Isoprenylated Flavonoids with PTP1B Inhibition from Macaranga denticulata
Lai-Bin Zhang.Chun Lei.Li-Xin Gao.Jing-Ya Li.Jia Li.Ai-Jun Hou
ORIGINAL ARTICLE
Isoprenylated Flavonoids with PTP1B Inhibition from Macaranga denticulata
Lai-Bin Zhang.Chun Lei.Li-Xin Gao.Jing-Ya Li.Jia Li.Ai-Jun Hou
ⒸThe Author(s)2016.This article is published with open access at Springerlink.com
Three new C-methylated and isoprenylated chalcone derivatives,dentichalcones A–C(1–3),together with six known compounds(4–9),were isolated from the twigs and leaves ofMacaranga denticulata.Their structures were elucidated by spectroscopic analysis,including 1D,2D NMR,and MS data.The known compounds,(2E)-1-(5,7-dihydroxy-2,2,6-trimethyl-2H-benzopyran-8-yl)-3-(4-methoxyphenyl)-2-propen-1-one (4),(2E)-1-(5,7-dihydroxy-2,2-dimethyl-2H-benzopyran-8-yl)-3-phenyl-2-propen-1-one(5),laxichalcone(6),macarangin (7),bonanniol A (8),and bonannione A(9),showed inhibitory activities against protein tyrosine phosphatase 1B(PTP1B)in vitro.
Graphical AbstractThree new C-methylated and isoprenylated chalcone derivatives,dentichalcones A–C(1–3),together with six known compounds,were isolated from the twigs and leaves ofMacaranga denticulata.Some compounds showed inhibitory activities against PTP1B in vitro.
Macaranga denticulata·Euphorbiaceae·Isoprenylated flavonoids·Dentichalcones A–C ·Protein tyrosine phosphatase 1B
1 Introduction
Isoprenylated flavonoids are a group of natural products with diverse structures and important bioactivities.A large number of new compounds have been isolated mainly from species of the Leguminosae,Moraceae,and Euphorbiaceae families,and some of the compounds showed antibacterial,antioxidant,anti-HIV,antidiabetic,and tyrosinase-inhibiting effects[1].The genusMacarangaThou.(Euphorbiaceae)comprises about 300 species mainly distributed in the tropical regions of Africa,Asia,Australia,and the Pacific islands[2].The leaves of someMacarangaspecies have been used as folk medicine for the treatment of swellings,cuts,sores,boils,and bruises[3].Macaranga denticulata(Bl.)Muell.Arg.is a tree with rich resources in Hainan and Xishuangbanna areas of China.Its roots have been used as traditional Chinese medicine against icteric hepatitis,eczema,and epigastric pain[4].Previous phytochemical studies on this plant resulted in the isolation of isoprenylated flavonoids and diterpenylated flavonoids or stilbenes,some of which showed antioxidant,acetylcholinesterase-inhibiting,and antiangiogenic activities[5–7].
Protein tyrosine phosphatase 1B(PTP1B)has been regarded as a promising target for treating type 2 diabetes and obesity[8].Discovery of effective PTP1B inhibitors is one of our research interests[9–12],and a few of isoprenylated phenolics including flavonoids and Diels–Alder adducts were found to have significant PTP1B inhibitory effects[9,10].In our continuing search for natural PTP1B inhibitors from plants,chemical investigations ofM.denticulatawere carried out.Fractionation of the ethanol extract afforded nine isoprenylated flavonoids,including three new C-methylated and isoprenylated chalcones,dentichalcones A–C(1–3),together with six known compounds,(2E)-1-(5,7-dihydroxy-2,2,6-trimethyl-2H-benzopyran-8-yl)-3-(4-methoxyphenyl)-2-propen-1-one(4),(2E)-1-(5,7-dihydroxy-2,2-dimethyl-2H-benzopyran-8-yl)-3-phenyl-2-propen-1-one(5),laxichalcone(6),macarangin(7),bonanniol A(8),and bonannione A(9)(Fig.1).The isolated compounds were tested in vitro for inhibition on PTP1B enzymatic activity.Compounds4–9showed significant inhibitory effects.This is the first report of C-methylated and isoprenylated chalcones from the genusMacaranga.Herein,we describe the structural elucidation and biological evaluation of these compounds.
