图像处理和特征分析在评估乳腺癌近期发病风险中的应用
郑 斌
(俄克拉荷马大学 电机和计算机工程系,俄克拉荷马州 诺曼市 73019,美国)
图像处理和特征分析在评估乳腺癌近期发病风险中的应用
郑斌
(俄克拉荷马大学 电机和计算机工程系,俄克拉荷马州 诺曼市73019,美国)
图像处理和特征分析在乳腺癌早期诊断中发挥着重要作用。为了将一个基于左右乳腺X光片图像密度特征不对称性的图像特征分析方法应用于乳腺癌近期发病风险的预测,训练开发了一个以多图像特征相融合的人工智能分辨模型来预测每个妇女在近期(比如,在最近一次的良性检测以后2年之内)由医学影像检测出来的早期癌症的风险程度。介绍这一个新癌症风险预测模型的基本原理,演示一个新型的人机交互式的计算机辅助图像特征分析和计算的工作平台。初步实验证明,应用这一个基于医学图像特征定量分析的模型能够取得比采用现行其他已知的乳腺癌风险因子有着显著提高的对于近期乳腺癌发病风险的预测精度,这将为建立更有效的个性化乳腺癌普查提供依据和帮助。
医学图像处理; 医学图像标志物; 乳腺X光图像特征的定量分析; 近期乳腺癌风险评估; 计算机辅助检测
Introduction
Medical imaging using X-ray,ultrasound,magnetic resonance and other imaging modalities plays a critical role in many diseases including cancer screening,detection,diagnosis,and prognosis or treatment efficacy evaluation.However,accurately and consistently reading and interpreting medical images by the radiologists still faces a number of challenges in the clinical practice due to the large inter-reader variability and lack of quantitative assessment capability.Hence,developing computer-aided image processing and quantitative image feature analysis methods has been attracting extensive research interest in medical imaging fields.This review article introduces and discusses an example of applying a new computer-aided X-ray mammographic image processing scheme to build a unique near-term breast cancer risk prediction model,which aims to help improve efficacy of breast cancer screening.
Breast cancer is the most prevalent cancer in women and it is also very heterogeneous[1].The majority of breast cancers are detected among women with no known cancer risk factors to date[2].Hence,a uniform and population-based mammography screening protocol is currently recommended for all women who are older that the qualified age in many countries[3].The cancer screening has played an important role in the early detection and treatment of breast cancer for the last four decades,which results in a substantial reduction of breast cancer mortality.A lthough breast X-ray imaging (mammography) is the most commonly used breast cancer screening modality,its performance is sharply limited due to the large variability of breast abnormalities and overlapped dense fibro-glandular tissue in the 2D projection images[4].For example,sensitivity of screening mammography is significantly lower among women who are younger (i.e.,< 50 years old)[5],have dense breasts[6],use hormone replacement therapy[7],and carry certain breast cancer susceptibility genes[8].One study reported that mammographic sensitivity declined from 87.0% in women with almost entirely fatty breasts to 62.9% in women with extremely dense breasts,or from 83.3% in women over 80 years old to 68.6% in women younger than 50 years old[9].In addition,the specificity of screening mammography is also low,which generates higher recall rates.One study reported that during a 10-year period more than half of screened women would receive at least one false-positive recall and 7% to 9% would have at least one benign biopsy[10].Besides the women’s anxiety that can cause long-term psychosocial consequences[11],the limited healthcare resource and associated high cost to the women and society are also major issues[12].As a result,the efficacy of current mammography screening remains quite low and controversial[13].
In order to overcome such limitations,developing a more effective personalized screening paradigm based on individualized risk assessment has been recently attracting significant research interest[14-15].Although several studies have tried to identify small groups of women with substantially higher-than-average risk for developing breast cancer based on the epidemiology study identified risk factors,genomic information and breast density[16-18],using the existing risk factors and/or prediction models based on epidemiology studies do not yield a clinically acceptable discriminatory power when applied at the individual level to decide who should be screened or not in the near-term[19].Therefore,the prerequisite of reaching the goal of establishing a new and optimal personalized cancer screening paradigm is to identify and develop more highly discriminative cancer risk factors and/or new risk prediction models,which aim to predict near-term cancer risk and thus enable to stratify women into different risk groups in which different screening methods and intervals can be recommended.
In our research group,we proposed and investigated a new approach to identify breast cancer risk factors based on bilateral mammographic density and image feature asymmetry between the left and right breasts of the same individual women.Unlike the existing risk breast cancer factors that assess the risk of a group of women with higher risk of developing breast cancer as comparing to the general population in their lifetime,the image feature based risk factors dynamically change as the increase of the emerging risk of developing breast cancer in a near-term.The hypothesis of our approach is supported by several underlying assumptions and observations,which include that (1) humans naturally show bilateral symmetry in paired morphological traits including breasts[20],(2) radiologists routinely examine bilateral mammographic tissue asymmetry pattern and the change in asymmetry over time when making clinical decisions[21-22],(3) computerized schemes are able to achieve more consistent results in assessing mammographic tissue density by virtual elimination of observer variability[23],and (4) computerized image features are more reliable than risk factors reported by patients themselves in the clinic[24].Thus,in the last several years our research group has been working to test this hypothesis by developing new computer-aided detection (CAD) schemes to process mammograms,compute image features related to the bilateral mammographic density or tissue feature asymmetry,and train multi-feature based machine learning classifiers or models.In the following section of this article,we briefly review our study procedures and preliminary testing results when applying our new near-term breast cancer risk prediction models to variety of image datasets[25-30].
