Skin whitening and anti-corrugation activities of glycoprotein fractions from liquid extracts of boiled sea cucumber
So Jung Kim, So Yun Park, Sun-Mee Hong, Eun-Hye Kwon, Taek-Kyun Lee✉
1Gyeongbuk Institute for Marine Bio-Industry, Uljin 36315, Republic of Korea
2South Sea Environment Research Department, Korea Institute of Ocean Science and Technology, Geoje 53201, Republic of Korea
Skin whitening and anti-corrugation activities of glycoprotein fractions from liquid extracts of boiled sea cucumber
So Jung Kim1#, So Yun Park2#, Sun-Mee Hong1, Eun-Hye Kwon1, Taek-Kyun Lee2✉
1Gyeongbuk Institute for Marine Bio-Industry, Uljin 36315, Republic of Korea
2South Sea Environment Research Department, Korea Institute of Ocean Science and Technology, Geoje 53201, Republic of Korea
ARTICLE INFO
Article history:
in revised form 3 June 2016
Accepted 15 Junce 2016
Available online 20 October 2016
Sea cucumber
Glycoprotein
Cytotoxicity
Tyrosinase
Elastase
Objective: To determine skin whitening and wrinkle improvement efficacy, glycoprotein fractions were extracted from liquid extracts of boiled sea cucumber and their effects on tyrosine and elastase inhibitory activities were assayed. Methods: Fractions above and below 50 kDa (>50 kDa and <50 kDa) were extracted via a series of steps invovling: boiling,filtering, desalting and freeze drying. Cytotoxicity, skin whitening and wrinkle-removing effects of boiled liquid were determined. Results: Our MTT data showed that neither glycoprotein fraction of boiled liquid induces cellular cytotoxicity up to a concentration of 10 mg/mL treatment of the mouse melanoma cell line, B16F10, with 10 mg/mL >50 kDa enhanced tyrosinase and elastase inhibitory activities by 50.84% and 28.78%, respectively. Correlations of the >50 kDa concentration with tyrosinase inhibitory (R2=0.968) and elastase inhibitory (R2=0.983) efficacy were significant. Conclusions: >50 kDa glycoprotein fraction isolated from liquid extracts of boiled sea cucumber, which can serve as a functional cosmetic ingredient for whitening and wrinkle improvement of skin.
Document heading doi: 10.1016/j.apjtm.2016.08.001
1. Introduction
Sea cucumbers are marine animals from the class Holothuroidea. About 1 200 known species of sea cucumber exist in worldwide,with using in food or folk remedies [1]. Sea cucumbers have traditionally been employed as medicines owing to their therapeutic efficacy in various diseases such as hypertension, asthma,rheumatism, incisions, burns, erectile dysfunction, and constipation. Over 300 compounds, including saponins, cerebrosides,polysaccharides, and peptides, have been identified in sea cucumber extract [2-5], with anti-angiogenic, anti-coagulation, anti-bacterial,anti-oxidation, and anti-tumor activities [6-10]. In terms of food science, sea cucumbers contain 90.5% water, 3.2% protein, 0.2% fat, and 3.5% minerals [11, 12]. In addition, these animals are known as “ginseng of the sea” due to their high saponin content known for its efficacious ginseng ingredient [6, 13]. The key components of dried sea cucumber are glycoprotein and chondroitin. Earlier research has focused on mucous-like polysaccharides and sulfate chondroitin structure [14]. In particular, glycoprotein, derived from marine organisms, has been shown to enhance anticancer activities and immune functions [15, 16]. However, the mechanisms underlying the effects of these compounds on diseases at the molecular level remain to be elucidated.
Liquid extracts of boiled sea cucumber are byproducts of the dried cucumber production process. In general, the majority of this liquid is discarded, and results in large economic losses and raising concerns regarding environmental pollution. In addition, despite the high content of functional ingredients (for instance, polyphenol,protein, and glycogen), most studies on sea cucumbers to date have been related to food product development [17] and limited research has focused on extracting functional ingredients from the aspect ofsea cucumber cooked liquid or developing functional products that based on the constituents.