2 Results and Discussion
Dentichalcone A(1)was assigned the molecular formula C21H20O5by HREIMS with anm/z352.1308[M]+(calcdfor C21H20O5,352.1311).The IR spectrum showed absorptions for OH(3417 cm-1),carbonyl(1625 cm-1),and aromatic(1605,1514,and 1445 cm-1)moieties.The1H NMR spectrum(Table 1)displayed a hydrogen-bonded hydroxylsignal atδH14.62(1H,s,OH-2′),twotrans-coupled olefinic protons at δH8.08(1H,d,J=15.6 Hz,H-α)and 7.76(1H,d,J=15.6 Hz,H-β),resonances of a 1,4-disubstituted benzene moiety atδH7.61(2H,d,J=8.6 Hz,H-2,6)and6.95(2H,d,J=8.6 Hz,H-3,5),a methylgroup at δH2.05(3H,s,H3-7′),and signals of a 2,2-dimethylpyran ring at δH6.70(1H,d,J=10.0 Hz,H-1′),5.59(1H,d,J=10.0 Hz,H-2′),and 1.56(6H,s,H3-4′,5′).The13C NMR spectrum(Table 1)exhibited 21carbon signals:three methyls,eightsp2methines,and ten quaternary carbons including a carbonyl,eightsp2,and one oxygenatedsp3.These NMR spectroscopic data indicated that1was a chalcone derivative with an isoprenoid and a C-methyl group.By interpretation of the HMBC and NOESY spectra(Fig.2),the structure of1was established.The HMBC cross-peaks of H-α/C-γ,C-1 and H-β/C-α,C-γ,C-1,C-6 verified the presence of chalcone skeleton.The hydroxyl group at δH14.62 were assigned to OH-2′by the HMBC correlations of OH-2′/C-1′,C-2′,C-3′.The methyl group at δH2.05(H3-7′)was located at C-3′by the HMBC correlations of H3-7′/C-2′,C-3′,C-4′.The 2,2-dimethylpyran group was fusedat C-5′andC-6′,asdeduced from the HMBC correlations of H-1′/C-4′,C-5′,C-6′and H-2′/C-5′,together with the key NOESY correlations of H3-4′,5′/H-α andH-2,6.Thus,the structure of1was elucidated as(2E)-1-(5,7-dihydroxy-2,2,6-trimethyl-2H-benzopyran-8-yl)-3-(4-hydroxyphenyl)-2-propen-1-one and named dentichalcone A.
Dentichalcone B(2)was assigned the molecular formula C21H22O5by HREIMS(m/z354.1465[M]+;calcd for C21H22O5,354.1467).Comparison of its NMR spectroscopic data(Table 1)with those of1showed that2was a dihydrochalcone derivative of1.This was confirmed by some diagnostic signals at δH3.40(2H,t,J=7.6 Hz,H2-α)and 2.89(2H,t,J=7.6 Hz,H2-β)and at δC46.6(C-α),and 30.4(C-β),and 207.3(C-γ)and by the HMBC correlations of H2-α/C-γ,C-1 and H2-β/C-α,C-γ,C-1,C-6(Fig.2).Thus,the structure of2was elucidated as 1-(5,7-dihydroxy-2,2,6-trimethyl-2H-benzopyran-8-yl)-3-(4-hydroxyphenyl)propan-1-one and named dentichalcone B.
