The Bioactive Secondary Metabolites from Talaromyces species
Ming-Ming Zhai.Jie Li.Chun-Xiao Jiang.Yan-Ping Shi.Duo-Long Di.Phillip Crews.Quan-Xiang Wu
REVIEW
The Bioactive Secondary Metabolites from Talaromyces species
Ming-Ming Zhai.Jie Li.Chun-Xiao Jiang.Yan-Ping Shi.Duo-Long Di.Phillip Crews.Quan-Xiang Wu
ⒸThe Author(s)2015.This article is published with open access at Springerlink.com
The focus of this review is placed on the chemical structures from the species of the genusTalaromycesreported with reference to their biological activities.221 secondary metabolites,including 43 alkaloids and peptides,88 esters,31 polyketides,19 quinones,15 steroid and terpenoids,and 25 other structure type compounds,have been included,and 66 references are cited.
Graphical Abstract
Talaromyces·Secondary metabolites·Biological activities
1 Introduction
The nameTalaromycesis derived from the Greek word for‘basket’,which aptly describes the body in which ascospores are formed.In the past,species producing sexual stages withPenicilliumanamorphs have been classified inEupenicilliumandTalaromyces.After July 2011,species formally classified in thePenicilliumsubgenusBiverticilliumwere classified inTalaromyces.The situation is complicated by the fact that many species now classified inTalaromyceswill continue to be sought asPenicilliumspecies in identifications[1].So in this review,all of thepapers which reported the secondary metabolites from the species namedTalaromyceswere covered.
The genusTalaromyces(Trichocomaceae)is an important fungal genus because of its ubiquity which were isolated from soil,plants,sponges,and foods.Some of the species are heat resistant.Some of the species are famous because of their enzymes applicable in the synthesis of saccharides,preparation of chiral building blocks or biotrans for mations,and forits application in pest biocontrol.Many of its species are used in food and agricultural production.Interestingly,theT.pinophilusstrainEMOO13–3isabletodegradeagriculturalwaste[2].However,although endemic inmaize,T.funiculosusalso occurs in a wide range of other foods and sometimes causes spoilage[1].Considering their importance,members of this genus have attracted the attention of chemists.Many studies have focused on the secondary metabolites.
2 The Secondary Metabolites
The secondary metabolites ofTalaromycesmainly include alkaloids,peptides,lactones,polyketides,and miscellaneous structure type compounds.T.flavus,a microorganism remarkable for its secondary metabolites with unique biological activities,is the commonest species of the genusTalaromyces[3].All of the natural products from the species of this genus are classified.The reported bioactivities are also represented below.nematicidal.The structures of alkaloids isolated fromTalaromycesspecies are mainly nitrogen heterocyclic derivatives.
Two prenylated indole alkaloids,talathermophilins A and B(1and2),were isolated from a thermophilic fungusT.thermophilusstrain YM1-3.And the ratio of1and2in the culture broths was unexpectedly rather constant(about 2:3),which even remained unchanged despite the addition of exogenous1or2suggesting that talathermophilins might be of special function for the extremophilic fungus.Those both compounds showed nematicidaltoxicity (ca.38 and 44% inhibition,respectively)toward the worms ofthe free-living nematodePanagrellus redivivusat a concentration of 400 μg/mL for 72 h.The family of prenylated indole alkaloids is a well-known group of secondary metabolites mainly produced byAspergillusandPenicilliumsp.This is a first report about pyranoindol alkaloids fromTalaromyces[4].Other fourindole alkaloids with various levels of prenylation,talathermophilins C–E(3–5)and cyclo(glycyltryptophyl)(6),from the thermophilic fungusT.thermophilusstrain YM3-4 which was collected in hot springs,were also elucidated by the same research group in 2011[5].Interestingly,authors found that only a very small group of amino acids(glycine,alanine,proline,and its derivatives)could be naturally chosen as a starting building block to form the 2,5-diketopiperazine with tryptophan[4,5].
2.1 Alkaloids and Peptides
Alkaloid is a kind of important natural products.Many alkaloids have various kinds of biological activities,such as antibacterial,antifungal,cytotoxic,and
Seven known indole alkaloids(7–12)were obtained from the culture of the alga-endophytic fungusTalaromycessp.cf-16.Bioassay results showed that9was more toxic to brine shrimp than the other compounds,and8,9,and10could inhibitStaphylococcus aureus[6].
Three known diketopiperazines,cyclo(L-proline-L-leucine)(13),cyclo(L-proline-L-phenylalanine)(14),and cyclo(L-tyrosine-L-phenylalanine)(15),were isolated from the methanolic extracts of the green Chinese onion-derived fungusT.pinophilusAF-02[7].
An unprecedented class of PKS-NRPS hybrid metabolites possessing a 13-membered lactam-bearing macrolactone,thermolides A–F(16–21),were also obtained fromT.thermophilusYM3-4.They showed that compounds16and17displayed potent inhibitory activity against three notorious nematodes with LC50values of 0.5–1 μg/mL,as active as commercial avermectins.This is the first report on the discovery of hybrid macrolides from a fungus origin[8].Afterwards,a combination of chemical screening,genome analyses,and genetic manipulation led to the identification of the thermolide biosynthetic genes from sister thermophilic fungiT.thermophilusandThermomyceslanuginosusC5.And a novel macrolactone,thermolide G(22),was obtained from the cultural broth ofThermomyces lanuginosusC5.Their results revealed the first fungal hybrid iterative polyketide synthase–nonribosomal peptide synthetase(PKS–NRPS)genes involved in the biosynthesis of bacterial-like hybrid macrolactones instead of typical fungal tetramic acids-containing metabolites[9].
Four new tetramic acid derivatives,talaroconvolutins A–D(23–26),along with a known mitorubrin derivative,ZG-1494R(27),were isolated from the strainT.convolutesby the group of Shun-ichi Udagawa in 2000.The antifungal activity of the talaroconvolutins against the pathogenic fungiAspergillus fumigates,A.niger,Cryptococcus albicans,andC.neoformans,was determined.And the results showed that talaroconvolutins B(24)and C(25)and ZG-1494R(27)inhibited the growth ofA.fumigatus,A.niger,andC.albicans[10].
Four new drimane sesquiterpene lactones conjugated withN-acetyl-L-valine,minioluteumides A–D(28–31),and three known compounds,purpuride(32),berkedrimane B(33),and purpuride B(34),were isolated from the marine fungus,T.minioluteus(P.minioluteum)by the group of Prasat Kittakoop.The structure28was elucidated by single crystal X-ray analysis.28,31and33showed cytotoxic activity against HepG2 with IC50ranges of 50.6–193.3 μM,but28–34did not shown any inhibit activity to caspase-3[11].
