动物繁殖相关lncRNA的研究新进展
贺小云,狄 冉,胡文萍,王翔宇,刘秋月,储明星
(中国农业科学院北京畜牧兽医研究所,农业部畜禽遗传资源与种质创新重点实验室,北京 100193)
动物繁殖相关lncRNA的研究新进展
贺小云,狄冉,胡文萍,王翔宇,刘秋月*,储明星*
(中国农业科学院北京畜牧兽医研究所,农业部畜禽遗传资源与种质创新重点实验室,北京 100193)
长链非编码RNA(Long noncoding RNA,lncRNA)是一类长度大于200 nt,且不具有蛋白编码潜能的RNAs,在真核生物基因表达调控中发挥着重要作用。不同物种性染色体和部分印记基因的转录能力受到lncRNA的顺式调控作用。寻找与繁殖相关的lncRNA分子,研究这些lncRNA分子及其靶基因的生物学功能,阐明lncRNA在动物繁殖中的调控机制,是动物繁殖研究的一个热点。本文主要对繁殖过程中配子发生、胎盘形成和妊娠建立、激素调节、早期胚胎发育以及性别决定和性腺发育等重要阶段lncRNA 的研究进行综述,为深入探究动物繁殖调控机制提供参考。
lncRNA;繁殖;配子发生;激素调节;妊娠
随着高通量测序技术的发展运用和大量基因组学研究的不断深入,传统遗传学所认为的基因表达调控模式已不能完全的解释生物的功能运转。自2000年人类基因组测序顺利完成以后,科学家们发现在人体中存在大量的非编码RNA(Non-coding RNA,ncRNA),这些ncRNA约占基因组全部转录产物的98%,虽然被转录,却不具有蛋白翻译功能[1]。
lncRNA是长度大于200 nt,且不具有蛋白编码功能的RNAs[2]。在生物体内,lncRNA的表达水平比正常的编码蛋白基因低,且功能多样。根据其在基因组上位置的不同,可以将lncRNA分为5类:双向(Divergent)lncRNA、正义链(Sense)lncRNA、反义链(Antisense)lncRNA、基因间(Intergenic)lncRNA、内含子间(Intronic)lncRNA[3]。部分lncRNA已被证实在基因表达调控等方面扮演着重要的角色,由于其来源和作用方式的不同,主要参与了生物体的X染色体失活、染色质重塑、组蛋白修饰、转录水平及转录后调控[4-11]等重要生命活动。对于其它动物及生物lncRNA的功能研究尚处于起步阶段,借助从人类医学上所取得的研究成果,科学家们通过高通量RNA-seq技术结合现代分子生物学等方法在模式动植物和畜禽中对lncRNA展开了全面的研究,本文主要对模式动物及畜禽中繁殖相关领域lncRNA的功能研究现状及方法进行综述,以期为深入研究与动物繁殖相关的lncRNA作用机制和功能,多层次、全面揭示动物生命活动提供参考。
1 lncRNA的作用机制
lncRNA作为基因表达调控的关键因子,通过不同的作用方式对基因表达进行调节[12-13](图1)。例如,lncRNA可以作为分子骨架抑制(如HOTAIR、PRC2)或激活(如Trithorax group)染色质修饰,从而抑制或激活目标基因表达[14-16];lncRNA在转录后的调控作用也被广为关注,主要集中在mRNA剪接[17]、翻译[18]和降解[19-20]。此外,还有部分lncRNA可以抑制miRNA的功能,间接地促进被miRNA下调的目标基因的表达,发挥促表达作用等[21-24],lncRNA作用机制的多样性使其在生物体不同生命进程中发挥着重要作用。

图1 lncRNA的作用机制[12]Fig 1 Mechanisms of lncRNA function[12]
2 动物繁殖领域lncRNA的研究现状
对lncRNA的发现、数目、功能和研究方法,科学家已经进行了详述[25-29],lncRNA对干细胞的维持和分化等发育过程[30-32]的调控以及性染色质的剂量补偿机制[33-35]也有了相应的总结。在人类疾病和模式动物繁殖现象的研究中,lncRNA在动物早期生殖细胞形成、早期胚胎的着床和发育以及有关激素调节中扮演着必不可少的角色(表1),深入了解各个阶段lncRNA的功能和作用能为人们更加准确的阐述繁殖机理提供帮助。
2.1卵子发生过程中的lncRNAs及部分功能