Dentichalcone C(3)was assigned the molecular formula C21H20O5by HRESIMS(m/z351.1237[M–H]-;calcd for C21H19O5,351.1238).The1H and13C NMR spectra indicated the presence of a chalc one skeleton with a hydrogenbonded hydroxyl and a methyl group,which was similar to that of1.Its NMR spectra also displayed signals of a monosubstituted benzene ring [δH7.86 (2H,brd,J=7.3 Hz,H-2,6),7.44(3H,m,H-3,4,5);δC136.7(C-1),130.8(C-4),129.7(C-2,6),and 129.5(C-3,5)]and resonances of a 2-hydroxymethyl-2-methylpyran moiety[δH6.79(1H,d,J=10.0 Hz,H-1′),5.52(1H,d,J=10.0 Hz,H-2′),3.90 and 3.61 (each 1H,d,J=11.9 Hz,H-4′a,b),1.57(3H,s,H3-5′); δC119.7(C-1′),122.5(C-2′),81.5(C-3′),66.5(C-4′),22.7(C-5′)].The planar structure of3was further constructed by the HMBC spectrum(Fig.2).The stereochemistry at C-3′′could not be assigned by the available data.Thus,the structure of3was elucidated as(2E)-1-[5,7-dihydroxy-2-(hydroxymethyl)-2,6-dimethyl-2H-benzopyran-8-yl]-3-phenyl-2-propen-1-one and named dentichalcone C.
The known compounds were identified as(2E)-1-(5,7-dihydroxy-2,2,6-trimethyl-2H-benzopyran-8-yl)-3-(4-methoxyphenyl)-2-propen-1-one(4)[13],(2E)-1-(5,7-dihydroxy-2,2-dimethyl-2H-benzopyran-8-yl)-3-phenyl-2-propen-1-one(5)[14],laxichalcone(6)[15],macarangin(7)[7],bonanniol A(8)[16],and bonannione A(9)[16](Fig.1)by comparison of their spectroscopic data with those reported.Compound4was a new natural product,which was previously reported as a synthetic molecule[13].
All the isolated compounds were tested in vitro for the inhibitory effects on PTP1B.Compounds4–9showed inhibition with IC50values ranging from 14.0±1.2 to 48.8 ± 5.5 μM(Table 2).Oleanolic acid,an effective natural PTP1B inhibitor[17],was used as the positive control(IC50=2.6 ± 0.6 μM).