A peptide analogueN-benzoylphenylalanyl-N-benzoylphenylalaninate(35)was isolated from the fungusT.thailandiasis,which was firstly found from a higher plant,Croton hieronymi[12].Two new cyclic peptides,talaromins A and B(36and37)were yielded from the endophytic fungusT.wortmannii,isolated fromAloe veraby the group of Peter Proksch and Abdessamad Debbab.Both cyclopeptides contain ring systems comprised of six α-amino acid residues connected to β-amino acid.The absolute configurations of the α-amino acids were determined by Marfey’s method.Both compounds showed no activity when evaluated for their cytotoxicity against L5178Y mouse lymphoma cells and no antibacterial activity against a broad spectrum of bacterial strains up to a concentration of 64 μg/mL[13].9-(3-L-alanylamino-3-carboxypropyl)adenine(NK374200,38)with a peptidyl adenine nucleus was isolated from the culture broth of the fungusTalaromycessp.,which had been isolated from a soil sample.38was screened in various biological assay systems,and found to have anti-mosquito larval activity[14].
Two quinazoline alkaloids,2-[(S)-hydroxy(phenyl)methyl]-3-methylquinazolin-4(3H)-one(39)and 2-[(R)-hydroxy(phenyl)methyl]-3-methylquinazolin-4(3H)-one(40),and a pyridone derivative(41),were isolated and identified in a culture of the alga-endophytic fungusTalaromycessp.cf-16 for the first time.Following chiral column chromatography,compounds39and40were identified as enantiomers by spectroscopic analyses and quantum chemical calculations[6].
(E)-3-(2,5-dioxo-3-(propan-2-ylidene)pyrrolidin-1-yl)acrylic acid(42)was isolated from the ethyl acetate extract of the culture broth ofT.verruculosus,a rhizosphere fungus ofStellera chamaejasmeL.In the antimicrobial activities,42gave slight active against the plant pathogenic fungi,Alternaria solani,Valsa mali,Curvularialunata,andBotryosphaeriaberengeriana,at 100 μg/mL and its MIC values against pathogenic bacteria,Straphylococcus aureusandEscherichia coli,were more than 100 μg/mL[15].Emerin(43)was obtained from the extract ofT.flavusIFM52668,and showed no activity against pathogenic filamentous fungi,Aspergillus fumigatusandA.niger,and pathogenic yeasts,Candida albicansandCryptococcus neoformans,at 200 μg/disc[16].
2.2 Esters
The secondary metabolites ofTalaromycesare mainly esters,including macrolides,linear polyesters,aromatic lactones,coumarins,phthalides,and five/six-membered saturated lactones.
Four novel 22-membered triene macrolides,wortmannilactones A–D(44–47),were obtained from the fungusT.wortmanniiwhich isolated from a soil sample collected in China’s Yunnan province.44–47were screened for cytotoxic activity against a panel of human cancer cell lines(HCT-5,HCT-115,A549,MDA-MB-231,and K562).The IC50values range from 28.7 to 130.5 μM[17].Vermiculine(48),a 16-membered macrolide dilactone antibiotic had been found in crystalline solid fromT.wortmannii,isolated from a soil sample[18].
Seven 15G256 macrolidepolyesters,15G256ι(49),15G256β(50),15G256α(51),talapolyesterE(52),15G256α-1(53),talapolyester F(54),and 15G256ω(55),were isolated from the wetland soil-derived fungusT.flavusBYD07-13 by Chinese researchers.Among these compounds,50and55exhibited significant activity against MCF-7cell line with the IC50of 3.27 and 4.32 μM,respectively[19].51[20,21]and53[22]were also isolated from the soil-derived fungusT.flavusFKI-0076 by Japanese researchers.In the course of screening for synergist effects with clinic-used miconazole as well as antifungal agent,51was showed that can inhibitBacillus subtilis(IC5015 mg/L),Staphyloccus aureus(IC5090 mg/L),Micrococcus luteus(IC50100 mg/L),Mucorracemosus(IC5040 mg/L)[20].As proposed by Schlingmann,15G256 polyesters are biosynthetically assembled by alternately linking 2,4-dihydroxy-6-(2-hydroxypropyl)benzoic acid and 3-hydroxybutyric acid moieties[23].
Four new linear polyesters,talapolyesters A–D(56–59),together with six known compounds(60–65),were isolated from the wetland soil-derived fungusT.flavusBYD07-13.Those compounds contained both 2,4-dihydroxy-6-(2-hydroxypropyl)benzoic acid or its derivatives and 3-hydroxybutyric acid or its derivatives.The cytotoxicity against five tumor cell lines of those compounds was examined,but all polyesters were inactive(IC50>40 μM)as compared to cisplatin[19].
Three new oxaphenalenone dimers,bacillosporins A–C(66–68),were isolated fromT.bacillosporusNHL 2660.66had the antibacterial activity againstBacillus subtilisandSarcina lutea[24].Other oligophenalenone dimers,bacillisporins D and E(69and70)and duclauxin(71),were isolated from the fungusT.bacillisporusfrom a soil sample.They were screened for in vitro cytotoxicity again three human tumor cell lines MCF-7,NCI-H-460 and SF-268,and71exhibited moderate inhibitory effects against all three cell lines but70showed little activity[25].In 2015,two new oxaphenalenone dimers,talaromycesone A(72)and talaromycesone B(73),were isolated from the culture broth and mycelia of a marine fungusTalaromycessp.strain LF458.72exhibited potent antibacterial activities with IC503.70 μM against human pathogenicStaphylococcusstrains,and72also displayed potent acetylcholinesterase inhibitory activities with IC507.49 μM[26].
Antibacterial binaphtho-α-pyrones,talaroderxines A and B(74and75)were isolated from a new heterothallic ascomycetous fungus,T.derxii,cultivated on rice.The antibacterial activities of the metabolites fromT.derxiiand their derivations againstBacillus subtilisindicated that only talaroderxine,the mixture of74and75,showed antibacterial activity,which was almost as strong as that of viriditoxin.And talaroderxine had inhibitory activity toward 5-lipoxygenase,its IC50value was determined as 3.8×10-6M[27].
Eight new dinapinones,AB1,AB2,AC1,AC2,AD1,AD2,AE1 and AE2(76–83)were obtained from the culture broth ofT.pinophilusFKI-3864.All these dinapinones possessed the same biaryl dihydronaphthopyranone skeleton consisting of a heterodimer with one monapinone A and one different monapinone.The effect of dinapinones was evaluated on the synthesis of[14C]triacylglycerol(TG)and[14C]cholesterol ester from[14C]oleic acid in CHO-K1 cells and the results indicated that dinapinone(77)showed potent inhibition of TG synthesis in intact mammalian cells with an IC50value of 1.17 μM,whereas the other dinapinones showed weak inhibition of TG synthesis[28].