在哺乳动物中,处于发育状态的卵母细胞通过细胞间隙与其周围的卵丘细胞进行物质运输和信息传递。利用RNA-Seq技术,通过对人卵丘细胞、卵丘卵母细胞复合体的lncRNAs进行检测,发现大量差异表达的lncRNAs,其中有45个反义lncRNAs,44个基因间lncRNAs,虽然对这些lncRNA的功能还没有进行研究,但反义lncRNA的存在暗示其对不同时期卵丘卵母细胞复合体的发育具有调控作用[58-60]。A.D.Macaulay等[61]利用共聚焦透射电子显微镜和RNA-Seq发现牛卵母细胞周围的卵丘细胞为成年母牛的卵母细胞运输大量的营养和物质,包括mRNA和lncRNA。在果蝇中,E.Nicolas等[62]研究发现卵巢营养细胞将RNA和细胞质的其它物质转移到卵母细胞中。在小鼠中,科学家发现了和果蝇中同样的现象,并指出这些外来体参与了lncRNA的转录调控等过程[63-65],由于卵母细胞及其周围细胞细胞质间的信息传递和物质运输方式有限,暗示这些差异表达的lncRNA可能对卵子的发生具有重要作用。
表1lncRNAs在繁殖中的功能
Table 1LncRNAs with identified functions in reproduction
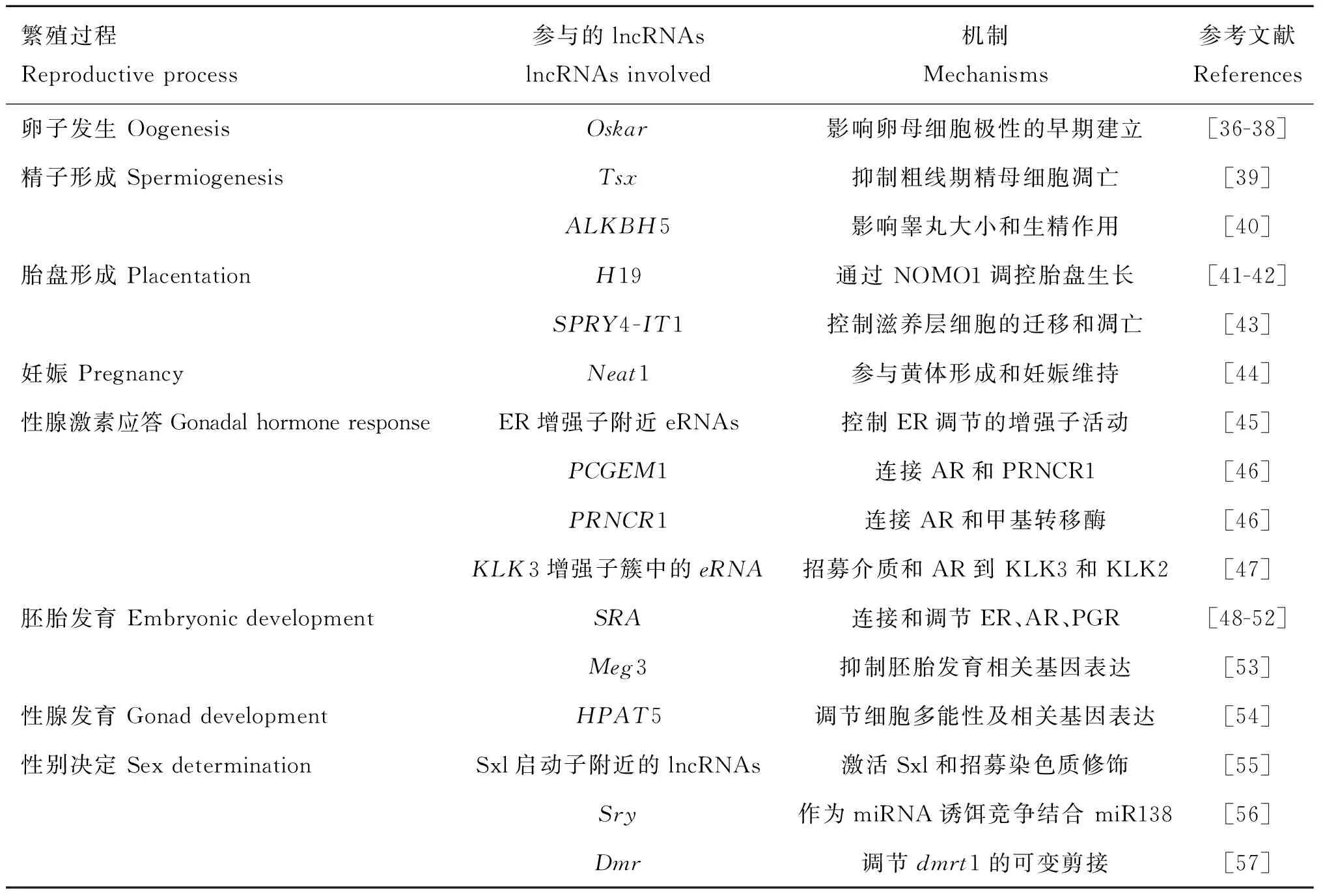
繁殖过程Reproductiveprocess参与的lncRNAslncRNAsinvolved机制Mechanisms参考文献References卵子发生OogenesisOskar影响卵母细胞极性的早期建立[36-38]精子形成SpermiogenesisTsx抑制粗线期精母细胞凋亡[39]ALKBH5影响睾丸大小和生精作用[40]胎盘形成PlacentationH19通过NOMO1调控胎盘生长[41-42]SPRY4-IT1控制滋养层细胞的迁移和凋亡[43]妊娠PregnancyNeat1参与黄体形成和妊娠维持[44]性腺激素应答GonadalhormoneresponseER增强子附近eRNAs控制ER调节的增强子活动[45]PCGEM1连接AR和PRNCR1[46]PRNCR1连接AR和甲基转移酶[46]KLK3增强子簇中的eRNA招募介质和AR到KLK3和KLK2[47]胚胎发育EmbryonicdevelopmentSRA连接和调节ER、AR、PGR[48-52]Meg3抑制胚胎发育相关基因表达[53]性腺发育GonaddevelopmentHPAT5调节细胞多能性及相关基因表达[54]性别决定SexdeterminationSxl启动子附近的lncRNAs激活Sxl和招募染色质修饰[55]Sry作为miRNA诱饵竞争结合miR138[56]Dmr调节dmrt1的可变剪接[57]
L.Yan等[66]利用单细胞测序技术在人着床前的胚胎和四分体时期(Metaphase-II)的卵母细胞中筛选出8 700个母系lncRNAs,其中差异表达的lncRNAs有660个。在小鼠着床前的胚胎中也发现了大量lncRNAs,这些lncRNAs可能对卵子到早期胚胎这一转化过程中某些基因的活化有调控作用,同时研究者在早期胚胎中还发现了至少1 000对lncRNA/mRNA对,其中发现一部分启动子相关ncRNAs(Promoter-associated noncoding RNAs,pancRNAs)[67-68]。J.B.Brown等[69]在对果蝇的卵巢研究中也发现了大量与启动子相关的反义lncRNAs,这些lncRNAs可能调控其同源基因的转录激活,对早期胚胎着床前发育有重大意义。目前,已经证实部分lncRNAs在卵子发生中发挥重要功能。例如,oskarmRNA就具有编码和不编码蛋白质的双重功能,oskarmRNA翻译后,其3′调控区介导发挥lncRNA功能,招募各种调控因子影响卵子形成后期极性的建立[36-38]。哺乳动物中,没有发现与果蝇oskar相似的lncRNA,可能是由于其结构不适合哺乳动物,但并不排除在哺乳动物中存在相似的lncRNA在卵子发生过程中发挥重要作用。
2.2lncRNA对精子生成至关重要
lncRNA以一种动态表达的模式影响精子的发生:精原细胞转录水平大大提高使细胞进入减数分裂,随着精母细胞增加,产生精子细胞,在精子细胞向精子转化的过程中,细胞内大量的RNA被消耗[70-73]。科学家在对精子形成不同阶段进行分析时发现,这些被消耗的RNA可能部分以lncRNA 的形式在精子发生中发挥调节作用。