In summary,this is the first report of C-methylated and isoprenylated chalcones from the genusMacaranga.This class of compounds is distributed limitedly in the family Euphorbiaceae,and onlyMallotus philippinensiswas reported to produce C-methylated or both C-methylated and isoprenylated chalcones[18–21].TheMacarangaandMallotusgenera are monophyletic sister groups in the family Euphorbiaceae,which show a remarkable resemblance in their phylogeny,habit,and geographical distribution[22].The present study indicates that the two genera also have some similarity in their secondary metabolites.Furthermore,isoprenylated flavonoids as potent PTP1B inhibitors for the therapy of obesity and type 2 diabetes need further studies.
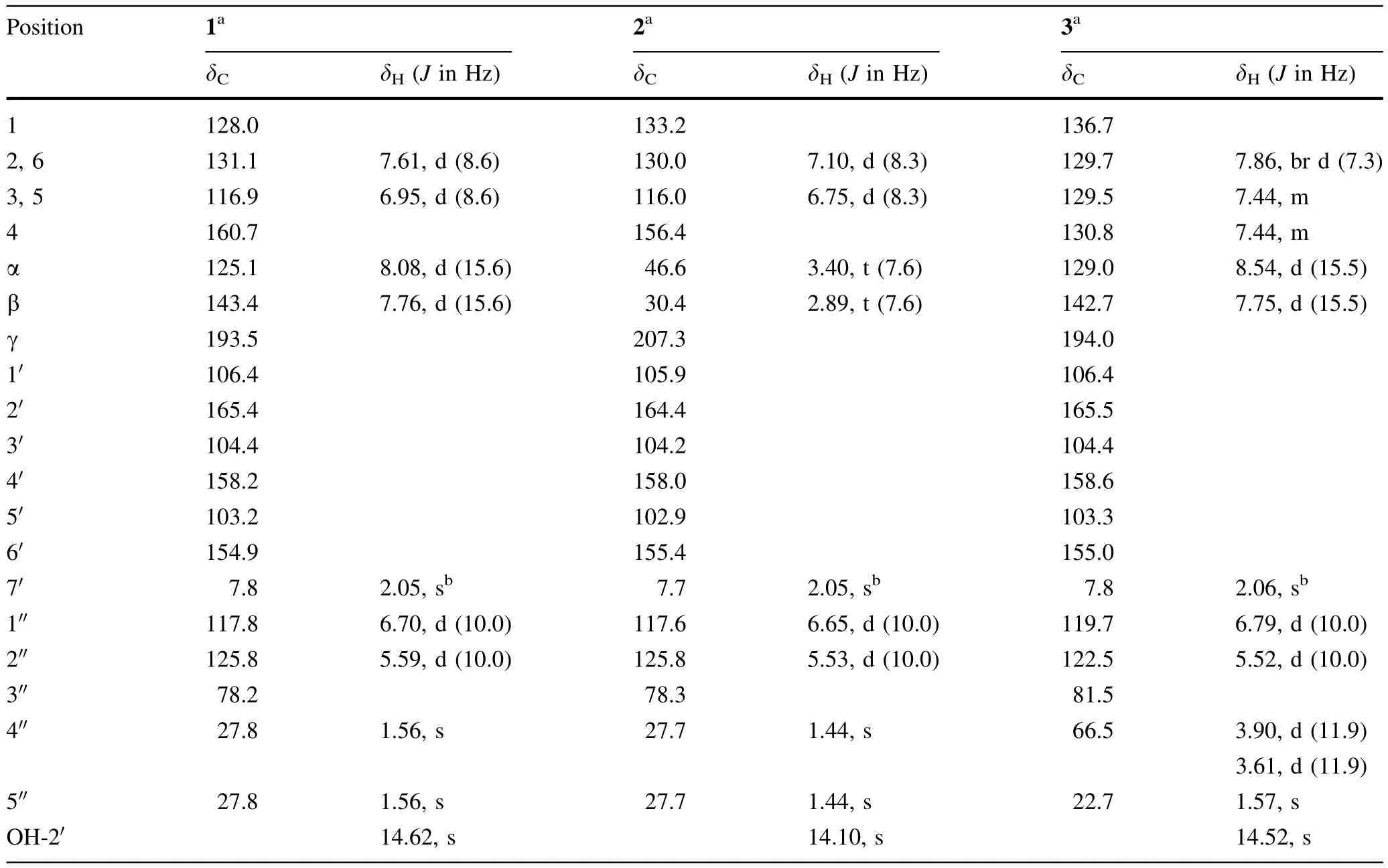
Table 11H and13C NMR spectroscopic data of compounds 1–3(in acetone-d6)
3 Experimental Section
3.1 General Experimental Procedures
Optical rotation was measured on a JASCO P-1030 digital polarimeter.UV spectra were recorded on a Hitachi U-2900 spectrophotometer.IR spectra were measured on a Nicolet Avatar-360 spectrometer with KBr pellets.NMR spectra were obtained on Varian Mercucy Plus 400 instruments.Chemical shifts were reported with TMS as internal standard or with respect to acetone-d6(δH2.04,δC206.0 ppm).EIMS(70 eV)and HREIMS were recorded on an Agilent 5973N and a Waters Micromass GCT mass spectrometer,respectively.ESIMS and HRESIMS were performed on an Agilent 1100 LC/MSD and a Bruker Daltonics ApexIII mass spectrometer,respectively.Semipreparative HPLC was performed on an Agilent 1200(Agilent Technologies,Palo Alto,CA,USA)and a Sepax Amethyst C18 column(150 × 10 mm,5 μm,Sepax Techologies,Inc.,Newark,DE,USA),using a UV detector set at 210 nm.Column chromatography(CC)was performed on silica gel(200-300 mesh,Yantai Institute of Chemical Technology,Yantai,People’s Republic of China),Diaion HP-20(Mitsubishi Chemical Co.,Tokyo,Japan),and Sephadex LH-20 gel(GE Healthcare Amersham Biosciences,Uppsala,Sweden).Fractions were monitored by TLC analysis run on precoated silica gel GF254 plates(10–40 μm,Yantai Institute of Chemical Technology,Yantai,People’s Republic of China).
