Detection of Multi-drug Resistant Acinetobacter Lwoffii Isolated from Soil of Mink Farm*
SUN Na, WEN Yong Jun, ZHANG Shu Qin, ZHU Hong Wei, GUO Li,WANG Feng Xue, CHEN Qiang, MA Hong Xia, and CHENG Shi Peng,#
Detection of Multi-drug Resistant Acinetobacter Lwoffii Isolated from Soil of Mink Farm*
SUN Na1, WEN Yong Jun1, ZHANG Shu Qin1, ZHU Hong Wei1, GUO Li1,WANG Feng Xue1, CHEN Qiang2, MA Hong Xia3, and CHENG Shi Peng1,#
There were 4 Acinetobacter lwoffii obtained from soil samples. The antimicrobial susceptibility of the strains to 16 antimicrobial agents was investigated using K-B method. Three isolates showed the multi-drug resistance. The presence of resistance genes and integrons was determined using PCR. The aadA1, aac(3')-IIc, aph(3')-VII,aac(6')-Ib, sul2, cat2, floR, and tet(K) genes were detected, respectively. Three class 1 integrons were obtained. The arr-3-aacA4 and blaPSE-1 gene cassette, which cause resistance to aminoglycoside and beta-lactamase antibiotics. Our results reported the detection of multi-drug resistant and carried resistant genes Acinetobacter lwoffii from soil. The findings suggested that we should pay close attention to the prevalence of multi-drug resistant bacterial species of environment.
Acinetobacter lwoffii (formerly known as Mimapolymorpha or Acinetoba ctercalcoaceticus var. lwoffii) is a gram-negative aerobic bacillus that is seen as a member of the normal flora that inhabits the oropharynx, human skin, and the perineum in approximately 20%-25% of the healthy individuals[1]. But besides that, these organisms are generally ubiquitous and are found frequently in soil, water,dry environments and hospitals. The main transmission way were contact and airborne transmission. Acinetobacter lwoffii is an opportunistic pathogens and can cause infections when patients have their immune system impaired or compromised[2-3]. For Acinetobacter lwoffii, antibiotic therapy can reduce the duration and severity of the illness. However, with the long-term overuse of antimicrobials, the resistant Acinetobacter lwoffii has prevailed all over the world. A number of studies have reported the antimicrobial susceptibility of Acinetobacter lwoffii[4-7]. In this study, 4 Acinetobacter lwoffii strains were isolated from soil of mink farm in Jilin Province in China in 2015. The antimicrobial susceptibility and the presence of the resistance genes were determined in the isolates.
Samples were collected from soil and seeded on Trypticase Soy Agar plates incubated at 37 °C. Suspected colonies with typical morphology were selected from each sample, and identified using PCR assay with 16s rRNA universal primers (F:5'-AGAGTTTGATCCTGGCTCAG-3'; R: 5'-GGTTACCT TGTTACGACTT-3'). Antimicrobial susceptibility of the isolates was tested by Kirby-Bauer antibiotic testing method (K-B method) in accord with the guidelines of the Clinical Laboratory Standards Institute (CLSI). The antimicrobial agents included ampicillin,cefazolin, ceftriaxone, ceftazidime, cefoxitin,streptomycin, kanamycin, gentamicin, amikacin,ciprofloxacin, levofloxacin, norfloxacin, chloramphenicol, florfenicol, tetracycline and trimethoprimsulfamethoxazole. The PCR amplification of beta-lactamase genes (blaTEM, blaSHV),aminoglycoside resistance genes [aac(3')-IIc,aac(3')-IV, aph(3')-II, aph(3')-IV, aph(3')-VII, aadA1,aadA2, aac(6')-Ib], chloramphenicol and florfenicol resistance genes (cat1, cat2, cmlA, cmlB, floR),sulfonamides resistance genes(sul2, sul3),tetracycline resistance genes [tet(A), tet(B), tet(C),tet(K), tet(M)], class 1, 2, 3 integrase genes and a variable region of class 1 integrons was carried out. The PCR products were tested using electrophoresis with a 1.2% agarose gel, and visualized under UV light. The positive PCR products were sequenced and confirmed using the BLAST program.