In this study, liquid extracts of boiled sea cucumber were extracted from Korean sea cucumbers and glycoproteins that separated by purifying the fractionated liquid based on molecular size. We evaluated the skin whitening and anti-corrugation activities of each glycoprotein fraction, with a view to determining the feasibility of using functional ingredients from sea cucumber cooked liquid for preparation of cosmetics.
2. Materials and methods
2.1. Production of liquid extracts
We used approximately 200 g of Korean sea cucumbers (Stichopus japonicus, Selenka) harvested on the day of testing. Animals were eviscerated via a 3 cm incision from the anus to the center of the back and cleaned using fresh water for removal of salt and impurities. About 50 kg of eviscerated sea cucumbers were placed in boiling water and the mixture continuously stirred, and causes leakage of liquid extracts. Heating temperature was maintained at 80 -90 ℃. After removal of solid materials, the collected liquid extracts were stored at -20 ℃ until separation of glycoprotein fractions.
2.2. Separation and purification of glycoprotein fractions
After defrosting of the liquid extracts at 4 -6 ℃, the first decompression and filtration steps were conducted using a SUPRAcap depth filter (K500 P grade), followed by second depression and filtration to remove impurities using a SUPRAcap depth filter 10 inch capsule. Glycoprotein fractions with molecular weights of above and below 50 kDa (>50 kDa and <50 kDa) were separated using by an ultrafiltration system (BTR), lyophilized, and used for bioactivity assay. The separation and purification steps of glycoprotein fractions are shown in Figure 1.
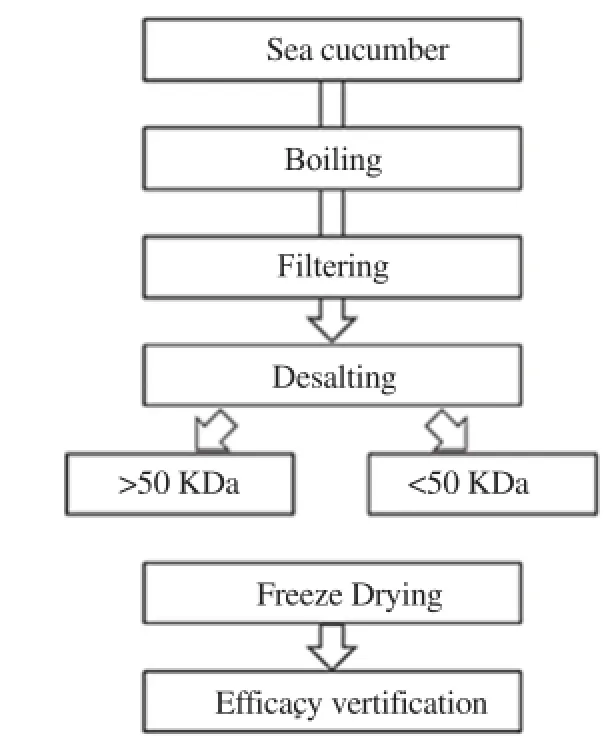
Figure 1. Schematic diagram of isolation of glycoprotein fractions from sea cucumber.
2.3. Cytotoxicity assay of glycoprotein fractions
Mouse melanoma cell line suspensions (100 μL; 5 000 cells/well)(B16-F10, ATCC LOT. 60508145) were dispensed into a 96-well plate and preincubated at 37 ℃ in 5% CO2for 24 h for cell adherence to the well plate. The control contained 100 μL Dulbecco's Modified Eagle Medium (ATCC) without cells. A 10 μL aliquot of fractions of >50 kDa and <50 kDa of liquid extracts was added to each well for each concentration (0.1, 1, 2, and 5 mg/mL). For the negative control, 10 μL medium was added and the mixtures reacted at 37 ℃in 5% CO2for 24 h. After incubation of each well with 10 μL CCK-8 solution for 1h, absorbance was measured at 450 nm.