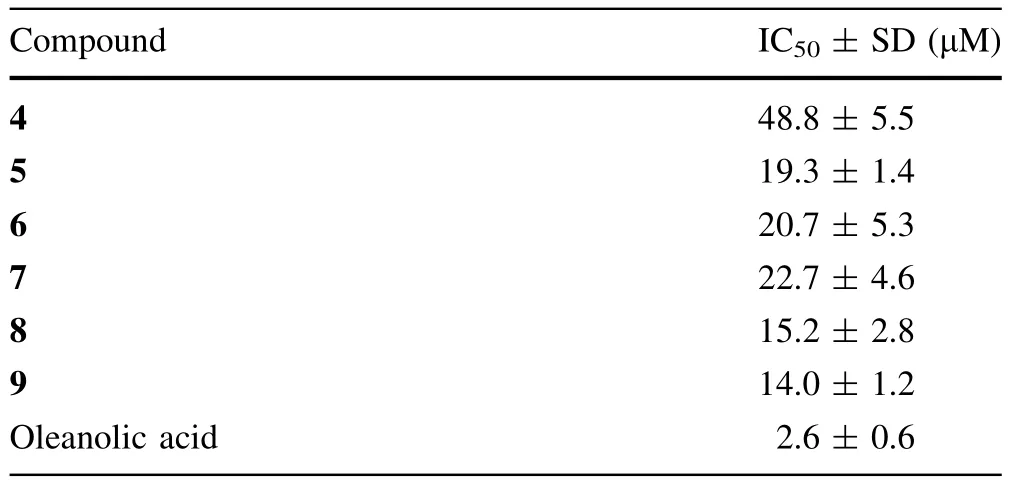
Table 2 Inhibitory activities of compounds 4–9 against PTP1B
3.2 Plant Material
The twigs and leaves ofM.denticulatawere collected in Hekou County,Yunnan Province,People’s Republic of China,in April 2011.The plant material was identified by Dr.Qin-Shi Zhao,Kunming Institute of Botany,Chinese Academy of Sciences,and a voucher specimen(TCM 11-04-15 Hou)has been deposited at the Herbarium of the Department of Pharmacognosy,School of Pharmacy,Fudan University.
3.3 Extraction and Isolation
The milled,air-dried twigs and leaves ofM.denticulata(5.0 kg)were percolated with 95%EtOH at room temperature(60 L).The filtrate was evaporated under reduced pressure to give a residue(500 g),which was suspended in H2O and extracted with CH2Cl2(4×1 L).The CH2Cl2extract(140 g)was subjected to CC on Diaion HP-20 eluted with 90%EtOH.The 90%EtOH fraction(95 g)was separated by CC on silica gel eluted with a gradient of petroleum ether–EtOAc(1:0,10:1,5:1,1:1,1:2)to give fractions A–J.Fraction D was separated by CC on Sephadex LH-20 eluted with CHCl3–MeOH(1:1)to afford fractions D1–D4.Fraction D4 was chromatographed over silica gel eluted with a gradient of petroleum ether–Me2CO(10:1,5:1)to afford fractions D4.1–D4.5.Fraction D4.3 was purified on a Sephadex LH-20 column eluted with MeOH to provide4(100 mg).Fraction E was chromatographed by silica gel eluted with a gradient of CH2Cl2–Me2CO(40:1,2:1)to afford fractions E1–E8.Fraction E4 was separated on silica gel eluted with a gradient of petroleum ether–Me2CO(12:1,10:1)to give fractions E4.1–E4.5.Fraction E4.5 was chromatographed by semi-preparative HPLC(CH3OH–H2O,90:10,flow rate 1 mL/min)to afford6(10 mg).Fraction F was isolated by CC over Sephadex LH-20 eluted with CHCl3–MeOH(1:1)to afford fractions F1–F5.Fractions F2 and F4 were chromatographed over silica gel eluted with a gradient of petroleum ether–EtOAc(10:1,2:1)to afford fractions F2.1–F2.6 and F4.1–F4.4,respectively.Fractions F2.2,F2.4,and F4.4 were purified by semi-preparative HPLC at flow rate 1 mL/min to afford2(4 mg;CH3OH–H2O,83:17),9(10 mg;CH3OH–H2O,90:10),and7(12 mg;CH3OH–H2O,91:9),respectively.Fraction F4.2 was separated by CC on Sephadex LH-20 eluted with CH3OH to provide1(15 mg).Fraction G was separated by CC on silica gel eluted with a gradient of CH2Cl2–Me2CO(30:1,2:1)to give fractions G1–G7.Fractions G2,G3,and G4 were chromatographed by semi-preparative HPLC at flow rate 1 mL/min to yield5(2 mg;CH3OH–H2O,88:12),3(3 mg;CH3OH–H2O,80:20),and8(12 mg;CH3OH–H2O,88:12),respectively.