1 Methods
Figure 1 shows a flow diagram of developing our CAD based image segmentation and feature computation scheme,as well as a multi-image feature fusion based near-term breast cancer risk prediction model.The details of this approach have been reported in reference[29].In brief,three steps are applied to develop our new quantitative image feature based breast cancer risk prediction model.First,CAD is applied to automatically segment the breast tissue area depicted on each image and detect two landmarks including nipple and chest wall[25].In brief,CAD first detects the orientation of an image of the left or right breast and automatically flips the image of right breast.Thus,the chest walls of the breasts always align on the left side (edge) of the image.Next,based on the grey level histogram of the image,CAD applies an iterative searching method to detect the smoothest curvature between breast tissue and the air background as representing the skin line that is the interface between breast tissue and air background.All air-background related pixels depicted on the image are removed from the segmented breast area.Then,CAD detects the chest wall.Since the chest wall is often outside the depicted field of view on the CC images,for the purpose of this work,our scheme is only applied to detect the chest wall depicted on each MLO view image.CAD horizontally scans the breast image from left to right and searches for the pixel with the maximum gradient.The chest wall is detected by employing linear regression to fit all identified pixels with the maximum gradient on each scan.After detecting the chest wall,all pixels located inside the chest wall (the pectoral muscle region) are also excluded from the segmented breast area.Finally,CAD detects the nipple by searching either a small protruding area or a relatively low density smooth area along the detected skin line.
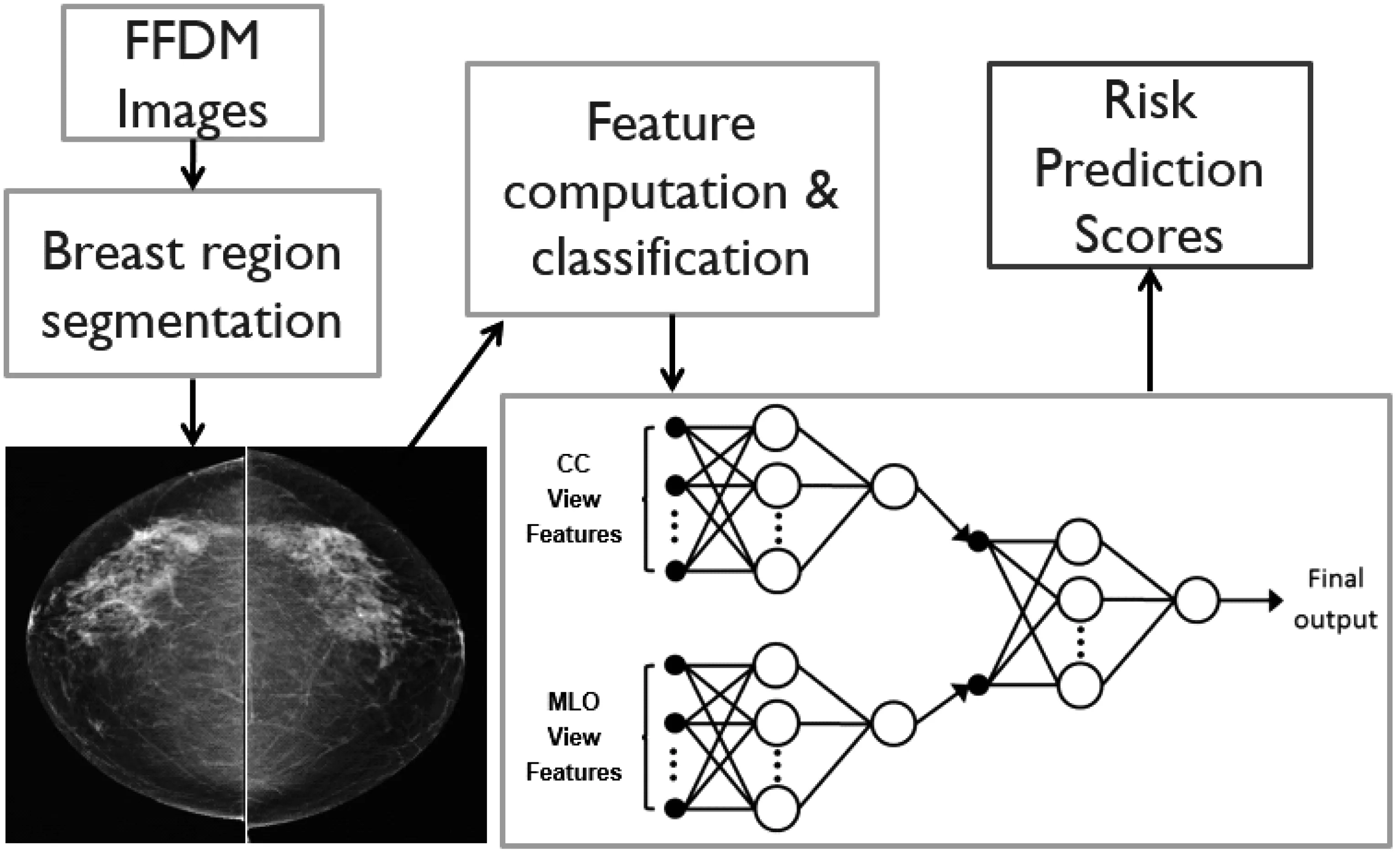
Fig.1 A flow diagram of our CAD-based quantitative image feature analysis scheme for assessing near-term breast cancer risk
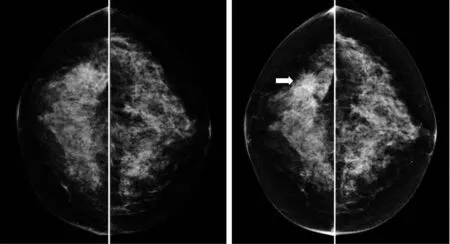
Fig.2 An example of two sets of bilateral mammograms acquired from one woman during the “prior” screening(left) in which the images were interpreted as negative and the “current” screening (one-year later) in which cancer was detected with a malignant mass as pointed by the arrow
Second,after breast region segmentation,we applied CAD scheme to compute a number of mammographic image features and difference (asymmetry) of the features between the left and right mammograms.In our studies,we computed density (or pixel value distribution) related feature asymmetry and variety of texture asymmetry based features.Figure 2 shows an example of two bilateral pairs of cranio-caudal (CC) view mammograms acquired from one woman in two sequential screenings.Our near-term breast cancer model is applied to the “prior” negative screening mammograms