Sixdiphenyl ether lactone derivatives(84,85and86–88)and AS-186c(89)were isolated from amarine fungusTalaromycessp.strain LF458.89exhibited potent antibacterial activities with IC501.34 μM against human pathogenicStaphylococcusstrains,potent acetylcholinesterase inhibitory activities with IC502.60 μM,and phosphodiesterase PDE-4B2 inhibitory activities with IC502.63 μM [26].Penicillide and dehydroisopenicillide(84and85)were isolated fromT.derxiicultivated on rice[29].Penicillide was also isolated from the methanolic extracts of the green Chinese onion-derived fungusT.pinophilusAF-02[7].
A coumarin90was obtained from the organic extracts of the soil fungusT.flavus[30].Two new coumarins,talacoumarins A(91)and B(92),were isolated from the ethyl acetate extract of the wetland soil-derived fungusT.flavusBYD07-13.They were evaluated for anti-Aβ42 aggregation,cytotoxic,and antimicrobial activities and the results showed that91and92had moderate anti-Aβ42 aggregation activity,and this was the first report on the Aβ42 inhibitory aggregation activity of coumarins[31].
AnO-methylated 3,4-dihydroisocoumarin93was isolated from a previously undescribed fungusT.thailandiasis[12].An isocoumarin derivate(94)was isolated from the ethyl acetate extract of the culture broth ofT.verruculosus,a rhizosphere fungus ofStellera chamaejasmeL.94exhibited the significant activities in vitro againstStaphylococcus aureusandEscherichia coli,with MIC values of 2.5 and 5.0 μg/mL,respectively.And for the plant pathogenic fungi,94disclosed significant growth inhibitions of 92.6±2.1,97.3±3.3,87.2±2.8 and 94.9±1.9%at 50 μg/mL againstAlternaria solani,Valsa mali,Curvularia lunataandBotryosphaeria berengeriana,respectively[15].Two isocoumarin derivates(95and96)were isolated from the organic extracts of the soil fungusT.flavus[30].Sclerotinin A(97)and alternariol(98)were isolated from the methanolic extracts of the green Chinese onion-derived fungusT.pinophilusAF-02[7].
Merodrimanes,thailandolides A(99)and B(100),a drimane linked through a tertiary oxygen to the dihydroisocoumarin,were isolated from a previously undescribed fungusT.thailandiasis[12].A new meroterpenoid,chrodrimanin C(101)together with chrodrimanins A and B(102and103)from the strain YO-2 ofTalaromycessp.Chrodrimanin B exhibited insecticidal activity with an LD50value of 10 μg/gofdiet,while chrodrimanins A and C were inactive[32].Four new meroterpenoids,named chrodrimanin D–G(104–107),and a known compound chrodrimanin H(108)were also isolated from the strain YO-2 ofTalaromycessp.Chrodrimanins D,E and F(104–106)showed insecticidal activity against silkworms with respective LD50values of 20,10 and 50 μg/g of diet[33].
A phthalide derivative109and a spiro-phthalide derivative110were obtained from the organic extracts and from the water extracts of the soil fungusT.flavus[30,34].Another phthalide compound FKI-0076 B,vermistatin111,was obtained fromTalaromycessp.during the screening programme for synergist of azoles antifungal antibiotics[20].111was also isolated from the extract ofT.flavusIFM52668[16],and from the culture brothT.flavusFKI-0076 which isolated from a soil sample[21].Other two analogues penisimplicissin(112)and hydroxydihydrovermistatin(113)were isolated from the fungusT.thailandiasis[12].
Three new phthalide derivatives,talaromycolides A–C(114–116),and a known compound rubralide C(117),were isolated from the methanolic extracts of the green Chinese onion-derived fungusT.pinophilusAF-02.Talaromycolides A–C are rare phthalide derivatives with a novel linkage position between the phenyl and phthalide moieties,and exhibited significant antibacterial activity in response to some of the tested strains,Bacillus subtilis,B.megaterium,Escherichia coli,Clostridium perfringens,Micrococcus tetragenus,and no activity against the strain of MRSA(methicillin-resistantStaphylococcus aureus)[7].
A six-membered ring lactone(118)was isolated from the water extracts of the soil fungusT.flavus[34].Two lactones(119and120)were isolated from an endophytic fungus,a close relative ofTalaromycessp.,found in association withCedrus deodara.They displayed a range of cytotoxicities against human cancer cell lines(HCT-116,A-549,HEP-1,THP-1,and PC-3),and induced apoptosis in HL-60 cells,as evidenced by fluorescence and scanning electron microscopy studies[35].In the course of screening for apoptosis inducers in ras dependent Ba/F3-V12 cells,a new active compound,rasfonin(121)was isolated from the fermented mycelium ofTalaromycessp.3656-A1.The cytotoxic activity indicated that rasfonin induced cell death in Ba/F3-V12 cells in an IL-3-free medium containing Dex(2×10-7M)with an IC50of 0.16 μg/mL and no cell death was observed in the presence of IL-3 at concentrations less than 1.25 μg/mL of rasfonin(IC501.8 μg/mL)[36].
Wortmannilactones E–H(122–125),from the culture of the soil filamentous fungusT.wortmannii,showed inhibitory activities against cathepsin B with IC50values of 4.3,6.5,13.0,and 6.0 μM,respectively[37].In screening for NADH-fumarate reductase inhibitors led to the isolation of a new ukulactone analog,ukulactone C(126),as a major polyene compound produced byTalaromycessp.FKI-6713.Ukulactone C possessed a potent inhibitory activity(IC5062 nM)against NADH-fumaratereductase of the roundwormAscaris suuminvitro[38].
D-Glucono-1,4-lactone(127)was obtained from the organic extracts of the soil fungusT.flavus[30].A new penicillic acid,coculnol(128)(five-membered ring lactone),was produced by a coculture ofFusarium solaniFKI-6853 andTalaromycessp.FKA-65.128showed an inhibitory effect(with IC50value of 283 μg/mL)against A/PR/8/34(H1N1)with weak cytotoxicity against MDCK cells(IC50value of 781 μg/mL)[39].Berkedienolactone(129)was isolated from the methanolic extracts of the green Chinese onion-derived fungusT.pinophilusAF-02[7].A new spiculisporic acid derivative,spiculisporic acid E(130),was isolated from the culture of the marine-sponge associated fungusT.trachyspermus(KUFA 0021)[40].The ethoxylated of spiculisporic acid E(131)was isolated from theT.panasenkoi[41].