A total of 4 Acinetobacter lwoffii were obtained from soil samples, named A1, A2, A3, and A4. The strains were resistant to at least nine antimicrobial agents except A1 isolates. The results of the antimicrobial susceptibility of the isolates were shown in Table 1. The results suggested that the Acinetobacter lwoffii isolates were multi-drug resistance. The high antimicrobial resistance in isolates may be due to the overuse and abuse of antimicrobials in practice and disease treatment. In the past, Acinetobacter spp. were regarded as saprophytes of little clinical significance. However,with the use of antibiotics in clinical practice and agriculture, antibiotic resistance-related communityand hospital-acquired Acinetobacter infections have emerged with increasing frequency[8-9]. The drug resistance of bacteria was formed under the antibiotic selection pressure for a long time. The multi-resistant Acinetobacter lwoffii isolates detected in this study pleads for the cautious use of antimicrobials.
Among the aminoglycoside resistance genes, 4 aminoglycoside modifying enzyme genes were identified. The aadA1 gene, aac(3')-IIc gene,aph(3')-VII gene and aac(6')-Ib gene which cause resistance to streptomycin, gentamicin and kanamycin were detected in 1, 2, 2, 1 isolates; the sul2, cat2, floR, tet(K) genes which cause resistance to trimethoprim-sulphamethoxazole, chloramphenicol, florfenicol, tetracycline were detected in 3, 4,3, 1 isolates, respectively. Three class 1 integrons were obtained in 4 strains. The arr-3-aacA4 gene cassette and blaPSE-1 gene cassette, which cause resistance to aminoglycoside and beta-lactamase antibiotics. Integrons play an important role in the dissemination of antimicrobial resistance and have been found to be common in Enterobacteriaceae. The results of drug resistance and the distribution of resistance genes and integrons were shown in Table 1. The figures of PCR results were in supplemental material (Figure S1, See the www.besjournal.com). There were many research about Acinetobacter lwoffii carried resistance genes,but the strains carried resistance genes isolated from soil were little. Antibiotics used to the environment were limited. So it was assumed that the multi-drug resistant and carried resistance genes Acinetobacter lwoffii strains of soil might derived from urine or feces of minks. It does't matter where the strains dated from, it will lead to great effects on the environment. Multi-drug resistant bacteria from human and animal were needed to test, but the bacteria in the environment were also taken into account.
In conclusion, the present study reported,detection of multi-drug resistant and carried resistance genes Acinetobacter lwoffii isolated from soil of mink farm. The strains represents a case or the early stage of a wide dissemination trend. The dissemination of the strains may cause human and animals infection. If the resistance genes were transmitted to other bacterial which infected human or animals, it would bring the difficulty to clinical treatment. Therefore, the findings highlight the need for monitor the prevalence of multi-drug resistant Acinetobacter spp. and other bacterial species of environment.
The authors are grateful to the professional editing service ‘LetPub' for improving writing and language style. WEN Yong Jun, ZHANG Shu Qin, and ZHU Hong Wei collected the samples and isolated the strains. GUO Li and WANG Feng Xue performed the analysis of antimicrobial susceptibility of the isolates. CHEN Qiang and MA Hong Xia detected the resistance genes. SUN Na and CHENG Shi Peng oversaw the experiment design and the whole experimental progress. We also wish to express our gratitude to WEN Yong Sheng, WANG Meng Hang, and LIU Ying for their assistance with experiments.
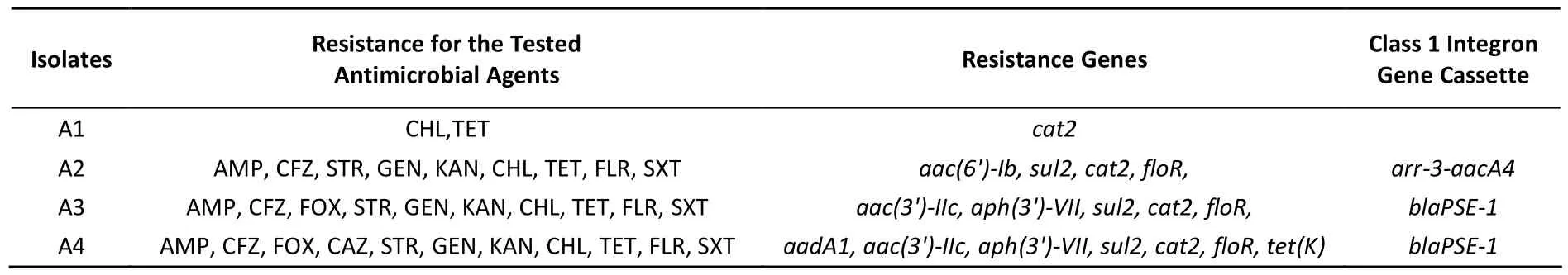
Table 1. The Results of Multidrug Resistance in Isolates
#Correspondence should be addressed to CHENG Shi Peng, Tel/Fax: 86-0431-81919840, E-mail: tcscsp@ 126.com
Biographical note of the first author: SUN Na, female,born in 1983, Doctor, Research Assistant, majoring in bacterial identify and drug resistance research.