to detect and analyze the mammographic density or tissue asymmetric patterns.Figure 3 shows bilateral asymmetric patterns of the computed mammographic density and the initial suspicious lesion regions detected by CAD scheme on the left and right “prior” mammograms as shown in Figure 2.Applying to this pair of images,CAD computed a set of features including the difference of (1) mean pixel values,(2) fibro-glandular tissue (FGT) ratios,(3) compact ratios of FGT regions,(4) center shift of FGT regions,as well as the number and areas of initial suspicious regions.Figure 4 shows two examples of the computed texture patterns.From the CAD-generated texture maps,we computed and assessed a number of structural similarity features between two bilateral mammograms (i.e.,Complex Wavelet SSIM (CW-SSIM) index metric).The details have been reported in our recent publication[30].
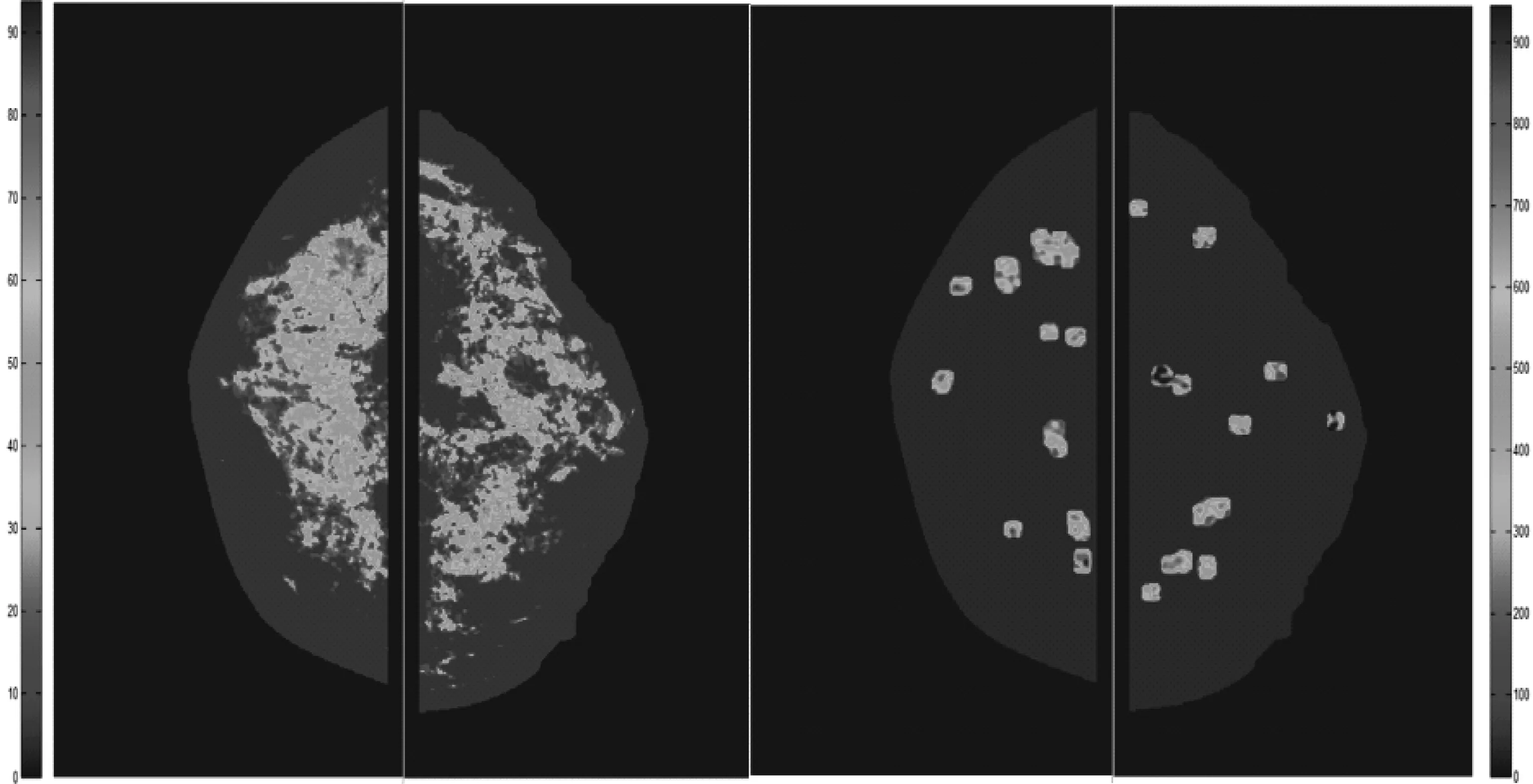
Fig.3 Illustration of computed asymmetry patterns of mammographic density (left) and CAD-detected initial suspicious lesion regions (right) between two bilateral CC view mammograms
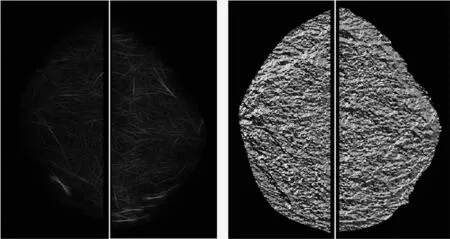
Fig.4 Illustration of computed texture patterns of bilateral mammograms including the amplitude of a Gabor filter (left) and phase of Weber Local Descriptor(right)
Third,after computing and normalizing all computed image features,we apply a machine learning method (i.e.,a best-first heuristic feature search and selection method[31]) to analyze and select the highly performed and also low redundant image features from the initial feature pool.Using the selected image features,we then train a two-layer artificial neural network (ANN) as shown in Figure 1.Specifically,the image features computed from the CC and mediolateral oblique (MLO)view of left and right mammograms are independently trained.Since the mammograms are two-dimensional projection images,the tissue density patterns in two projection (CC and MLO) views may not be the same or highly correlated.Thus,fusion of two ANN-generated risk prediction scores based on image features computed independently from the CC and MLO view images has potential to further improve the prediction performance.
After developing this quantitative mammographic image feature based near-term breast cancer risk prediction model,we have tested its performance using several image datasets.Two evaluation indices,the areas under the receiver operating characteristic (ROC) curves (AUC) and odds ratios (OR),were used to assess performance.To compute AUC as a summary measure of discriminative power,the feature values computed from all cases in each of the compared groups (e.g.,cancer-detected versus screening negative) were used to fit ROC performance curves based on maximum likelihood statistical data analysis (ROCKIT,http://www-radiology.uchicago.edu/krl/).To assess OR as a summary index of calibration performance,we sorted the model-generated risk prediction scores in ascending order and selected five threshold values to segment all the cases into five subgroups/bins with an equal number of cases within each subgroup.We then used a publically-available statistical computing software package (R version 2.1.1,http://www.r-project.org) to compute and analyze the adjusted ORs in all subgroups including an OR increasing risk trend.