2.3 Polyketides
Polyketides,pyrones,xanthones,are both a major focus of many research efforts and a rich source of novel metabolites ofTalaromyces.
Hydroxymethylmaltol(132)was isolated from the water extracts of the soil fungusT.flavus[34].Funicone(133)and a new funicone derivative,9,14-epoxy-11-deoxyfunicone (134),were isolated from the strainT.flausIFM52668.As the results of the antifungal assay showed that133had the characteristic inhibition against a human pathogenic filamentous fungus,A.fumigates(11-mm inhibition zone at 100 μg/disc),whereas134showed the weak antifungal activity againstA.niger(10-mm inhibition zone at 200 μg/disc)[16].Deoxyfunicone(135)and actofunicone(136)were obtained from the culture brothT.flavusFKI-0076 which isolated from a soil sample.135and136showed no effect on the growth ofCandida albicansup to 300 μM,and a slight inhibition(35%)was observed at that concentration for NG-012.But in the absence of the funicones,the IC50value of miconazole againstC.albicanswas calculated to be 19 μM,however,in combination with the funicones(50 μM),the IC50values were decreased to 1.6–3.7 μM,demonstrating that they reinforced the inhibitionC.albicansactivity of miconazole[20,21].
Abenzopyrone derivate137was isolated from the organic extracts of the soil fungusT.flavus[30].Benzopyrone derivatives138and139were isolated from a culture broth of a fungus,Talaromycessp.138exhibited the weak anti-HBV activity with an IC50value of 72.4 μM[42].
Two xanthones,norlichexanthone(140)and secalonic acid A(141),were obtained from the extract of the mangrove endophytic fungusTalaromycessp.ZH-154 which was isolated from the stem bark ofKandeliacandel(L.)Druce,Rhizophoraceae.141exhibited high activities against six selected strains.Moreover,in vitro cytotoxic activities indicated that141displayed very strong cytotoxicity against KB and KBv200 cell lines with IC50valuesof0.63 and 1.05 μg/mL,closed to those of the positive control(0.56 and 0.78 μg/mL).Whereas,the xanthone dimer141showed higher bioactivity than the xanthone monomer140[43].
A new isopentenylxanthenone,talaroxanthenone(142),was isolated from the culture broth and mycelia of a marine fungusTalaromycessp.strain LF458.142displayed potent acetylcholinesterase inhibitory activities with IC501.61 μM.Interestingly,phosphodiesterase PDE-4B2 was inhibited by compounds142(IC507.25 μM)[26].A new xanthone dimer talaroxanthone143was isolated fromTalaromycessp.which collected in the Amazonian rainforest from the medicinal plantDuguetia stelechantha[44].
Two newpolyketides,7-epiaustdiol(144)and 8-O-methylepiaustdiol(145),were obtained from the extract of the mangrove endophytic fungusTalaromycessp.ZH-154 which was isolated from the stem bark ofKandelia candel(L.)Druce,Rhizophoraceae.144showed significant inhibitory activity toPseudomonas aeruginosawith a MIC value of 6.25 μg/mL [43].Two new polyketides,TL-1 and-2(luteusins A and B)(146and147)with monoamine oxidase(MAO)inhibitory effect were isolated from an ascomyceteT.lutcus[45].Three new azaphilones,luteusins C,D,and E (148–150),together with146and147,were isolated from an Ascomycete,T.luteus.As regards MAO-inhibitory activity,the IC50values of146and147were 6.6 and 11 μM,respectively[46].
Kasanosins A(151)and B(152),novel azaphilones,were isolated from cultures ofTalaromycessp.derived from the seaweed.151and152selectively inhibited the activities of eukaryotic DNA polymerases β and λ (pols β and λ)in family X of pols,and151was a stronger inhibitor than152,and the IC50values of151on rat pol β and human polλ were 27.3 and 35.0 μM,respectively.And the results also suggested that151and152could identifythe inhibition between pols β,λ,and terminal deoxynucleotidyl transferase(TdT)in family X[47].Kasanosin C(153)and entonaemin A(154)were isolated from the solid fermentation ofTalaromycessp.T1BF derived from the old bast tissue ofTaxus yunnanensis[48].A known polyketide(155)was isolated from the strainT.wortmanii[49].Deacetylisowortmin(156)was isolated from the endophytic fungusT.wortmanniiLGT-4[50].
A new azaphilone derivative,monomethyl-(+)-mitorubrin(157),was isolated from the ascomata ofT.ardifaciensderived from the paddy soil from Bhaktapur,Nepal[51].Four new chlorinated azaphilones,helicusins A–D(158–161),were isolated fromT.helices.158–161showed weak MAO-inhibitory effects[52].Diazaphilonic acid(162)was obtained fromT.flavusPF1195.162inhibited DNA amplification by polymerase chain reaction(PCR)withThermus thermophilusDNA polymerase and the IC50value was 2.6 μg/mL.162dosedependently inhibited the telomerase activity of MT1(human leukemia)and almost completely inhibited the activity at 50 μM.But162showed no antimicrobial activity[53].
2.4 Quinones
Three pigments,emodin(163),ω-hydroxyemodin(164),and emodic acid(165),were obtained from the strainT.avellaneus[54].Emodin,erythroglaucin(166),and catenarin(167),were isolated from the strainT.stipitatus[55].A new atropisomer,biemodin(168),as well as five known metabolites(165and169–172),was isolated from the strainT.wortmannii,an endophyte ofAloe vera.169and171exhibited considerable antibiotic activity against Gram positive pathogenic bacteria with MIC values ranging between 4 and 16 μg/mL.168also showed strong activity against Gram positive bacteria,especially against MRSA,but was less active compared to compounds169and171[49].Emodin(163)and skyrin(169)were also isolated from the extract of the mangrove endophytic fungusTalaromycessp.ZH-154 derived fromKandelia candel(L.)Druce[43].Skyrin(169)was also isolated from the strainT.wortmannii,an endophyte ofAloe vera[56].
Two bisdihydroanthracenone atropodiastereomeric pairs,homodimeric flavomannin A (173)and flavomannin B(174),two new unsymmetrical dimers175and176,and two new mixed dihydroanthracenone/anthraquinone dimers177and178,were isolated fromT.wortmannii,an endophyte ofAloe vera.The compounds exhibited antibacterial activity,including(multi)drugresistant clinical isolates and compounds173–178were predominantly active againstStaphylococci,with MIC values from 4 to 8 μg/mL.Reporter gene analyses indicated induction of the SOS response for some of the derivatives,suggesting interference with DNA structure or metabolism.But the compounds showed no cytotoxic activity,encouraging their further evaluation as potential starting points for antibacterial drug development[56].