1. Rathinavelu S, Zavros Y, Merchant JL. Acinetobacter lwoffii infection and gastritis. Microbes Infect, 2003; 5, 651-7.
2. Ku SC, Hsueh PR, Yang PC, et al. Clinical and microbiological characteristics of bacteremia caused by Acinetobacter lwoffii. Ero J Clin Microbiol Infect Dis, 2000; 19, 501-5.
3. Regalado NG, Martin G, Antony SJ. Acinetobacter lwoffii:bacteremia associated with acute gastroenteritis. Travel Med Infect Dis, 2009; 7, 316-7.
4. Wang Y, Wu C, Zhang Q, et al. Identification of New Delhi metallo-beta-lactamase 1 in Acinetobacter lwoffii of food animal origin. PLoS One, 2012; 7, e37152.
5. Singla P, Sikka R, Deeep A, et al. Co-production of ESBL and AmpC beta-Lactamases in Clinical Isolates of A. baumannii and A. lwoffii in a Tertiary Care Hospital From Northern India. J Clin Diagn Res, 2014; 8, 16-9.
6. Mittal S, Sharma M, Yadav A, et al. 'Acinetobacter lwoffii an emerging pathogen in neonatal ICU'. Infect Disord Drug Targets, 2015; 15, 184-8.
7. Xu L, Lv R, Wang H, et al. Fitness costs of blaNDM-1bearing plasmid pNDM-BJ01 in Acinetobacter lwoffii. Wei Sheng Wu Xue Bao, 2013; 53, 99-104. (In Chinese)
8. BergogneBerezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: Microbiological, clinical, and epidemiological features. Clinical Microbiology Reviews, 1996;9, 148.
9. Guardabassi L, Dalsgaard A, Olsen JE. Phenotypic characterization and antibiotic resistance of Acinetobacter spp. isolated from aquatic sources. Journal of Applied Microbiology,1999; 87, 659-67.
10.3967/bes2016.068
April 1, 2016;
*This research was supported by the Innovation Project Foundation of Chinese Academy of Agricultural Sciences (20140204066NY); Development Plan of Science and Technology in Jilin Province (20150520128JH); and the Special Fund for Agro-Scientific Research in the Public Interest from the Ministry of Agriculture, China (201303042).
1. State Key Laboratory for Molecular Biology of Special Economic Animals, Institute of Special Economic Animals and Plants,Chinese Academy of Agricultural Sciences CAAS, Changchun 130112, Jilin, China; 2. JL TEYAN Biological Technology Limited Liability Company, Changchun 130000, Jilin, China; 3. Jilin Agricultural University, Changchun 130000, Jilin, China
Accepted: May 4, 2016
 Biomedical and Environmental Sciences2016年7期
Biomedical and Environmental Sciences2016年7期
- Biomedical and Environmental Sciences的其它文章
- ldeal Cardiovascular Health Metrics and Coronary Artery Calcification in Northern Chinese Population:A Cross-sectional Study*
- p21 is Responsible for Ionizing Radiation-induced Bypass of Mitosis*
- Autophagy Attenuates MnCl2-induced Apoptosis in Human Bronchial Epithelial Cells*
- Lack of Association between rs4331426 Polymorphism in the Chr18q11.2 Locus and Pulmonary Tuberculosis in an Iranian Population*
- Green Tea Polyphenols Alleviate Autophagy Inhibition Induced by High Glucose in Endothelial Cells*
- Cytotoxic Responses and Apoptosis in Rat Kidney Epithelial Cells Exposed to Lead*