2 Results
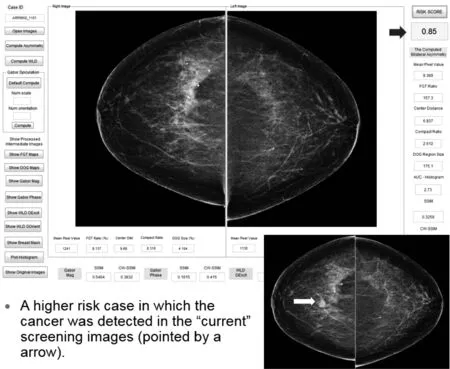
Fig.5 Illustration of our risk model working platform and prediction result applying to a high risk cases
Figures 5 and 6 demonstrate the working platform of our current interactive image feature analysis based near-term breast cancer risk assessment model and two examples of applying the model to compute risk prediction scores of these two women who are likely to have image-detectable cancer in the next sequential (“current”) screening mammography examinations.Figure 5 shows that due to the higher bilateral mammographic density or tissue asymmetry,the computed risk score is 0.85,which is much higher that the risk score of 0.38 when applying the model to another case shown in Figure 6,although mammographic density of case shown in Figure 6 is substantially higher than the case shown in Figure 5.These examples support our hypothesis that it is the bilateral mammographic density or tissue asymmetry plays important role in predicting near-term breast cancer risk.
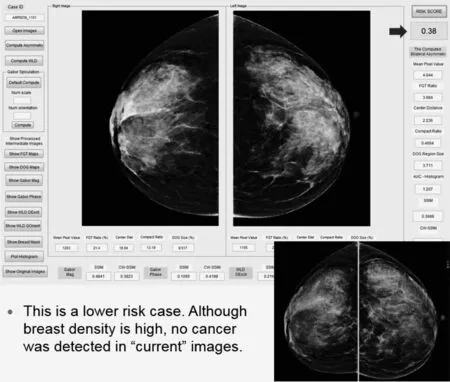
Fig.6 Illustration of our risk model working platform and prediction result applying to a lower risk cases
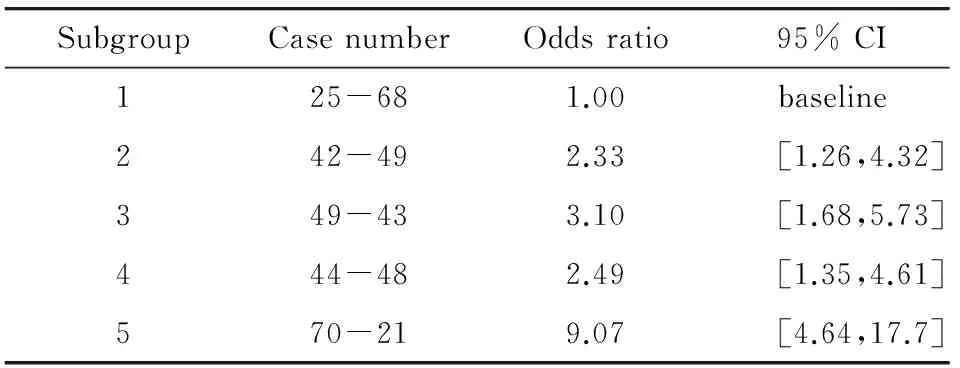
SubgroupCasenumberOddsratio95%CI125-681.00baseline242-492.33[1.26,4.32]349-433.10[1.68,5.73]444-482.49[1.35,4.61]570-219.07[4.64,17.7]
Table 1 summarizes the computed odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) computed for 5 subgroups (bins) of an image dataset including 460 sets of “prior” negative screening images.Among them,230 were positive (cancer-detected) in the “current” screening,while other 230 remained negative (cancer-free).The result showed a significantly increasing trend between the model-predicated risk scores and the detection results in the “current” screening with a p-value of 0.037[28].When applying 3 other common risk factors (namely women’s age,breast density as subjectively rated by radiologists,and reported family history of breast cancer) to predict the near-term cancer risk in this testing dataset,the computed ORs are substantially lower (ORs ≤ 2.74) and the regression based trend analysis also showed that the slopes of adjusted OR values versus increase of risk factor values were not statistically significantly different from a zero slope indicating that these risk factors have little discriminatory power in predicting near-term risk of women developing an image-detectable breast abnormality or cancer during the next subsequent examination as compared to the “negative” cases.