2013年,M.Soumillon等[74]发现lncRNA在初生小鼠与成年小鼠睾丸精原细胞中的数量基本一致,在减数分裂过程中,精母细胞中大量lncRNA转录。例如,在粗线期,睾丸特异性lncRNATsx高表达,Tsx敲除后小鼠睾丸体积减小,并且在该时期精母细胞的凋亡增加,但并不影响繁殖后代[39]。除此之外,敲除ALKBH5也会使小鼠的睾丸体积减小,影响精子产生[40]。最近关于成熟精子核内及其周围细胞质转录组分析发现,精子的RNA大部分位于细胞质而不是细胞核(大约34%)中,而MALAT1在成熟精子的细胞核中显著高表达,暗示它可能是调节雄性生殖细胞核染色质组装的主要lncRNA之一。特定时期精子内的lncRNA还不能够确定是否对配子产生或受精后的胚胎的功能有调节作用,需要进一步探索。
2.3lncRNA促进动物胎盘形成和妊娠建立
胎盘是母体妊娠建立后胎儿生长发育的主要场所,是动物繁殖的关键器官。虽然与之相关的lncRNA研究数据较少,但部分lncRNA已经进行了准确的定位和功能研究,并且取得了显著地成果。例如,H19基因,转录产物为 2.3 kb的非编码RNA分子,可以作为miR675的前体,而miR675可以直接负调控节点调制器1(Nodal modulator,NOMO1),抑制滋养层细胞增殖[41-42]。正常的胎盘中H19和miR675抑制NOMO1介导的滋养层细胞增殖,而在惊厥前期的孕妇胎盘中,H19和miR675表达受到抑制,导致滋养层细胞大量增殖,胎盘过度生长,妊娠受阻。快速生长发育因子同源蛋白4的内含子转录本1,SPRY4-IT1(Sprouty homolog 4,intronic transcript 1,)是胎盘中表达的另一个lncRNA,研究发现其在惊厥前期的孕妇胎盘过量表达,siRNA干扰后,其表达恢复正常[43]。黄体的形成是雌性动物妊娠的重要标志,N.Shinichi等[44]研究胚胎着床前发育时发现,Neat1基因在黄体组织中高表达,Neat1基因敲除的雌性小鼠黄体功能发生障碍,体内孕酮功能低于正常妊娠水平,导致虽然能正常排卵的雌性小鼠不能妊娠,这暗示Neat1基因对于黄体形成至关重要,是雌性动物妊娠建立和维持必不可少的一部分。
2.4lncRNA介导性腺激素发挥功能
目前,主要采用雌激素受体(ER)、孕酮受体(PGR)、雄激素受体(AR)等核受体研究lncRNA对性腺类固醇激素的调节。lncRNA主要是通过G蛋白偶联受体和胞浆信号转导的级联效应发挥作用,而作为信号分子刺激促性腺激素(LH和FSH)还没有相关报道。
关于ER和AR应答的lncRNA功能研究主要集中在增强子RNAs(eRNA)和其它lncRNAs。W.Li等[45]发现ER连接的增强子转录产生的eRNAs招募转录激活物致使ER基因附近效应基因的表达。同样AR与PCGEM1(前列腺特异转录本)和PRNCR1(前列腺癌相关的lncRNA)也有着同样的作用模式,敲除PCGEM1和PRNCR1使得很多AR的靶基因转录水平降低,与此同时启动子与增强子间的相互作用也有所减弱[46]。2014年,C.L.Hsieh等[47]在前人的基础上发现,在前列腺特异抗原3(Kallikrein-related peptidase 3,KLK3)启动子区上游4 kb有一簇增强子,AR以一种激素依赖方式连接在增强子上发挥作用,并且这些增强子产生的eRNA可以增加KLK3及其下游KLK2的转录活性。这些研究暗示ER和AR的其它靶基因可能也受到lncRNAs的调节。
类固醇受体RNA激活剂(Steroid receptor RNA activator,SRA)是研究人类PGR时最早发现的lncRNA[48],后来研究证明SRA直接作用于激素受体(ER、AR、PGR)调节其活性[49-50],同时它还可以剪接后翻译产生SRA蛋白(SRAP),增强类固醇激素对基因表达的调控作用[51]。为了深入了解SRA的生理机制,G.P.Vicent等[52]在乳腺癌细胞中发现,PGR和其它核染色质修饰因子(如CBX5/HP1、KDM1A/LSD1和HDAC、RCOR1/CoREST)组成的抑制组蛋白修饰复合物与SRAP协作致使部分激素诱导基因沉默。尽管SRAP可以增强各种类固醇激素受体的活性,但是对于SRA自身转录调控和剪切翻译产生SRAP的机制还不清楚,需要进一步研究。
2.5lncRNA参与动物早期胚胎发育
