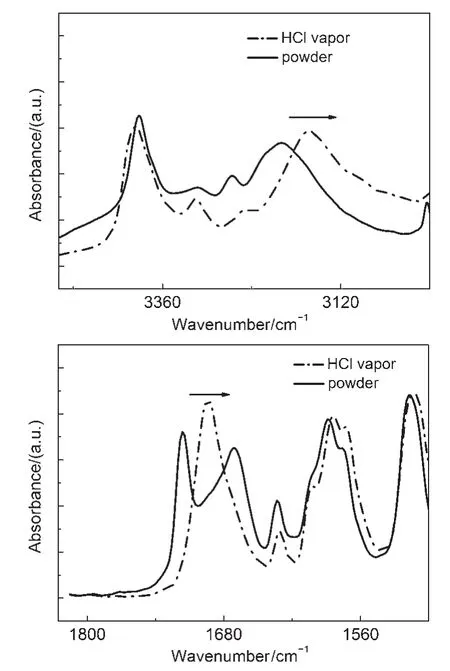
Fig.7 IR spectra of CDMPA in crystalline form(solid line)and its protonated form(dash line)
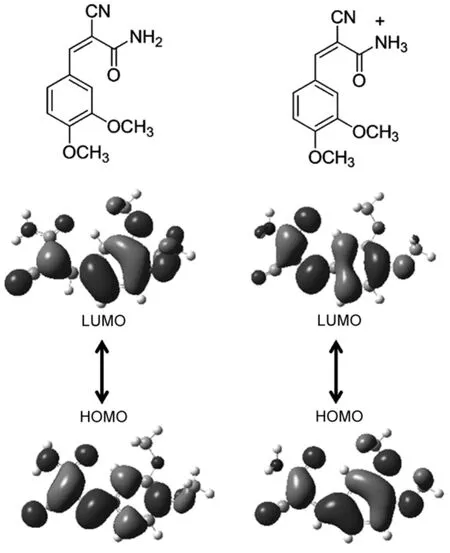
Fig.8 Molecular structures and theoretical calculated frontier orbit at contributions of CDMPAand protonated CDMPA
The highest occupied molecular orbital(HOMO)and the lowest unoccupied molecular orbital(LUMO)of CDMPA were calculated based on B3LYP/6-31G**as shown in Fig.8.The HOMO was mainly located on the phenyl propenamide,while the LUMO was partially distributed over the molecule.In contrast,we calculated the orbitals of the protonated CDMPAmolecule.Since the protonation of its nitrogen atom enhanced the electron-withdrawing ability of amino group,the LUMO of one protonated molecule was spread over the whole molecule with an increased electron cloud density on amino group and the LUMO should be more delocalized in the protonated molecules.Generally,the delocalization of the electron cloud was beneficial for stabilizing the molecule excited state39,40,resulting in a decreased band gap. Consequently,the delocalization of the LUMO stabilized the excited molecule at a lower band gap and contributed to the bathochromic in emission of protonated CDMPA41.
4 Conclusions
We designed and synthesized a multi-stimuli responsive fluorescence molecule CDMPA,which exhibited remarkeble fluorescence changes under pressure and acid stimuli.According to the X-ray diffraction pattern and fluorescence lifetime analysis,the piezochromic behavior of the CDMPA samples originated from the changes in their aggregation state and the formation of strong intermolecular π-π interaction under pressure stimiuli.The protonation process of CDMPA was confirmed by photophysic and computational studies and indicated that fumigation with acid vapor led to a transfomation of the compound′s frontier molecular orbitals,resulting in red-shift phenomenon.The reversible and different multistimuli-response fluorescence switch for the molecule CDMPA promised their potential applications in the fluorescent sensors in the future.