Two new tricyclic polyketides,vanitaracin A(179)and B(180),were isolated from a culture broth of a fungus,Talaromycessp.179and180were evaluated for anti-HBV activity using HBV-susceptible HepG2-hNTCP-C4 cells and179exhibited the strong anti-HBV activity with an IC50value of 10.5 μM[42].Stemphyperylenol(181)was isolated from the extract of the mangrove endophytic fungusTalaromycessp.ZH-154,and showed inhibitory activity againstSarcina ventriculiwith a MIC value of3.12 μg/mL,lower than that of ampicillin(12.5 μg/mL)[43].
2.5 Steroids and Terpenoids
A steroid182was isolated from the genus ofTalaromycessp.T1BF for the first time which isolated from an endophyte fromTaxus yunnanensisby chromatography techniques[57].A new natural product 3-acetyl ergosterol 5,8-endoperoxide(183)was isolated from the culture of the marine-sponge associated fungusT.trachyspermus(KUFA 0021)[40].Secovironolide(184)was purified from the culture broth ofT.wortmanniand is the first example of a furanosteroid scaffold bearing a five-membered B ring.Additional known viridian derivatives(185–188,190)were isolated,including the new epoxide containing compound,epoxyvirone(189).Isolates were tested and showed only weak MAO inhibitory activity[50].
A new nardosinane-type sesquiterpene,talaflavuterpenoid A(191),wasisolatedfromthewetlandsoil-derivedfungusT.flavusBYD07-13.191was tested for the cytotoxic activity against five human tumor cell lines and the antimicrobial activity,however,191showed no cytotoxic(IC50>40 μM)and antimicrobial activities(MIC>1.0 mg/mL)[58].Four new norsesquiterpene peroxides,named talaperoxides A–D(192–195),as well as a known analogue,steperoxide B(196),had been isolated from a mangrove endophytic fungus,T.flavus.Cytotoxic activitiesof192–196were evaluated in vitro against human cancer cell lines MCF-7,MDA-MB-435,HepG2,HeLa,andPC-3.193and195showed activity against the five human cancer cell lines with IC50values between0.70 and 2.78 μg/mL[59].
2.6 Others
(-)-Epoformin(197)and(1S*,3R*,5R*)-3-methyl-2-oxabicyclo[3.3.1]nonan-7-one(198)were isolated from an endophytic fungusTalaromycessp.,found in association withCedrusdeodara.The sulforhodamine B cytotoxicity assay indicated that197was found to be the most active followed by compound198[35].Four new spiroketaltalaromycins(199–202)had been isolated from the strainT.stipitatus[60].A new metabolite,trachyspic acid(203)that inhibited heparanase,was isolated from the culture broth ofT.trachyspermusSANK 12191.Its structure was determined from NMR spectral analyses and chemical reactions as a tricarboxylic acid derivative containing a spiroketal.The IC50value of trachyspic acid against heparanase was 36 μM[61].
A novel benzene derivative(204)was isolated from a culture broth of a fungus,Talaromycessp.,and it was evaluated for anti-HBV activity using HBV-susceptible HepG2-hNTCP-C4 cells,but204exhibited the weak anti-HBV activity[42].5-Hydroxymethylfurfural(205)and two benzene derivatives206and207were isolated from the organic extracts of the soil fungusT.flavus[30].207was also evaluated for its ability to inhibit HIV-1 integrase in coupled and strand-transfer assays and the data indicated that207with IC50values of 19 μM in the coupled assay and 25 μM in the strand-transfer assay[62].Two benzene derivatives208and209from the genus ofTalaromycessp.T1BF which isolated from an endophyte fromTaxus yunnanensisby chromatography techniques[57].
Three diphenyl ether derivatives including two new natural products,tenelates A(210)and B(211),together with the known compound,tenellic acid C(212),were isolated from the mangrove endophytic fungusTalaromycessp.(SBE-14),from the South China Sea[63].Three new derivatives ofp-hydroxybenzoic acid(213–215)had been isolated from the culture filtrate ofT.derxii[64].
A new long-chain dicarboxylic acid,2-hydroxyradiclonic acid(216),and four known compounds,benzoic acid(217),(Z)-3-phenylpropenal(218),2-formyl-3,5-dihydroxy-4-methylbenzoic acid(219),and radiclonic acid(220),were isolated from the methanolic extracts of the green Chinese onion-derived fungusT.pinophilusAF-02.216showed significant antibacterial activities againstE.coli[7].
A new antibiotic,fosfonochlorin(221),was found in the culture filtrate of four strains of fungi freshly isolated from soil samples includingT.flavus.The biological activity indicated that it was active againstProteus mirabilisandP.vulgarisand weakly active againstSalmonella enteritidis,Klebsiella pneumoniaeandProvidencia rettgeri,and its synergistic effect with glucose-6-phosphate was observed onStaphylococcus aureusandEscherichia coli[65].
A new antifungal antibiotic,named talaron,had been isolated from the culture ofT.vermiculatus(M-3224).Talaron is water-soluble acidic polysaccharide containing nitrogen and phosphorus,and its molecular weight was estimated to be 7000–8000.Talaron had strong fungicidal activity against filamentous dermatophytes and exhibited inhibitory activity against the spore germination ofTrichophyto asteroidesand showed cytotoxic effect at 1 mcg/mL on HeLa cells,and at 0.2 mcg/mL on mouse embryo fibroblast cells,but no antibacterial activity[66].
3 Conclusions
TheTalaromycesgenus includes many species with a variety of uses,some of which are important in the food products and agriculture.Since,several anthraquinone metabolites fromT.avellaneuswere isolated in 1965[54],lots of secondary metabolites described in this report were obtained from this genus fungi which from a soil sample,from the plant,or from a marine sponge.The 221 compounds,including 43 alkaloids and peptides,88 esters,31 polyketides,19 quinones,15 steroid and terpenoids,and 25 other structure compounds,described in this review were isolated from 28 species,which 19 species have been determined and 9 species were not given the specific names(Table 1).The secondary metabolite studies were mainly performed on the commonest species of the genus,T.flavus[3].The stereochemistry of many compounds was determined via circular dichroism spectrum[7],Mosher’s analysis method[8],Marfey’s method[13],a single-crystal X-ray diffraction experiment using Cu Kα radiation[59],or quantum chemical calculation[6].Those fungi were cultivated with varying media:potato dextrose,barley grains[10],rice[25],WSP30[26],ISP2broth[44],or other modified medium.