科学家们通过RNA-Seq对人、小鼠、牛、猪等受精卵与受精前的配子、不同发育阶段的早期胚胎间的比较,发现大量与不同胚胎发育时期相关的lncRNAs[66-67,75-78]。关于早期胚胎发育的研究主要集中在胚胎干细胞,体外培养的小鼠胚胎干细胞至少表达226个 lncRNAs,其中137个lncRNAs已经被证明能够影响基因表达,26个lncRNAs能够抑制胚胎干细胞分化和维持其多能性[79]。例如,Meg3与JARID2基因互作从而招募PRC2以反式作用抑制胚胎发育相关基因表达[53]。同时,灵长类特异的ncRNAHPAT5可以调节细胞多能性及某些相关基因的表达,在人类胚胎着床前发育和细胞核重编程中起重要作用[54]。
2.6lncRNA性别决定和性腺发育
多方面研究显示,lncRNA对于性别决定和性腺发生有着重要作用,性染色质的数量和同源性是决定性别的典型遗传因素。例如,雌性果蝇的发育依赖于性别致死基因(Sex-lethal,Sxl)的早期表达,而Sxl的表达取决于X染色体与常染色体的比率。如果常染色体数大于X染色体,某些特定的蛋白质会结合到Sxl启动子区抑制其表达,而在该基因启动子区上游约1 000 bp存在的lncRNAs对Sxl表达具有激活作用[77]。
Y染色体编码基因Sry的表达对于雄性性别决定十分重要。小鼠体外试验发现,Sry基因的RNA以miRNA海绵的作用机制竞争结合miR138,从而对雄性小鼠的形成发挥正调控作用[78]。另外,在许多脊椎动物和非脊椎动物中,dmrt1已被证实对性别决定起着重要作用。如在小鼠中,dmrt1的转录本和lncRNAdmr参与反式剪接,产生一个新的转录本,该转录本编码一个羧基末端改变的新蛋白,在原始足细胞中过表达dmr,使得羧基端改变的新蛋白的数量增加,导致dmrt1靶基因的表达受损,表现出dmrt1功能丧失的表型[79]。虽然这些研究并不能完全揭示lncRNAs在性别决定中的作用,但lncRNA在性别决定中发挥着不可替代的功能。
3 小结与展望
虽然lncRNAs已经公认是调控生殖细胞命运和功能的重要介质,但与繁殖相关的lncRNAs大多基于描述和假设,并且主要是集中在特定生物的特定生殖现象中,对于大多数动物繁殖相关lncRNAs功能的深度解析尚未开始。想要准确定位和更多挖掘lncRNAs的功能,从3方面入手:(1)采用过表达和敲除等方法改变其表达量来观察表型的变化;(2)采用生物信息学分析、RNA纯化的染色质分离(Chromatin isolation by RNA purification,CHIRP)和RNA结合蛋白免疫沉淀技术(RNA immunoprecipitation,RIP)等科学的方法确定lncRNAs在体内的结构特点,阐明化学修饰等对其发挥功能的影响;(3)准确识别与lncRNAs相关的蛋白和其它作用因子,开展联合分析。通过多物种间及个体差异的研究,有助于揭示生物进化过程中影响繁殖和遗传变异的lncRNAs调节机制和生物功能。现阶段对于人类和模式动物繁殖相关lncRNAs的研究表明,研究其对家畜繁殖等重要经济性状的调控作用机理,深层次理解家畜繁殖机制,对改善家畜繁殖力提供重要的理论基础。
[1]BIRNEY E,STAMATOYANNOPOULOS J A,DUTTA A,et al.Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project[J].Nature,2007,447(7146):799-816.
[2]SPIZAO R,ALMEIDA M I,COLOMBATTI A,et al.Long non-coding RNAs and cancer:a new frontier of translational research[J].Oncogene,2012,31(43):4577-4587.
[3]ZHAO X Y,LIN J D.Long noncoding RNAs:A new regulatory code in metabolic control[J].TrendsBiochemSci,2015,40(10):586-596.
[4]HE S,LIU S,HAO Z.The sequence,structure and evolutionary features of HOTAIR in mammals[J].BMCEvolBiol,2011,11(1709):1-14.