3.4 Dentichalcone A(1)
Red,amorphous powder;UV(MeOH) λmax(log ε)229(4.43),244(4.42)(sh),289(4.30),368(4.66)nm;IR(KBr)νmax3417,2972,2915,1625,1605,1541,1514,1445,1167,830,536 cm-1;1H NMR and13C NMR data,see Table 1;EIMSm/z352[M]+(30),337(64),217(100),91(15),77(7);HREIMSm/z352.1308[M]+(calcd for C21H20O5,352.1311).
3.5 Dentichalcone B(2)
Yellow,amorphous powder;UV(MeOH)λmax(log ε)222(3.79),284(3.82)nm;IR(KBr)νmax3419,2975,2924,1638,1606,1515,1427,1218,1165,1133,827,548 cm-1;1H NMR and13C NMR data,see Table 1;EIMSm/z354[M]+(49),339(100),233(35),219(33),191(36),107(47),91(16),77(21),65(8),43(8);HREIMSm/z354.1465[M]+(calcd for C21H22O5,354.1467).
3.6 Dentichalcone C(3)
3.7 Assay of PTP1B Activity
The bioassay procedure was the same as that reported previously[10,23].The result of PTP1B inhibition was expressed as IC50,which was calculated with Prism 4 software(Graphpad,San Diego,CA).
AcknowledgmentsFinancial support from the National Natural Science Foundation of China(Nos.81222045,21372049),the Specialized Research Fund for the Doctoral Program of Higher Education of China(20130071120104),and the Shu Guang Project(No.12SG02)from Shanghai Municipal Education Commission and Shanghai Education Development Foundation is gratefully acknowledged.
Compliance with ethical standards
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License(http://creativecommons.org/licenses/by/4.0/),which permits unrestricted use,distribution,and reproduction in any medium,provided you give appropriate credit to the original author(s)and the source,provide a link to the Creative Commons license,and indicate if changes were made.
1.B.Botta,A.Vitali,P.Menendez,D.Misiti,G.D.Monache,Curr.Med.Chem.12,713–739(2005)
2.G.Webster,Ann.Missouri Bot.Garden81,33–144(1994)
3.A.Nick,T.Rali,O.Sticher,J.Ethnopharmacol.49,147–156(1995)
4.S.J.Wei,J.Guangxi.Tradit.Chin.Med.15,36–39(1992)
5.D.S.Yang,Z.L.Li,X.Wang,H.Yan,Y.P.Yang,H.R.Luo,K.C.Liu,W.L.Xiao,X.L.Li,RSC Adv.5,13886–13890(2015)
6.D.S.Yang,Z.L.Li,W.B.Peng,Y.P.Yang,X.Wang,K.C.Liu,X.L.Li,W.L.Xiao,Fitoterapia103,165–170(2015)
7.S.Sutthivaiyakit,S.Unganont,P.Sutthivaiyakit,A.Suksamrarn,Tetrahedron58,3619–3622(2002)
8.B.J.Goldstein,A.Bittner-Kowalczyk,M.F.White,M.Harbeck,J.Biol.Chem.275,4283–4289(2000)
9.M.Wang,L.X.Gao,J.Wang,J.Y.Li,M.H.Yu,J.Li,A.J.Hou,Phytochemistry109,140–146(2015)
10.M.Wang,B.W.Yu,M.H.Yu,L.X.Gao,J.Y.Li,H.Y.Wang,J.Li,A.J.Hou,Chem.Biodivers.12,937–945(2015)