The similar results were confirmed in another study in which we increased the size of our dataset to 870 cases[29].The ORs monochromatically increased from 1.00 to 11.77 with a 95% CI of [7.07,19.59] and p-value smaller than 0.01.The computed AUC value in this dataset was 0.725±0.026.Applying a middle point of 0.5 as an operation threshold,we yielded an overall prediction accuracy of 65.4% (569/870) with a positive and a negative predictive values of 72.6% and 61.9%,respectively.
In another unique application,we applied our near-term risk model to assess the risk of women harboring the mammography-occult breast lesions (or cancer) in the “negative” screening mammography.In a preliminary study[32],the model was applied to an image dataset of 30 women who had both digit mammography and breast MRI screening examinations.Each woman took screening mammography and immediately followed by breast MRI.All mammograms were interpreted as negative.In diagnosis of breast MRI,5 cancers were detected and later verified by biopsies,while 25 women remained negative.Table 2 presents a computed confusion matrix,which summarizes the overall accuracy of case stratification into the high and low risk groups.
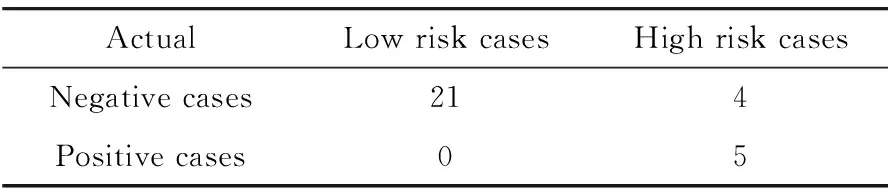
Tab.2 A confusion matrix obtained when applying our risk prediction model to classify 30 negative screening mammography cases into high and low risk groups of harboring mammography-occurred breast cancers
The result shows that our risk prediction model yielded an overall prediction accuracy of 86.7% in which 26 of 30 cases were correctly classified.All 5 positive cases were classified into the high risk group (with 100% sensitivity),while 4 negative cases were also classified into high risk group resulting in a false-positive rate of 16% (4/25).The maximum cancer detection yield of breast MRI was 55.6% (5/9) after using the case selection based on the prediction scores of our risk model,which substantially increased from the original yield of 16.7% (5/30) in this dataset.Using our risk model also showed potential to eliminate 84% (21/25) of unnecessary (negative) breast MRI screening examinations.The result suggested the potential of applying this new risk model to help significantly increase cancer detection yield using breast MRI as an alternative breast cancer screening modality[33].
3 Discussion
Developing precision medicine[34]is an ultimate goal in modern medicine,which will bring great benefit to the individual people and whole society by more accurately diagnose and treat patients,which can avoid or minimize the over-diagnosis and over-treatment,and thus increases efficacy of healthcare and reduces the cost.Recently,a new field of Radiomics has also been emerged in biomedical research field[35].Studies conducted by different research groups (including our own) have demonstrated the potentially high association between the quantitative image features and genomic biomarkers[36-37].Hence,quantitative medical image feature analysis can play an important role in developing precision medicine.In the cancer screening field,the goal is to develop an optimal and more effective personalized screening paradigm to replace current uniform and population-based screening methods[14-15].In this review article,we presented a new method and a unique quantitative image feature analysis based risk model to help identify which women have higher risk of harboring early breast cancer or developing image-detectable cancer in the near-term after a negative mammography screening.From our studies,we observed a number of valuable characteristics of applying this new model in future breast cancer screening practice.
First,although breast density is one of the important breast cancer risk factors with the second highest discriminatory power behind the age in many epidemiology study based breast cancer risk models[2],it has little discriminatory power to predict near-term cancer risk as we reported in our studies[26].However,bilateral mammographic density asymmetry does not correlate with the breast or mammographic density.It changes as the reduction of time delay between the negative and positive screening examinations.The trend of increasing bilateral mammographic density asymmetry as the decreasing time delay to the positive detection has also been detected and reported in our recent study[30].In addition,accurately assessing breast density from mammograms is difficult or unreliable without knowing the exact X-ray exposure values and the compressed breast thickness during imaging[38].On the other hand,computing bilateral mammographic density asymmetry may be more robust in this regard as many of the uncontrollable imaging factors should similarly affect both breasts.Therefore,the image features that are based on the subtracted values of two bilateral mammograms are more likely to be valid for assessing near-term breast cancer risk.