[5]MATTICK J S.The central role of RNA in human development and cognition[J].FEBSLett,2011,585(11):1600-1616.
[6]MCHUGH C A,CHEN K C,CHOW A,et al.The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3[J].Nature,2015,521(7551):232-236.
[7]NAGANO T,FRASER P.No-nonsense functions for long noncoding RNAs[J].Cell,2011,145(2):178-181.
[8]NGUYEN V T,KISS T,MICHELS A A,et al.7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes[J].Nature,2001,414(6861):322-325.
[9]PETERLIN B M,PRICE D H.Controlling the elongation phase of transcription with P-TEFb:molecular cell[J].MolCell,2006,23(3):297-305.
[10]YANG Z,ZHU Q,LUO K,et al.The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription[J].Nature,2001,414(6861):317-322.
[11]GUPTA R A,NILAY S,WANG K C,et al.Long noncoding RNA HOTAIR reprograms chromatin state to promote cancer metastasis[J].Nature,2010,464(7291):1071-1076.
[12]HU W,ALVAREZ-DOMINGUEZ J R,LODISH H F.Regulation of mammalian cell differentiation by long non-coding RNAs[J].EMBORep,2012,13(11):971-983.
[13]GUTTMAN M,RINN J L.Modular regulatory principles of large non-coding RNAs[J].Nature,2012,482(7385):339-346.
[14]LI L,DANG Q,XIE H,et al.Infiltrating mast cells enhance prostate cancer invasion via altering LncRNA-HOTAIR/PRC2-androgen receptor (AR)-MMP9 signals and increased stem/progenitor cell population[J].Oncotarget,2015,6(16):14179-14190.
[15]WONGTRAKOONGATE P,RIDDICK G,FUCHAROEN S,et al.Association of the long non-coding RNA steroid receptor RNA activator (SRA) with TrxG and PRC2 complexes[J].PLoSGenet,2015,11(10):e1005615.
[16]CHASE J,GREMBECKA J,MAILLARD I.Trithorax group genes in hematopoiesis[J].Oncotarget,2015,6(20):17855-17856.
[17]GONZALEZ I,MUNITA R,AGIRRE E,et al.A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature[J].NatStructMolBiol,2015,22(5):370-376.