In the early years of secondary metabolite of those genus species research was less emphasis on biological testing,but increasingly there has been a focus on the biological properties of these compounds.Inhibitory activity to tumour cells[17],bacteria[7],fungi[10],HBV[42],nematode[8],HIV-1-integrase[62],caspase-3[11],mosquito larval[14],5-lipoxygenase[27],and other activities were performed.Some of the isolated compounds have been used as pigments.
Studies on total synthesis and biotransformation of some of those compounds have been described.Structure–activity relationships have also been undertaken.Recently,there has been great interest in the study of biosynthesis genes based on secondary metabolites from the genus.However,systematic secondary metabolites–biosynthesis genes relationship might give insight into the molecular level,seem to be absent.This might be a promising direction in which work in the field of the secondary constituents from this genus fungi may proceed.
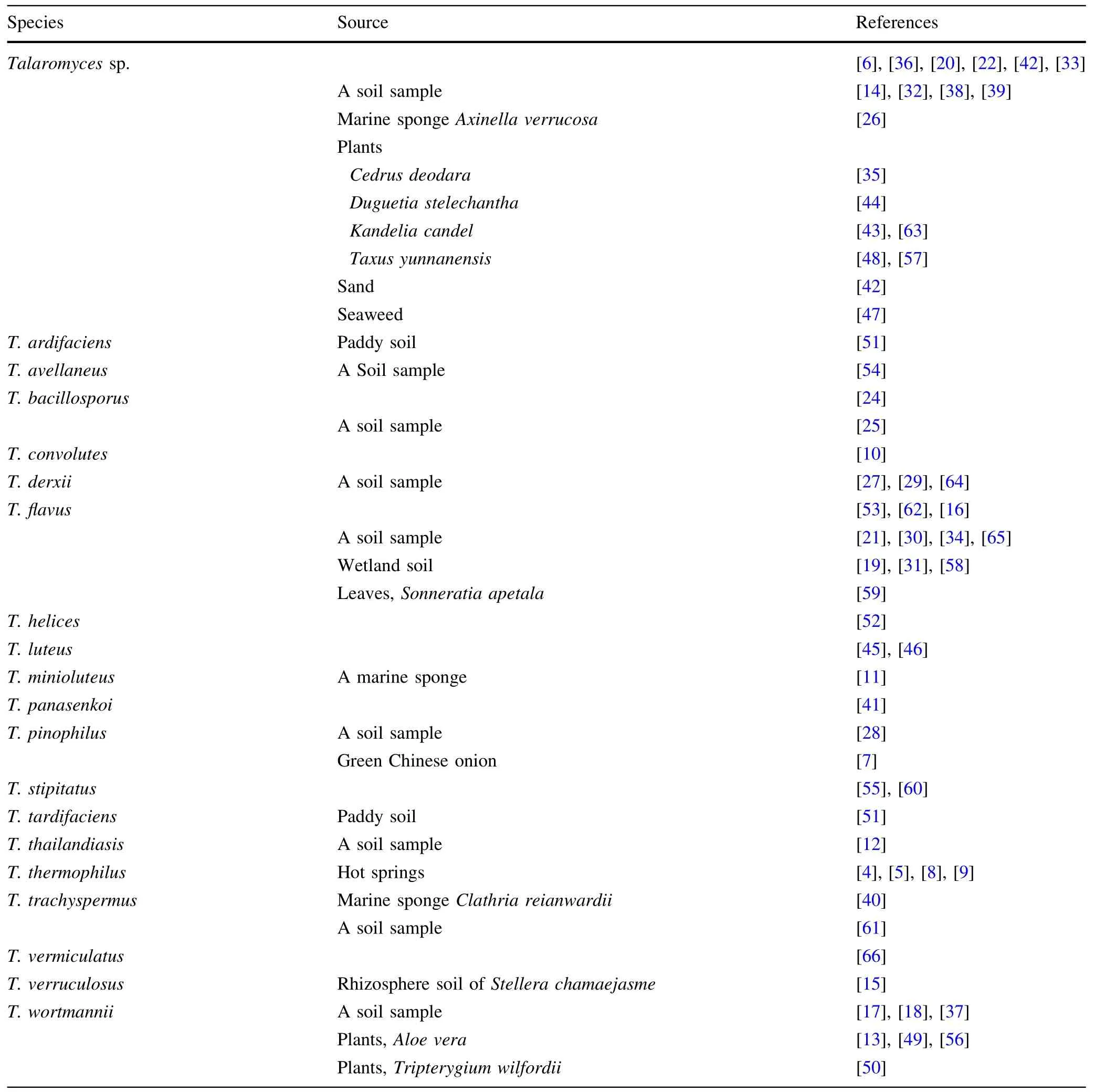
Table 1 The source of Talaromyces species
AcknowledgmentsAuthors are thankful to financially supporting by the National Natural Science Foundation of China(Nos.21202075 and 21272103),the 111 Project,the Scientific Research Foundation for Returned Overseas Students(No.45),the Scienti fic Research Ability Training of Undergraduate Students Majoring in Chemistry by the Two Patters Based on the Tutorial System and Top Students Project(J1103307).
Compliance with ethical standards
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License(http://creativecommons.org/licenses/by/4.0/),which permits unrestricted use,distribution,and reproduction in any medium,provided you give appropriate credit to the original author(s)and the source,provide a link to the Creative Commons license,and indicate if changes were made.