References
(1)Irie,M.;Fukaminato,T.;Sasaki,T.;Tamai,N.;Kawai,T. Nature 2002,420,759.doi:10.1038/420759a
(2)Lim,S.J.;An,B.K.;Jung,S.D.;Chung,M.A.;Park,S.Y. Angew.Chem.Int.Edit.2004,43,6346.doi:10.1002/ anie.200461172
(3)Hirata,S.;Watanabe,T.Adv.Mater.2006,18,2725.doi: 10.1002/adma.200600209
(4)Babu,S.S.;Kartha,K.K.;Ajayaghosh,A.J.Phys.Chem. Lett.2010,1,3413.doi:10.1021/jz101219y
(5)Che,Y.K.;Yang,X.M.;Zang,L.Chem.Commun.2008, 1413.doi:10.1039/B719384J
(6)Sagara,Y.;Kato,T.Nat.Chem.2009,1,605.doi:10.1038/ nchem.411
(7)Weder,C.J.Mater.Chem.2011,21,8235.doi:10.1039/ c1jm90068d
(8)Chi,Z.G.;Zhang,X.Q.;Xu,B.J.;Zhou,X.;Ma,C.P.; Zhang,Y.;Liu,S.W.;Xu,J.R.Chem.Soc.Rev.2012,41, 3878.doi:10.1039/C2CS35016E
(9)Dong,Y.J.;Xu,B.;Zhang,J.B.;Tan,X.;Wang,L.J.;Chen,J. L.;Lv,H.G.;Wen,S.P.;Li,B.;Ye,L.;Zou,B.;Tian,W.J. Angew.Chem.Int.Edit.2012,51,10782.doi:10.1002/ anie.201204660
(10)Yoon,S.J.;Chung,J.W.;Gierschner,J.;Kim,K.S.;Choi,M. G.;Kim,D.;Park,S.Y.J.Am.Chem.Soc.2010,132,13675. doi:10.1021/ja1044665
(11)Suzuki,T.;Shinkai,S.;Sada,K.Adv.Mater.2006,18,1043. doi:10.1002/adma.200502552
(12)Takahashi,E.;Takaya,H.;Naota,T.Chem.Eur.J.2010,16, 4793.doi:10.1002/chem.200903403
(13)Dautel,O.;Robitzer,M.;Lere-Porte,J.P.;Serein-Spirau,F.; Moreau,J.J.Am.Chem.Soc.2006,128,16213.doi:10.1021/ ja065434u
(14)Anthony,S.P.ChemPlusChem 2012,77,518.doi:10.1002/ cplu.201200073
(15)Sagara,Y.;Kato,T.Angew.Chem.Int.Edit.2008,47,5175. doi 10.1002/ange.200800164
(16)Yamaguchi,S.;Shirasaka,T.;Akiyama,S.;Tamao,K.J.Am. Chem.Soc.2002,124,8816.doi:10.1021/ja026689k
(17)Zhang,Z.L.;Yao,D.D.;Zhou,T.L.;Zhang,H.Y.;Wang,Y. Chem.Commun.2011,47,7782.doi:10.1039/c1cc11882j
(18)Mizoshita,N.;Tani,T.;Inagaki,S.Adv.Mater.2012,24,3350. doi:10.1002/adma.201201064
(19)Kunzelman,J.;Kinami,M.;Crenshaw,B.R.;Protasiewicz,J. D.;Weder,C.Adv.Mater.2008,20,119.doi:10.1002/ adma.200701772
(20)Sagara,Y.;Mutai,T.;Yoshikawa,I.;Araki,K.J.Am.Chem. Soc.2007,129,1520.doi:10.1021/ja0677362
(21)Zhao,Y.F.;Gao,H.Z.;Fan,Y.;Zhou,T.L.;Su,Z.M.;Liu,Y.; Wang,Y.Adv.Mater.2009,21,3165.doi:10.1002/ adma.200803432
(22)Pucci,A.;Ruggeri,G.J.Mater.Chem.2011,21,8282.doi: 10.1039/C0JM03653F
(23)Zhang,J.B.;Chen,J.L.;Xu,B.;Wang,L.J.;Ma,S.Q.;Dong, Y.J.;Tian,W.J.Chem.Commun.2013,49,3878.
(24)Xue,P.C.;Chen,P.;Jia,J.H.;Xu,Q.X.;Sun,J.B.;Yao,B. Q.;Zhang,Z.Q.;Lu,R.Chem.Commun.2014,50,2569.doi: 10.1039/C3CC49208G
(25)Xue,P.C.;Lu,R.;Jia,J.H.;Takahuji,M.;Ihara,H.Chem. Eur.J.2012,18,3549.doi:10.1002/chem.201103566
(26)Dou,C.D.;Han,L.;Zhao,S.S.;Zhang,H.Y.;Wang,Y. J.Phys.Chem.Lett.2011,2,666.doi:10.1021/jz200140c
(27)Varghese,S.;Das,S.J.Phys.Chem.Lett.2011,2,863.doi: 10.1021/jz200099p
(28)Zhang,Y.;Wang,K.;Zhuang,G.L.;Xie,Z.Q.;Zhang,C.; Cao,F.;Pan,G.;Chen,H.F.;Zou,B.;Ma,Y.Chem.Eur.J. 2015,21,2474.doi:10.1002/chem.201405348
(29)Zhang,H.Y.;Zhang,Z.L.;Ye,K.Q.;Zhang,J.Y.;Wang,Y. Adv.Mater.2006,18,2369.doi:10.1002/adma.200600704
(30)Wei,R.R.;Song,P.S.;Tong,A.J.J.Phys.Chem.C 2013, 117,3467.doi:10.1021/jp311020w
(31)Mutai,T.;Satou,H.;Araki,K.Nat.Mater.2005,4,685.doi: 10.1038/nmat1454
(32)Anthony,S.P.ChemPlusChem 2012,77,518.doi:10.1002/ cplu.201200073
(33)Zhang,X.Q.;Chi,Z.G.;Xu,B.J.;Chen,C.J.;Zhou,X.; Zhang,Y.;Liu,S.W.;Xu,J.R.J.Mater.Chem.2012,22, 18505.doi:10.1039/C2JM33140C
(34)Ouyang,M.;Yu,C.H.;Zhang,Y.J.;Hu,B.;Lü,X.J.;Sun,J. W.;Zhang,C.Acta Phys.-Chim.Sin.2012,28,2944.[欧阳密,俞春辉,张玉建,胡彬,吕晓静,孙璟玮,张诚.物理化学学报,2012,28,2944.]doi:10.3866/PKU.WHXB201208012
(35)Zhang,Y.J.;Zhuang,G.L.;Ouyang,M.;Hu,B.;Song,Q.B.; Sun,J.W.;Zhang,C.;Gu,C.;Xu,Y.X.;Ma,Y.G.Dyes Pigments 2013,98,486.doi:10.1016/j.dyepig.2013.03.017