[18]PANG W J,LIN L G,XIONG Y,et al.Knockdown of PU.1 AS lncRNA inhibits adipogenesis through enhancing PU.1 mRNA translation[J].JCellBiochem,2013,114(11):2500-2512.
[19]CHENGUANG G,MAQUAT L E.lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements[J].Nature,2011,470(7333):284-288.
[20]GIOVARELLI M,BUCCI G,RAMOS A,et al.H19 long noncoding RNA controls the mRNA decay promoting function of KSRP[J].ProcNatlAcadSciUSA,2014,111(47):5023-5028.
[21]JALALI S,BHARTIYA D,LALWANI M K,et al.Systematic transcriptome wide analysis of lncRNA-miRNA interactions[J].PLoSOne,2013,8:e53823.
[22]LI G,YANG Z,SHENG Y,et al.An integrated evolutionary analysis of miRNA-lncRNA in mammals[J].MolBiolRep,2014,41(1):201-207.
[23]KARRETH F A,TAY Y,PERNA D,et al.Invivoidentification of tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma[J].Cell,2011,147(4):382-395.
[24]MARCELLA C,DAVIDE C,IVANO L,et al.A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA[J].Cell,2011,147(2):358-369.
[25]JOHNSSON P,LIPOVICH L,GRANDER D,et al.Evolutionary conservation of long non-coding RNAs:sequence,structure,function[J].BBA-GenSubjects,2014,1840(3):1063-1071.
[26]WANG K C,CHANG H Y.Molecular mechanisms of long noncoding RNAs[J].MolCell,2011,43(6):904-914.
[27]FATICA A,BOZZONI I.Long non-coding RNAs:new players in cell differentiation and development[J].NatRevGenet,2014,15(1):7-21.
[28]IWAKKIRI J,HAMADA M,ASAI K.Bioinformatics tools for lncRNA research[J].BBA-GeneRegulMec,2015,1859(1):23-30.
[29]KASHI K,HENDERSON L,BONETTI A,et al.Discovery and functional analysis of lncRNAs:Methodologies to investigate an uncharacterized transcriptome[J].BBA-GeneRegulMec,2015,151(6):690-693.
[30]GHOSAL S,DAS S,CHAKRABARTI J et al.Long noncoding RNAs:new players in the molecular mechanism for maintenance and differentiation of pluripotent stem cells[J].StemCellsDev,2013,22(16):2240-2253.
[31]FLYNN RA,CHANG H Y.Long noncoding RNAs in cell-fate programming and reprogramming[J].CellStemCell,2014,14(6):752-761.
[32]BATISTA P J,CHANG H Y.Long noncoding RNAs:cellular address codes in development and disease[J].Cell,2013,152(6):1298-1307.
[33]AUTUORO J M,PIRNIE S P,CARMICHAEL G G.Long noncoding RNAs in imprinting and X chromosome inactivation[J].Biomolecules,2014,4(1):76-100.
[34]DENG X,BERLETCH J B,NGUYEN D K,et al.X chromosome regulation:diverse patterns in development,tissues and disease[J].NatRevGenet,2014,15(6):367-378.
[35]GALUPA R,HEARD E.X-chromosome inactivation:new insights into cis and trans regulation[J].CurrOpinGenetDev,2015,31:57-66.
[36]MERCER T R,DAGMER W,DINGER M E,et al.Expression of distinct RNAs from 3′ untranslated regions[J].NucleicAcidsRes,2011,39(6):2393-2403.
[37]RYU Y H,MACDONALD P M.RNA Sequences required for the noncoding function of oskar RNA also mediate regulation of oskar protein expression by Bicoid stability factor[J].DevBiol,2015,407(2):211-223.
[38]DAMIEN U,CLAIRE F,FLORENT H.When one is better than two:RNA with dual functions[J].Biochimie,2011,93(4):633-644.
[39]ANGUERA M C,MA W,CLIFT D,at el.Tsx produces a long noncoding RNA and has general functions in the germline,stem cells,and brain[J].PLoSGenet,2011,7:e1002248.
[40]ZHANG G,DAHL J A,NIU Y,at el.ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility[J].MolCell,2013,49(1):18-29.
[41]GAO W L,LIU M,YANG Y,at el.The imprinted H19 gene regulates human placental trophoblast cell proliferation via encoding miR-675 that targets nodal modulator 1 (NOMO1)[J].RNABiol,2012,9(7):1002-1010.
[42]ANDREW K,DAVID O,PAUL M,at el.The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r[J].NatCellBiol,2012,14(7):659-665.
[43]ZOU Y,JIANG Z,YU X,et al.Upregulation of long noncoding RNA SPRY4-IT1 modulates proliferation,migration,apoptosis,and network formation in trophoblast cells HTR-8SV/neo[J].PLoSOne,2013,8(11):e79598.