Second,the risk assessment performance of using our image model does not depend on the breast or mammographic density.Figure 7 shows an example of 4 ROC curves that were generated using 4 groups of cases that were subjectively rated by the radiologists based on the Breast Imaging Reporting and Data System (BIRADS).The AUC values are 0.734±0.079,0.725±0.029,0.715±0.023,0.712±0.090 for BIRADS 1~4 case groups,respectively[29].Since sensitivity of detecting breast lesions that may lead to cancer development heavily depends on mammographic density[9],this observation is encouraging.It suggests that using our new risk factor based on bilateral asymmetry of mammographic density can help detect more early or “mammography-occult” cancers among the women of younger age and having dense breasts.In addition,we did not observe the significant difference of risk prediction performance among the cases subgroups with different types of malignant breast lesions (i.e.,masses,micro-calcification clusters,or both).
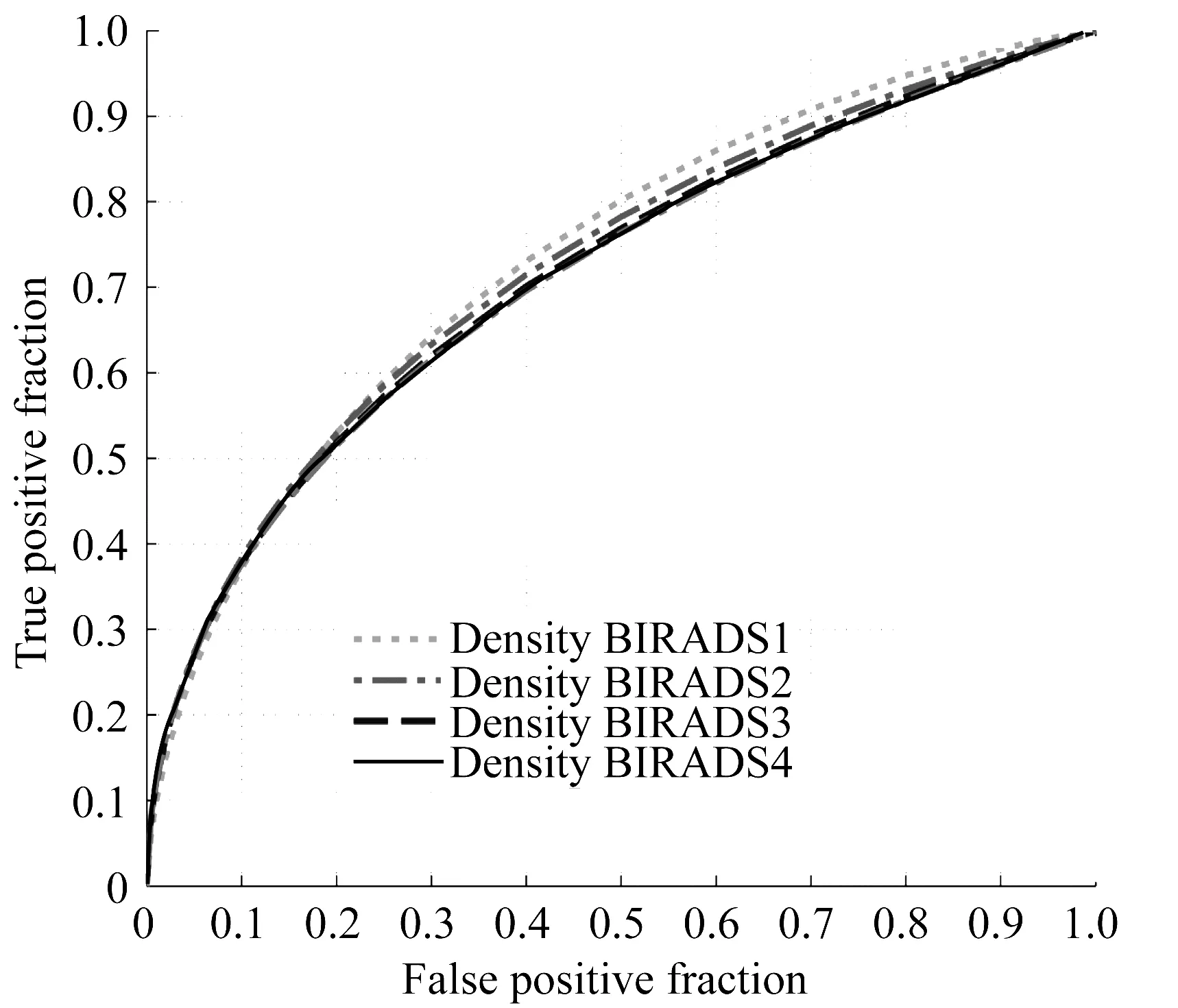
Fig.7 An example showing that performance of the near-term breast cancer risk prediction does not depend on breast density as rated by BIRADS
In summary,our studies demonstrated the potentially added clinical value of developing new quantitative image feature analysis approaches and schemes (or models) in helping establish a new personalized cancer screening paradigm.Although researchers have been working on identifying and detecting more effective biomarkers aiming to assess individualized cancer risk from biological and/or molecular tests,as supported by Radiomics concept,our study also demonstrated that developing quantitative image markers and models could provide valuable and/or supplementary information to the genomic biomarkers,whichg is a more cost-effective approach without introducing new invasive and/or expensive tests on the women who participated in breast cancer screening.This is another major advantage of developing and applying new image marker or image feature analysis based breast cancer risk prediction models in future clinical practice.