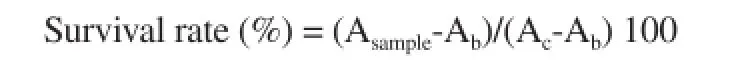
Asample: sample absorbance, Ab: blank absorbance (value obtained with sample containing no cells), Ac: negative control absorbance(value obtained with distilled water containing cells). Experiments were performed in triplicate, and the results recorded as means ± SD.
2.4. Measurement of tyrosinase inhibitory activity
Tyrosinase inhibitory activity was assessed according to the method of Kim et al. [18]. A 500 μL cell suspension (50 000 cells/ well) was dispensed into a 24-well plate (B16-F10, ATCC LOT. 60508145), and cells adhered by preincubating at 37 ℃ in 5% CO2for 24 h. Fractions >50 kDa and <50 kDa of liquid extracts and vitamin C were added to each well for all the concentrations tested and incubated at 37 ℃in 5% CO2for 24 h. The pellet obtained from trypsin treatment was dissolved in 1% Triton X-100 PBS (0.5 mL),mixed with 0.2% L-DOPA (0.1 M) and 0.5 mL sodium phosphate buffer, and incubated at 37 ℃ for 2 h. Absorbance was measured at 490 nm.
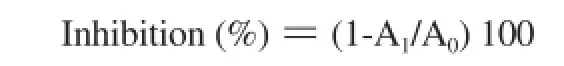
(A0=control group absorbance, A1=experimental group absorbance). Experiments were performed in triplicate, and the results recorded as means ± SD.
2.5. Elastase inhibitory activity assay
The elastase inhibitory effect was measured using the method of Kim et al. [19]. Fractions >50 kDa and <50 kDa of liquid extracts and vitamin C were prepared, and an aliquot of 10 μL pipetted into a test tube and mixed with 50 μL porcine pancreas elastase (10 μg/mL)solution dissolved in 50 mM Tris-HCl buffer (pH 8.6). Next, 100 μL N-succinyl-(L-Ala)3-p-nitroanilide (0.5 mg/mL) dissolved in 50 mM Tris-HCl buffer (pH 8.6) was added as a substrate and reacted for 20 min. Elastase inhibitory activity was determined as the reduction in absorbance between groups with and without sample solutions.

(A0=control group absorbance, A1=experimental group absorbance). Experiments were performed in triplicate, and the results recorded as means ± SD.
2.6. Statistical analysis
Statistical analysis was performed using SPSS statistical software. Normality and homogeneity of data were verified using analysis of variance (ANOVA), and the differences among experimental groups evaluated using one-way ANOVA and Duncan's multiple range tests.
3. Results
3.1. Cytotoxicity of glycoprotein fractions
Cytotoxicity of sea cucumber liquid extracts was analyzed using the MTT assay (Figure 2). Cells were treated with fractions above and below 50 kDa (>50 kDa and <50 kDa) derived from liquid extracts at concentrations of 0.1, 1, 2, and 5 mg/mL, and viability measured. Cell viability remained >90% with all treatment concentrations, suggesting no significant cytotoxicity of sea cucumber glycoprotein fractions at the cellular level. In contrast,positive controls (treated with 100% ethanol) showed <10% cell viability.
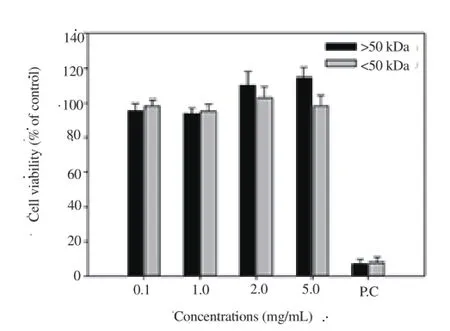
Figure 2. Cytotoxicity assay of fractions isolated from sea cucumber.