[44]SHINICHI N,MASAYUKI S,KAORI Y,et al.The lncRNA Neat1 is required for corpus luteum formation and the establishment of pregnancy in a subpopulation of mice[J].Development,2014,141(23):4618-4627.
[45]LI W,NOTANI D,MA Q,et al.Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation[J].Nature,2013,498(7455):162-190.
[46]YANG L,LIN C,JIN C,et al.lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs[J].Nature,2013,500(7464):598-602.
[47]HSIEH C L,FEI T,CHEN Y,et al.Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation[J].ProcNatlAcadSciUSA,2014,111(20):7319-7324.
[48]LANZ R B,MCKENNA N J,ONATE S A,et al.A steroid receptor coactivator,SRA,functions as an RNA and is present in an SRC-1 complex[J].Cell,1999,97(1):17-27.
[49]LANZ R B,RAZANI B,GOLDBERG A D,et al.Distinct RNA motifs are important for coactivation of steroid hormone receptors by steroid receptor RNA activator (SRA)[J].ProcNatlAcadSciUSA,2002,99(25):16081-16086.
[50]DANIEL A R,GAVIGLIO A L,KNUTSON T P,et al.Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1-and estrogen receptor-containing transcription complexes[J].Oncogene,2015,34(4):506-515.
[51]KAWASHIMA H,TAKANO H,SUGITA S,et al.A novel steroid receptor co-activator protein (SRAP) as an alternative form of steroid receptor RNA-activator gene:expression in prostate cancer cells and enhancement of androgen receptor activity[J].BiochemJ,2003,369:163-171.
[52]VICENT G P,NACHT A S,ZAURIN R,et al.Unliganded progesterone receptor-mediated targeting of an RNA-containing repressive complex silences a subset of hormone-inducible genes[J].GenesDev,2013,27(10):1179-1197.
[53]KANEKO S,SON J,SHEN S S,et al.PRC2 binds active promoters and contacts nascent RNAs in embryonic stem cells[J].NatStructMolBiol,2013,20(11):1258-1264.
[54]DURRUTHY-DURRUTHY J,SEBASTIANO V,WOSSIDLO M,et al.The primate-specific noncoding RNA HPAT5 regulates pluripotency during human preimplantation development and nuclear reprogramming[J].NatGenet,2016,48(1):44-52.
[55]MULVEY B B,OLCESE U,CABRERA J R,et al.An interactive network of long non-coding RNAs facilitates the Drosophila sex determination decision[J].BBA-GeneRegulMec,2014,1839(9):773-784.
[56]HANSEN T B,JENSEN T I,CLAUSENl B H,et al.Natural RNA circles function as efficient microRNA sponges[J].Nature,2013,495(7441):384-388.
[57]LEI Z,HENG L,DAZHUAN X,et al.A novel ncRNA gene from mouse chromosome 5 trans-splices with Dmrt1 on chromosome 19[J].BiochemBiophResCo,2010,400(4):696-700.
[58]YOKOO M,SATO E.Cumulus-oocyte complex interactions during oocyte maturation[J].IntRevCytol,2004,235:251-291.
[59]YERUSHALMI G M,SALMIN-DIVON M,YUNG Y,et al.Characterization of the human cumulus cell transcriptome during final follicular maturation and ovulation[J].MolHumReprod,2014,20(8):719-735.
[60]XU X,LI J,CAO Y,et al.Differential expression of long noncoding RNAs in human cumulus cells related to embryo developmental potential:A microarray analysis[J].ReprodSci,2014,22(6):672-678.
[61]MACAULAY A D,ISABELLE G,JULIETA C,et al.The gametic synapse:RNA transfer to the bovine oocyte[J].BiolReprod,2014,91(4):90-90.
[62]NICOLAS E,CHENOUARD N,OLIVO-MARIN J C,et al.A dual role for actin and microtubule cytoskeleton in the transport of Golgi units from the nurse cells to the oocyte across ring canals[J].MolBiolCell,2009,20(1):556-568.
[63]CRISTINA C,LUANA L,LETIZIA A,et al.Soma-to-germline transmission of RNA in mice xenografted with human tumour cells:possible transport by exosomes[J].PLoSOne,2014,9:e101629.
[64]EVANGELOS P,JIGUANG W,GERSON R,et al.RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity[J].Cell,2015,161(4):774-789.
[65]GEZER U,ÖZGÜR E,CETINKAYA M,et al.Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes[J].CellBiolInt,2014,38(9):1076-1079.
[66]YAN L,YANG M,GUO H,et al.Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells[J].NatStructMolBiol,2013,20(9):1131-1139.
[67]HAMAZAKI N,UESAKA M,NAKASHIMA K,et al.Gene activation-associated long noncoding RNAs function in mouse preimplantation development[J].Development,2015,142(5):910-920.