Despite the encouraging study results,many issues of developing optimal and robust image feature analysis based cancer risk prediction models remain uninvestigated and/or unsolved to date.For example,our current risk model only computes and incorporates the global bilateral mammographic density or tissue asymmetry features.The local or regional bilateral asymmetric mammographic image features may also provide very useful information as suggested by a recent study[39].How to compute the local and regional image features and then optimally integrate them into the risk assessment models need to be investigated in the future studies.In addition,previous studies are mainly single institution based retrospective studies with significantly higher cancer prevalence rates than that in the real cancer screening environment.Hence,the actual performance or robustness,as well as the clinical potential of applying this new type of image feature analysis based risk assessment models need to be further tested and validated in the future prospective or cohort studies.
[1]SIEGEL R,NAISHADHAM D,JEMAL A.Cancer statistics,2013[J].CA:A Cancer Journal for Clinicians,2013,63(1):11-30.
[2]AMIR E,FREEDMAN O C,SERUGA B,et al.Assessing women at high risk of breast cancer:a review of risk assessment models[J].Journal of the National Cancer Institute,2010,102(10):680-691.
[3]SMITH R A,COKKINDES V,BROOKS D,et al.Cancer screening in the United States,2011[J].CA:A Cancer Journal for Clinicians,2011,61(1):8-30.
[4]FENTON J J,EGGER J,CARNEY P A,et al.Reality check:perceived versus actual performance of community mammographers[J].American Journal of Roentgenology,2006,187(1):42-46.
[5]PEER P G M,VERBEEK A LM,STRAATMAN H,et al.Age-specific sensitivities of mammographic screening for breast cancer[J].Breast Cancer Research and Treatment,1996,38(2):153-160.
[6]MANDELSON M,OESTREICHER N,PORTER P L,et al.Breast density as a predictor of mammographic detection:Comparison of interval- and screen-detected cancers[J].Journal of the National Cancer Institute,2000,92(13):1081-1087.
[7]LAYA M B,LARSON E B,TAPLIN S H,et al.Effect of estrogen replacement therapy on the specificity and sensitivity of screening mammography[J].Journal of the National Cancer Institute,1996,188(10):643-649.
[8]KRIEGE M,BREKELMANS C T,BOETES C,et al.Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition[J].New England Journal of Medicine,2004,351(5):427-437.
[9]CARNEY P A,MIGLIORETTID L,YANKASKAS B C,et al.Individual and combined effects of age,breast density,and hormone replacement therapy use on the accuracy of screening mammography[J].Annals of Internal Medicine,2003,138(3):168-175.
[10]HUBBARD R A,KERLIKOWSKE K,FLOWERS C I,et al.Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography:A cohort study[J].Annals of Internal Medicine,2011,155(8):481-492.
[11]BRODERSEN J,SIERSMA V D.Long-term psychosocial consequences of false-positive screening mammography[J].The Annals of Family Medicine,2013,11(2):106-115.
[12]BUIST D S M,ANDERSON M L,HANEUSE S J P A,et al.Influence of annual interpretive volume on screening mammography performance in the United States[J].Radiology,2011,259(1):72-84.
[13]BERLIN L,HALL F M.More mammography muddle:emotions,politics,science,costs and polarization[J].Radiology,2010,255(2):311-316.
[14]GAIL M H.Personalized estimates of breast cancer risk in clinical practice and public health[J].Statistics Medicine,2011,30(10):1090-1104.
[15]BRAWLEY O W.Risk-based mammography screening:an effort to maximize the benefits and minimize the harms[J].Annals of Internal Medicine,2012,156(9):662-663.
[16]SCHOUSBOE J T,KERLIKOWSKE K,LOH A,et al.Personalizing mammography by breast density and other risk factors for breast cancer:analysis of health benefits and cost-effectiveness[J].Annals of Internal Medicine,2011,155(1):10-20.
[17]NELSON H D,ZAKHER B,CANTOR A,et al.Risk factors for breast cancer for women aged 40 to 49 years:a systematic review and meta-analysis[J].Annals of Internal Medicine,2012,156(9):635-648.
[18]PANKRATZ V S,DEGNIM A C,FRANK R D,et al.Model for individualized prediction of breast cancer risk after a benign breast biopsy[J].Journal of Clinical Oncology,2015,33(8):923-929.
[19]GAIL M H,MAI P L.Comparing breast cancer risk assessment models[J].Journal of the National Cancer Institute,2010,102(10):665-668.
[20]SCUTT D,LANCASTER G A,MANNING J T.Breast asymmetry and predisposition to breast cancer[J].Breast Cancer Research,2006,8:R14,doi:10.1186/bcr1388.
[21]BLANKS R G,WALLIS M G,GIVEN-WILSON R M.Observer variability in cancer detection during routine repeat (incident) mammographic screening in a study of two versus one view mammography[J].Journal of Medical Screening,1999,6(3):152-158.
[22]SUMKIN J H,HOLBERT B L,HERMANN J S,et al.Optimal reference mammography:a comparison of mammograms obtained 1 and 2 years before the present examination[J].American Journal of Roentgenology,2003,180(2):343-346.