1.J.Pitt,inPenicilliumandTalaromyces:Introduction,Penicillium,Encyclopedia of Food Microbiology(2nd edn)(2014),pp.6–13
2.N.E.A.El-Naggar,S.A.Haroun,E.A.Oweis,A.A.Sherief,Prep.Biochem.Biotechnol.45(7),712–729(2015)
3.B.Proksa,Chem.Pap.64(6),696–714(2010)
4.Y.S.Chu,X.M.Niu,Y.L.Wang,J.P.Guo,W.Z.Pan,X.W.Huang,K.Q.Zhang,Org.Lett.12(19),4356–4359(2010)
5.J.P.Guo,J.L.Tan,Y.L.Wang,H.Y.Wu,C.P.Zhang,X.M.Niu,W.Z.Pan,X.W.Huang,K.Q.Zhang,J.Nat.Prod.74(10),2278–2281(2011)
6.H.B.Yang,F.Li,N.Y.Ji,Chin.J.Oceanol.Limnol.(2015).doi:10.1007/s00343-015-4316-2
7.M.M.Zhai,H.T.Niu,J.Li,H.Xiao,Y.P.Shi,D.L.Di,P.Crews,Q.X.Wu,J.Agric.Food Chem.63(43),9558–9564(2015)
8.J.P.Guo,C.Y.Zhu,C.P.Zhang,Y.S.Chu,Y.L.Wang,J.X.Zhang,D.K.Wu,K.Q.Zhang,X.M.Niu,J.Am.Chem.Soc.134(50),20306–20309(2012)
9.X.M.Niu,L.Chen,Q.Yue,B.L.Wang,J.X.Zhang,C.Y.Zhu,K.Q.Zhang,G.F.Bills,Z.Q.An,Org.Lett.16(14),3744–3747(2014)
10.S.Suzuki,T.Hosoe,K.Nozawa,K.I.Kawai,T.Yaguchi,S.I.Udagawa,J.Nat.Prod.63(6),768–772(2000)
11.S.Ngokpol,W.Suwakulsiri,S.Sureram,K.Lirdprapamongkol,T.Aree,S.Wiyakrutta,C.Mahidol,S.Ruchirawat,P.Kittakoop,Mar.Drugs13(6),3567–3580(2015)
12.T.Dethoup,L.Manoch,A.Kijjoa,M.Pinto,L.Gales,A.M.Damas,A.M.S.Silva,G.Eaton,W.Herz,J.Nat.Prod.70(7),1200–1202(2007)
13.R.Bara,A.H.Aly,V.Wray,W.H.Lin,P.Proksch,A.Debbab,Tetrahedron Lett.54(13),1686–1689(2013)
14.T.Morino,M.Nishimoto,A.Masuda,S.Fujita,T.Nishikiori,S.Saito,J.Antibiot.48(12),1509–1510(1995)
15.F.Miao,R.Yang,D.D.Chen,Y.Wang,B.F.Qin,X.J.Yang,L.Zhou,Molecules17(12),14091–14098(2012)
16.S.I.Komai,T.Hosoe,T.Itabashi,K.Nozawa,K.Okada,G.M.de Campos Takaki,M.Chikamori,T.Yaguchi,K.Fukushima,M.Miyaji,K.I.Kawai,Mycotoxins54(1),15–19(2004)
17.Y.S.Dong,J.S.Yang,H.Zhang,J.Lin,X.Ren,M.Liu,X.H.Lu,J.G.He,J.Nat.Prod.69(1),128–130(2006)
18.D.Jones,H.A.Anderson,J.D.Russell,A.R.Fraser,Trans.Br.Mycol.Soc.83(4),718–721(1984)
19.J.W.He,Z.Q.Mu,H.Gao,G.D.Chen,Q.Zhao,D.Hu,J.Z.Sun,X.X.Li,Y.Li,X.Z.Liu,X.S.Yao,Tetrahedron70(29),4425–4430(2014)
20.H.Y.Wang,M.Alai,H.Tomoda,S.Omura,Chin.J.Antibiot.26(2),89–93(2001)
21.M.Aral,H.Tomoda,T.Okuda,H.Y.Wang,N.Tabata,R.Masuma,Y.Yamaguchi,S.O¯mura,J.Antibiot.55(2),172–180(2002)
22.C.H.Sun,M.Alai,H.Tomoda,S.Omura,Chin.J.Antibiot.27(2),80–83(2002)
23.G.Schlingmann,L.Milne,G.T.Carter,Tetrahedron58(34),6825–6835(2002)
24.M.Yamazaki,E.Okuyama,Chem.Pharm.Bull.28(12),3649–3655(1980)
25.T.Dethoup,L.Manoch,A.Kijjoa,M.S.J.Nascimento,P.Puaparoj,A.M.S.Silva,G.Eaton,W.Herz,Planta Med.72(10),957–960(2006)
26.B.Wu,B.Ohlendorf,V.Oesker,J.Wiese,S.Malien,R.Schmaljohann,J.F.Imhoff,Mar.Biotechnol.17(1),110–119(2015)
27.K.Suzuki,K.Nozawa,S.Nakajima,S.I.Udagawa,K.I.Kawai,Chem.Pharm.Bull.40(5),1116–1119(1992)
28.M.Kawaguchi,R.Uchida,S.Ohte,N.Miyachi,K.Kobayashi,N.Sato,K.Nonaka,R.Masuma,T.Fukuda,T.Yasuhara,H.Tomoda,J.Antibiot.66(3),179–189(2013)
29.K.Suzuki,K.Nozawa,S.I.Udagawa,S.Nakajima,K.I.Kawai,Phytochemistry30(6),2096–2098(1991)
30.W.A.Ayer,J.S.Racok,Can.J.Chem.68(11),2085–2094(1990)
31.J.W.He,D.P.Qin,H.Gao,R.Q.Kuang,Y.Yu,X.Z.Liu,X.S.Yao,Molecules19(12),20880–20887(2014)
32.H.Hayadhi,Y.Oka,K.Kai,K.Akiyama,Biosci.Biotechnol.Biochem.76(4),745–748(2012)
33.H.Hayadhi,Y.Oka,K.Kai,K.Akiyama,Biosci.Biotechnol.Biochem.76(9),1765–1768(2012)
34.W.A.Ayer,J.S.Racok,Can.J.Chem.68(11),2095–2101(1990)
35.M.Kumar,M.Qadri,P.R.Sharma,A.Kumar,S.S.Andotra,T.Kaur,K.Kapoor,V.K.Gupta,R.Kant,A.Hamid,S.Johri,S.C.Taneja,R.A.Vishwakarma,S.Riyaz-Ul-Hassan,B.A.Shah,J.Nat.Prod.76(2),194–199(2013)
36.T.Tomikawa,K.Shin-Ya,K.Furihata,T.Kinoshita,A.Miyajima,H.Seto,Y.Hayakawa,J.Antibiot.53(8),848–850(2000)
37.Y.S.Dong,J.Lin,X.H.Lu,Z.H.Zheng,X.Ren,H.Zhang,J.G.He,J.S.Yang,Helv.Chim.Acta92(3),567–574(2009)
38.S.Kaifuchi,M.Mori,K.Nonaka,R.Masuma,S.O¯mura,K.Shiomi,J.Gen.Appl.Microbiol.61(2),57–62(2015)
39.K.Nonaka,T.Chiba,T.Suga,Y.Asami,M.Iwatsuki,R.Masuma,S.O¯mura,K.Shiomi,J.Antibiot.68(8),530–532(2015)
40.D.Kumla,T.Dethoup,S.Buttachon,N.Singburaudom,A.M.S.Silva,A.Kijjoa,Nat.Prod.Comm.9(8),1147–1150(2014)
41.H.Fujimoto,Y.Jisai,Y.Horie,M.Yamazaki,Mycotoxins27,15–19(1988)