[68]LI J,CAO Y,XU X,et al.Increased new lncRNA-mRNA gene pair levels in human cumulus cells correlate with oocyte maturation and embryo development[J].ReprodSci,2015,22(8):1008-1014.
[69]BROWN J B,NATHAN B,ROBERT E,et al.Diversity and dynamics of the Drosophila transcriptome[J].Nature,2014,512(7515):393-399.
[70]BAO J,WU J,SCHUSTER A S,et al.Expression profiling reveals developmentally regulated lncRNA repertoire in the mouse male germline[J].BiolReprod,2013,89(5):107-118.
[71]CHALMEL F,LARDENOIS A,EVRARD B,et al.High-resolution profiling of novel transcribed regions during rat spermatogenesis[J].BiolReprod,2014,91(1):5-5.
[72]LIANG M,LI W,TIAN H,et al.Sequential expression of long noncoding RNA as mRNA gene expression in specific stages of mouse spermatogenesis[J].SciRep,2014,4:5966-5966.
[73]MARGOLIN G,KHIL P P,KIM J,et al.Integrated transcriptome analysis of mouse spermatogenesis[J].BMCGenomics,2014,15(1):1-19.
[74]SOUMILLON M,NECSULEA A,WEIER M,at el.Cellular source and mechanisms of high transcriptome complexity in the mammalian testis[J].CellRep,2013,3(6):2179-2190.
[75]CABALLERO J,GILBERT I,FOURNIER E,et al.Exploring the function of long non-coding RNA in the development of bovine early embryos[J].ReprodFertDev,2014,27(1):40-52.
[76]PARANJPE S S,JACOBI U G,HEERINGEN S J V,et al.A genome-wide survey of maternal and embryonic transcripts during Xenopus tropicalis development[J].BMCGenomics,2013,14(1):600-601.
[77]ZHANG K,HUANG K,LUO Y,er al.Identification and functional analysis of long non-coding RNAs in mouse cleavage stage embryonic development based on single cell transcriptome data[J].BMCGenomics,2014,15(1):1-12.
[78]WANG Y,XUE S,LIU X,et al.Analyses of long non-coding RNA and mRNA profiling using RNA sequencing during the pre-implantation phases in pig endometrium[J].SciRep,2016,6:20238.
[79]GUTTMAN M,DONAGHEY J,CAREY B W,et al.lincRNAs act in the circuitry controlling pluripotency and differentiation[J].Nature,2011,477(7364):295-300.
(编辑程金华)
New Research Progress in Animal Reproduction-related Long Noncoding RNAs
HE Xiao-yun,DI Ran,HU Wen-ping,WANG Xiang-yu,LIU Qiu-yue*,CHU Ming-xing*
(KeyLaboratoryofFarmAnimalGeneticResourcesandGermplasmInnovationofMinistryofAgriculture,InstituteofAnimalScience,ChineseAcademyofAgriculturalSciences,Beijing100193,China)
Long noncoding RNA (lncRNA) is one kind of RNA which is longer than 200 nt and does not show the potential of coding protein.It has been known to play vital roles in eukaryotic gene regulation.Transcriptional capacities of sex chromosomes and some imprinted genes is regulated incisby lncRNAs which vary among species.The researches of lncRNAs regulation in reproduction mainly focused on screening lncRNAs candidates,identifying their target genes and the biological functions,clarifying the molecular mechanisms of those lncRNAs regulation.The present review concerned with the role of lncRNAs in processes vital to reprodution,such as gametogenesis,placentation and pregnancy,sex hormone responses,early embryonic development,sex determination and gonad development stages during reproduction process.These results can give scientists new insights into the regulation mechanism of animal reproduction.
lncRNA;reproduction;gametogenesis;hormone regulation;pregnancy
10.11843/j.issn.0366-6964.2016.09.002
2016-04-25
国家自然科学基金项目(31472078;31402041;31572371);宁夏农林科学院科技创新先导资金项目(DWJLC-2016001);中国农业科学院科技创新工程(ASTIP-IAS13);国家肉羊产业技术体系专项(CARS-39);中央级公益性科研院所基本科研业务费专项(2013ywf-zd-1;2015ywf-zd-2;2015ywf-zd-8);内蒙古自治区科技重大专项;内蒙古自治区战略性新兴产业发展专项资金计划
贺小云(1990-),男,山西运城人,硕士生,主要从事动物分子育种研究,E-mail: hedayun@sina.cn
刘秋月,博士,副研究员,E-mail: liuqiuyue@caas.cn;储明星,博士,研究员,博士生导师,主要从事羊优异繁殖性状的分子机理研究,E-mail: mxchu@263.net
S821.3
A
0366-6964(2016)09-1749-08