[23]WEI J,CHANG H P,WU Y T,et al.Association of computerized mammographic parenchymal pattern measure with breast cancer risk:a pilot case-control study[J].Radiology,2011,260(1):42-49.
[24]DOMCHEK S M,ANTONIOU A.Cancer risk models:translating family history into clinical management[J].Annals of Internal Medicine,2007,147(7):515-517.
[25]WANG X W,LEDERMAN D,TAN J,et al.Computerized prediction of risk for developing breast cancer based on bilateral mammographic breast tissue asymmetry[J].Medical Engineering & Physics,2011,33(8):934-942.
[26]ZHENG B,SUMKIN J H,ZULEY M L,et al.Bilateral mammographic density asymmetry and breast cancer risk:a preliminary assessment[J].European Journal of Radiology,2012,81(11):3222-3228.
[27]TAN M,ZHENG B,RAMALINGAM P,et al.Prediction of near-term breast cancer risk based on bilateral mammographic feature asymmetry[J].Academic Radiology,2013,20(12):1542-1550.
[28]ZHENG B,TAN B,RAMALINGAM P,et al.Association between computed tissue density asymmetry in bilateral mammograms and near-term breast cancer risk[J].The Breast Journal,2014,20(3):249-257.
[29]TAN M,PU J T,CHENG S,et al.Assessment of a four-view mammographic image feature based fusion model to predict near-term breast cancer risk[J].Annals of Biomedical Engineering,2015,43(10):2416-2428.
[30]TAN M,ZHENG B,LEADER J K,et al.Association between changes in mammographic image features and risk for near-term breast cancer development[J].IEEE Transactions on Medical Imaging,2016(99):1,doi:10.1109/TMI.2016.2527619.
[31]BURNS E,LEMONS S,RUML W,et al.Best-first heuristic search for multicore machines[J].Journal of Artificial Intelligence Research,2010,39(1):689-743.
[32]TAN M,AGHAEI F,HOLLINGSWORTH A B,et al.Increasing cancer detection yield of breast MRI using a new CAD scheme of mammograms[C]//Proceedings of SPIE Medical Imaging 2016:Computer-Aided Diagnosis.San Diego,California:SPIE,2016,doi:10.1117/12.2208246.
[33]HOLLINGSWORTH A B,STOUGH R G.An alternative approach to selecting patients for high-risk screening with breast MRI[J].The Breast Journal,2014,20(2):192-197.
[34]COLLINS F,VARMUS H.A new initiative on precision medicine[J].New England Journal of Medicine,2015,372(9):793-795.
[35]LAMBIN P,RIOS-VELAZQUEZ E,LEIJENAAR R,et al.Radiomics:extracting more information from medical images using advanced feature analysis[J].European Journal of Cancer,2012,48(4):441-446.
[36]AERTS H J W L,VELAZQUEZ E R,LEIJENAAR R T H,et al.Decoding tumour phenotype by noninvasive imaging using a quantitative Radiomics approach[J].Nature Communications,2014,5:4006.
[37]EMAMINEJAD N,QIAN W,GUAN Y B,et al.Fusion of quantitative image and genomic biomarkers to improve prognosis assessment of early stage lung cancer patients[J].IEEE Transactions on Biomedical Engineering,2016,63(5):1034-1043.
[38]KOPANS D B.Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk[J].Radiology,2008,246(2):348-353.
[39]CASTI P,MENCATTINI A,SALMERI M,et al.Analysis of structural similarity in mammograms for detection of bilateral asymmetry[J].IEEE Trans Med Imaging,2015,34:662-671.
(编辑:刘铁英)
Applying quantitative medical image processing and feature analysis to assess near-term breast cancer risk
ZHENG Bin
(School of Electrical and Computer Engineering,University of Oklahoma,Norman,Oklahoma 73019,USA)
Medical image processing and quantitative feature analysis play an important role in accurate detection of early breast cancer.In order to apply a new image feature analysis method based on the detection of the bilateral mammographic density and tissue asymmetry to effectively predict the near-term risk of the individual women developing breast cancer,our research group recently developed and tested a new multiple image feature based model to predict the risk or likelihood of a woman having or developing an image detectable early breast cancer in the near-term (i.e.,within the 2 years after a negative mammography screening).In this review paper,we introduce the basic concept of this new image feature based risk prediction model,and demonstrate a graphic user interface (GUI) platform of interactively applying this new risk prediction model to process negative screening mammograms.Our preliminary testing results using several image datasets have demonstrated that applying this new risk prediction model could yield significantly higher discriminatory power in predicting near-term breast cancer risk than using other existing breast cancer risk factors and prediction models.If successful,applying our new model has potential to help establish a new and more effective personalized breast cancer screening paradigm in the future.
medical image processing; medical image markers; quantitative mammographic image feature analysis; near-term breast cancer risk prediction model; computer-aided detection (CAD)
2016-03-10
美国国家健康科学院National Institutes of Health Mammographic Density and Tissue Asymmetry based Breast Cancer Risk Stratification(R01 CA160205)
郑斌(1959—),男,美国科学院医学与生物工程院院士,研究员,主要从事光学仪器设计,医学图像特征的定量计算和分析等方面的研究。E-mail:bin.zheng-1@ou.edu
1005-5630( 2016) 03-0189-11
TP751
A
10.3969/j.issn.1005-5630.2016.03.001