11.C.C.Liu,C.Lei,Y.Zhong,L.X.Gao,J.Y.Li,M.H.Yu,J.Li,A.J.Hou,Tetrahedron70,4317–4322(2014)
12.H.B.Liao,C.Lei,L.X.Gao,J.Y.Li,J.Li,A.J.Hou,Org.Lett.17,5040–5043(2015)
13.L.Xia,Y.R.Lee,Bull.Korean Chem.Soc.32,2921–2927(2011)
14.A.G.Kanthasamy,G.A.Kraus,V.Anantharam,U.S.Pat.Appl.Publ.US 20110112182 A1 20110512(2011)
15.Y.L.Lin,Y.L.Chen,Y.H.Kuo,Chem.Pharm.Bull.40,2295–2299(1992)
16.M.Bruno,G.Savona,L.Lamartina,F.Lentini,Heterocycles23,1147–1153(1985)
17.Y.N.Zhang,W.Zhang,D.Hong,L.Shi,Q.Shen,J.Y.Li,J.Li,L.H.Hu,Bioorg.Med.Chem.16,8697–8705(2008)
18.R.R.Kulkarnia,S.G.Tupeb,S.P.Gamplec,M.G.Chandgudea,D.Sarkarc,M.V.Deshpandeb,S.P.Joshia,Nat.Prod.Res.28,245–250(2014)
19.A.Daikonya,S.Katsuki,S.Kitanaka,Chem.Pharm.Bull.52,1326–1329(2004)
20.T.Tanaka,T.Ito,M.Iinuma,Y.Takahashi,H.Naganawa,Phytochemistry48,1423–1427(1998)
21.M.Furusawa,Y.Ido,T.Tanaka,T.Ito,K.Nakaya,I.Ibrahim,M.Ohyama,M.Iinuma,Y.Shirataka,Y.Takahashi,Helv.Chim.Acta88,1048–1058(2005)
22.K.K.M.Kulju,S.E.C.Sierra,S.G.A.Draisma,R.Samuel,P.C.van Welzen,Am.J.Bot.94,1726–1743(2007)
23.L.Shi,H.P.Yu,Y.Y.Zhou,J.Q.Du,Q.Shen,J.Y.Li,J.Li,Acta Pharmacol.Sin.29,278–284(2008)
1 December 2015/Accepted:15 December 2015/Published online:20 January 2016
L.-B.Zhang ·C.Lei·A.-J.Hou(✉)
Department of Pharmacognosy,School of Pharmacy,Fudan
University,826 Zhang Heng Road,Shanghai 201203,People’s Republic of China
e-mail:ajhou@shmu.edu.cn
L.-B.Zhang
Department of Chinese Medicine,School of Pharmacy,Xinxiang Medical University,601 Jin Sui Road,Xinxiang 453003,
People’s Republic of China
L.-X.Gao ·J.-Y.Li·J.Li
National Center for Drug Screening,State Key Laboratory of Drug Research,Shanghai Institute of Materia Medica,Chinese Academy of Sciences,189 Guo Shou Jing Road,Shanghai
201203,People’s Republic of China
 Natural Products and Bioprospecting2016年1期
Natural Products and Bioprospecting2016年1期
- Natural Products and Bioprospecting的其它文章
- The Bioactive Secondary Metabolites from Talaromyces species
- Antiproliferative Triterpenoid Saponins from Leptaulus citroides Baill.from the Madagascar Rain Forest
- The Use of a Combination of RDC and Chiroptical Spectroscopy for Determination of the Absolute Configuration of Fusariumin A from the Fungus Fusarium sp.
- Koninginins N-Q,Polyketides from the Endophytic Fungus Trichoderma koningiopsis Harbored in Panax notoginseng
- Formosins A–F:Diterpenoids with Anti-microbial Activities from Excoecaria formosana