3.2. Measurement of tyrosinase inhibitory activity
Upon treatment of cells with >50 kDa and <50 kDa fractions at concentrations of 0.1, 1, 5, and 10 mg/mL, the inhibitory activity of the >50 kDa fraction was 20.54%-50.84% higher, whereas that of the <50 kDa fraction was not significant (Figure. 3). In particular,tyrosinase inhibitory activity (50.84%) of the >50 kDa fraction (10 mg/mL) was high accounting for 73% activity (69.95%; P<0.05) of ascorbic acid used as the control.
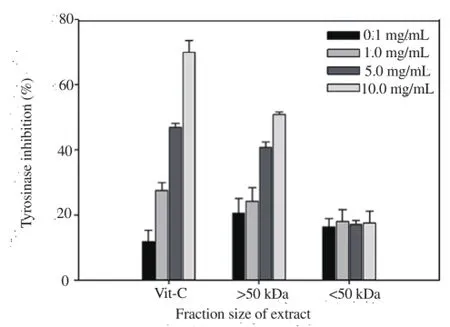
Figure 3. Tyrosinase inhibitory activities of fractions isolated from sea cucumber.
3.3. Analysis of elastase inhibitory activity
Upon treatment of cells with fractions >50 kDa and <50 kDa fractions at concentrations of 0.1, 1.0, 5.0, and 10.0 mg/mL, the elastase inhibitory activity of the >50 kDa fraction increased significantly to 5.75%-28.78% higher, and that of the <50 kDa fraction to 3.49%-10.40% (Figure 4). In particular, the elastase inhibitory activity of the >50 kDa fraction (28.78%) was over 2.8 times that of the <50 kDa fraction (10.40%) (P<0.05).
3.4. Correlation analysis
Correlation analyses of tyrosinase and elastase inhibitory activities with concentrations of >50 kDa glycoprotein fraction were conducted. High correlation was observed between >50 kDa levels and tyrosinase inhibitory activity (R2=0.968; Y=3.096X+21.652) as well as elastase inhibitory activity (R2=0.983, Y=2.468X+4.731), as shown in Figures 5 and 6.
4. Discussion
In this study glycoproteins from sea cucumber cooked liquid were separated according to molecular size and their cytotoxicity,whitening and anti-corrugation activities determined via MTT,tyrosinase inhibitory and elastase inhibitory assays, respectively,MTT assay is a cytotoxicity analysis technique that measures the amount of MTT-formazan, which generated from MTT by dehydrogenase present in mitochondria [20]. The whitening activity of the liquid extract obtained from boiling sea cucumber was determined by measuring dopachrome formed from L-tyrosine or L-DOPA at 490 nm [21]. Tyrosinase inhibition activity, which is effectively associated with suppression of melanin synthesis in the skin, and is useful for the development of skin whitening products[22]. Glycoprotein fractions of boiled sea cucumber (>50kDa and <50 kDa) at concentrations of 0.1, 1.0, 2.0, and 5.0 mg/mL induced no cytotoxicity and tyrosinase inhibitory activity of the >50 kDa fraction was higher than that of the <50 kDa fraction (Figure 3),suggesting a significant whitening effect.
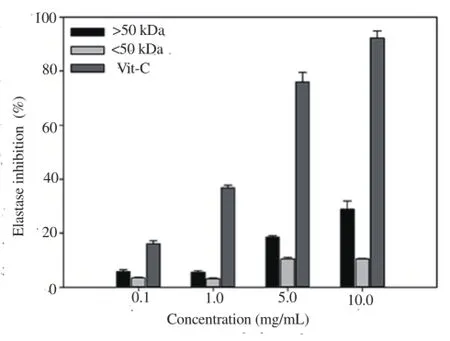
Figure 4. Elastase inhibitory activities of fractions isolated from sea cucumber.