(36)Song,Q.B.;Wang,Y.S.;Zhang,Y.J.;Sun,J.W.;Zhang,C. New J.Chem.2015,39,659.doi:10.1039/C4NJ01492H
(37)Li,H.Y.;Zhang,X.Q.;Chi,Z.G.;Xu,B.J.;Zhou,W.;Liu,S. W.;Zhang,Y.;Xu,J.R.Org.Lett.2011,13,556.doi:10.1021/ ol102872x
(38)Zhang,Y.J.;Sun,J.W.;Zhuang,G.L.;Ouyang,M.;Yu,Z. W.;Cao,F.;Pan,G.X.;Tang,P.S.;Zhang,C.;Ma,Y.G. J.Mater.Chem.C 2014,2,195.doi:10.1039/C3TC31416B
(39)Bernard,V.Molecular Fluorescence:Principles and Applications;WCH:Weinheim,Germany,2001;p 54.
(40)Yamaguchi,S.;Shirasaka,T.;Akiyama,S.;Tamao,K.J.Am. Chem.Soc.2002,124,8816.doi:10.1021/ja026689k
(41)Sun,H.T.;Tian,X.H.;Yuan,Y.Z.;Sun,J.Y.;Sun,Z.R.; Zhuo,X.L.Acta Phys.-Chim.Sin.2011,27,1847.[孙海涛,田晓慧,元以中,孙金煜,孙真荣,卓小玲.物理化学学报, 2011,27,1847.]doi:10.1039/C3TC31416B
Reversible Piezochromism and Protonation Stimuli-Response of (Z)-2-Cyano-3-(3,4-dimethoxyphenyl)acrylamide
BIAN Gao-Feng1,2HUANG Hua3ZHAN Ling-Ling1LÜ Xiao-Jing1CAO Feng3ZHANG Cheng1,*ZHANG Yu-Jian3,*
(1College of Chemical Engineering,Zhejiang University of Technology,Hangzhou 310014,P.R.China;2Key Laboratory of Organosilicon Chemistry and Material Technology,Hangzhou Normal University,Hangzhou 310012,P.R.China;3Department of Materials Chemistry,Huzhou University,Huzhou 313000,Zhejiang Province,P.R.China)
A3-aryl-2-cyano acrylamide derivative(Z)-2-cyano-3-(3,4-dimethoxy-phenyl)acrylamide(CDMPA) was designed and synthesized,which exhibited piezochromism and acidchromism properties.Under external mechanical force stimuli,CDMPAshowed a red-shift of 20 nm in its fluorescence emission and the mechanically induced luminescence color could return to the original state via heating or solvent vapor treatment.Powder X-ray diffraction(XRD)and fluorescence lifetime experiments indicated that the piezochromic luminescence could be attributed to the transformation from the crystalline to the amorphous phase.Additionally,the fluorescence color changed from blue to yellow with a red-shift of 33 nm using the stimulus of protonation.The emission color was recovered upon fuming with dimethyl formamide(DMF)vapor.Infrared(IR)spectra ofCDMPApowder and theoretical calculation of the frontier molecular orbitals showed that protonation of the amino moieties in CDMPAhad a significant effect on the frontier molecular orbitals and,thus,caused the acidchromism phenomenon.This study provides comprehensive insight into the stimuli-responsive luminescent mechanisms within this type of compound and the reversible switching of emission color may enable discovery of novel applications of CDMPAfor detection and sensing devices.
Acrylamide derivative;Multi-stimuli response;Fluorescence switching;Frontier molecular orbital;Fluorescence lifetime
October 14,2015;Revised:December 3,2015;Published on Web:December 8,2015.
O641
*Corresponding authors.ZHANG Cheng,Email:czhang@zjut.edu.cn;Tel:+86-571-88320508.ZHANG Yu-Jian,Email:sciencezyj@foxmail.com. The project was supported by the National Natural Science Foundation of China(51203138,51273179,51573165,51406030),Natural Science
Foundation of Zhejiang Province,China(LY15E030006,LY15E030002),Natural Science Foundation of Zhejiang University of Technology,China (1401101002408),andNatural Science Foundation of Huzhou,China(2014YZ02).
国家自然科学基金(51203138,51273179,51573165,51406030),浙江省自然科学基金(LY15E030006,LY15E030002),浙江工业大学自然科学基金(1401101002408)及湖州自然科学基金(2014YZ02)资助项目
©Editorial office ofActa Physico-Chimica Sinica