42.H.Matsunaga,S.Kamisuki,M.Kaneko,Y.Yamaguchi,T.Takeuchi,K.Watashi,F.Sugawara,Bioorg.Med.Chem.Lett.25(19),4325–4328(2015)
43.F.Liu,X.L.Cai,H.Yang,X.K.Xia,Z.Y.Guo,J.Yuan,M.F.Li,Z.G.She,Y.C.Lin,Planta Med.76(2),185–189(2010)
44.H.H.F.Koolen,L.S.Menezes,M.P.Souza,F.M.A.Silva,F.G.O.Almeida,A.Q.L.de Souza,A.Nepel,A.Barison,F.H.da Silva,D.E.Evangelista,A.D.L.de Souza,J.Braz.Chem.Soc.24(5),880–883(2013)
45.H.Fujimoto,T.Matsudo,A.Yamaguchi,M.Yamazaki,Heterocycles30(1),607–616(1990)
46.E.Yoshida,H.Fujimoto,M.Yamazaki,Chem.Pharm.Bull.44(2),284–287(1996)
47.T.Kimura,M.Nishida,K.Kuramochi,F.Sugawara,H.Yoshida,Y.Mizushina,Bioorg.Med.Chem.16(8),4594–4599(2008)
48.L.Q.Li,Y.G.Yang,Y.Zeng,C.Zou,P.J.Zhao,Molecules15(6),3993–3997(2010)
49.R.Bara,A.H.Aly,A.Pretsch,V.Wray,B.G.Wang,P.Proksch,A.Debbab,J.Antibiot.66(8),491–493(2013)
50.H.E.Ding,Z.D.Yang,L.Sheng,S.Y.Zhou,S.Li,X.J.Yao,K.K.Zhi,Y.G.Wang,F.Zhang,Tetrahedron Lett.56(48),6754–6757(2015)
51.K.Nozawa,R.Saito,S.I.Udagawa,S.Nakajima,K.I.Kawai,Phytochemistry39(3),719–721(1995)
52.E.Yoshida,H.Fujimoto,M.Baba,M.Yamazaki,Chem.Pharm.Bull.43(8),1307–1310(1995)
53.Y.Tabata,S.Ikegami,T.Yaguchi,T.Sasaki,S.Hoshiko,S.Sakuma,K.Shin-Ya,H.Seto,J.Antibiot.52(4),412–414(1999)
54.S.Natori,F.Sato,S.I.Udagawa,Chem.Pharm.Bull.13(3),385–386(1965)
55.G.W.van Eijk,Experientia29(5),522–523(1973)
56.R.Bara,I.Zerfass,A.H.Aly,H.Goldbach-Gecke,V.Raghavan,P.Sass,A.Ma´ndi,V.Wray,P.L.Polavarapu,A.Pretsch,W.H.Lin,T.Kurta´n,A.Debbab,H.Bro¨tz-Oesterhelt,P.Proksch,J.Med.Chem.56(8),3257–3272(2013)
57.L.Q.Li,Y.G.Yang,Y.Zeng,C.Zou,P.J.Zhao,Guangxi Zhiwu31(5),699–701(2011)
58.J.W.He,H.X.Liang,H.Gao,R.Q.Kuang,G.D.Chen,D.Hu,C.X.Wang,X.Z.Liu,Y.Li,X.S.Yao,J.Asian Nat.Prod.Res.16(10),1029–1034(2014)
59.H.X.Li,H.B.Huang,C.L.Shao,H.R.Huang,J.Y.Jiang,X.Zhu,Y.Y.Liu,L.Liu,Y.J.Lu,M.F.Li,Y.C.Lin,Z.G.She,J.Nat.Prod.74(5),1230–1235(2011)
60.N.J.Phillips,R.J.Cole,D.G.Lynn,Tetrahedron Lett.28(15),1619–1622(1987)
61.H.Shiozawa,M.Takahashi,T.Takatsu,T.Kinoshita,K.Tanzawa,T.Hosoya,K.Furuya,S.Takahashi,J.Antibiot.48(5),357–362(1995)
62.S.B.Singh,H.Jayasuriya,R.Dewey,J.D.Polishook,A.W.Dombrowski,D.L.Zink,Z.Q.Guan,J.Collado,G.Platas,F.Pelaez,P.J.Felock,D.J.Hazuda,J.Ind.Microbiol.Biotechnol.30(12),721–731(2003)
63.F.Liu,Q.Li,H.Yang,X.L.Cai,X.K.Xia,S.P.Chen,M.F.Li,Z.G.She,Y.C.Lin,Magn.Reson.Chem.47(5),453–455(2009)
64.K.Nozawa,M.Takada,S.I.Udagawa,S.Nakajima,K.I.Kawai,Phytochemistry28(2),655–656(1989)
65.M.Takeuchi,M.Nakajima,T.Ogita,M.Inukai,K.Kodama,K.Furuya,H.Nagaki,T.Haneishi,J.Antibiot.42(2),198–205(1989)
66.K.Mizuno,A.Yagi,M.Takada,K.Matsuura,K.Yamaguchi,K.Asano,J.Antibiot.27(7),560–563(1974)
16 November 2015/Accepted:7 December 2015/Published online:8 January 2016
M.-M.Zhai·J.Li·C.-X.Jiang ·Q.-X.Wu(✉)
State Key Laboratory of Applied Organic Chemistry,College of Chemistry and Chemical Engineering,Lanzhou University,
Lanzhou 730000,People’s Republic of China
e-mail:wuqx@lzu.edu.cn
Y.-P.Shi·D.-L.Di·Q.-X.Wu
Key Laboratory of Chemistry of Northwestern Plant Resources and Key Laboratory for Natural Medicine of Gansu Province,Lanzhou Institute of Chemical Physics,Chinese Academy of
Sciences,Lanzhou 730000,People’s Republic of China
P.Crews
Department of Chemistry and Biochemistry,University of
California Santa Cruz,Santa Cruz,CA 95064,USA
 Natural Products and Bioprospecting2016年1期
Natural Products and Bioprospecting2016年1期
- Natural Products and Bioprospecting的其它文章
- Isoprenylated Flavonoids with PTP1B Inhibition from Macaranga denticulata
- Antiproliferative Triterpenoid Saponins from Leptaulus citroides Baill.from the Madagascar Rain Forest
- The Use of a Combination of RDC and Chiroptical Spectroscopy for Determination of the Absolute Configuration of Fusariumin A from the Fungus Fusarium sp.
- Koninginins N-Q,Polyketides from the Endophytic Fungus Trichoderma koningiopsis Harbored in Panax notoginseng
- Formosins A–F:Diterpenoids with Anti-microbial Activities from Excoecaria formosana