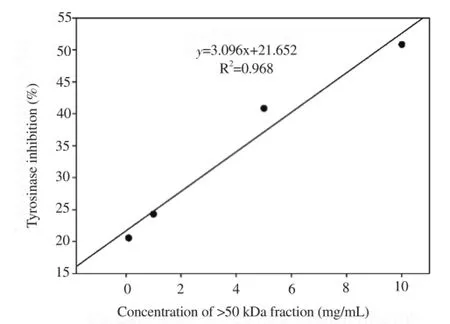
Figure 5. Correlation between the >50 kDa concentration and tyrosinase inhi-bitory activity.
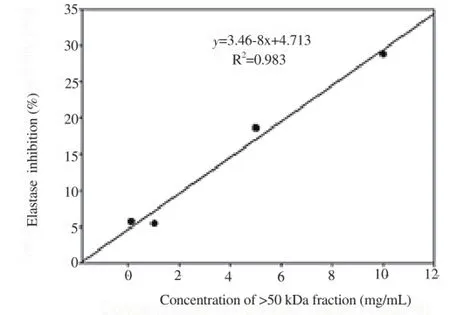
Figure 6. Correlation between the >50 kDa concentration and elastase inhibitory activity
The connective tissues and elastic fibers of tendons mainly consist of elastin, and the elastase is key enzyme hydrolyzing the protein of key connective tissue substrate. Inhibition of elastase activity prevents damage to elastic fibers due to UV or free radicals by protecting the skin from aging [23]. In our experiments, elastase inhibitory activity of the >50 kDa fraction was higher with increasing concentrations(Figure 4), clearly indicative of anti-corrugation activity. The anti-corrugation activity of glycoprotein, derived from sea cucumber steaming liquid, is proposed to be attributable to its antioxidant activity [24]. However, further studies are warranted to confirm this theory. Our findings collectively support the utility of sea cucumber extracts >50 kDa of as a valuable natural marine resource with potential functional application as a whitening and anti-corrugation ingredients in cosmetics.
Conflict of interest statement
The authors disclose no conflicts.
Acknowledgements
This research was supported by the Public Welfare & Safety Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013M3A2A1067529).
[1] Domínguez-Godino JA, Slater MJ, Hannon CGonzález-Wangüermert M. A new species for sea cucumber ranching and aquaculture: Breeding and rearing of Holothuria arguinensis. Aquaculture 2015; 438: 122-128.
[2] Antonov AS, Avilov SA, Kalinovsky AI, Dmitrenok PS, Kalinin VI,Taboada S, et al. Triterpene glycosides from Antarctic sea cucumbers III. Structures of liouvillosides A4 and A5, two minor disulphated tetraosides containing 3-O-methylquinovose as terminal monosaccharide units from the sea cucumber Staurocucumis liouvillei (Vaney). Nat Prod Res 2011;25(14): 1324-1333.
[3] Guo Y, Ding Y, Xu F, Liu B, Kou Z, Xiao WZhu J, et al. Systems pharmacology-based drug discovery for marine resources: An exampleusing sea cucumber (Holothurians). J Ethnopharmacol 2015; 165: 61-72.
[4] Wu M, Huang R, Wen D, Gao N, He J, Li Z, et al. Structure and effect of sulfated fucose branches on anticoagulant activity of the fucosylated chondroitin sulfate from sea cucumber Thelenata ananas. Carbohydr Polym 2012; 87(1): 862-868.
[5] Pérez-Vega JA, Olivera-Castillo L, Gómez-Ruiz JA, Hernández-Ledesma B. Release of multifunctional peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus). J Funct Foods 2013; 5(2): 869-877.
[6] Xue Z, Li H, Wang X, Li X, Liu Y, Sun J, et al. A review of the immune molecules in the sea cucumber. Fish Shellfish Immunol 2015; 44(1): 1-11.
[7] Chen S, Hu Y, Ye X, Li G, Yu G, Xue C, et al. Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochim Biophys Acta 2012; 1820(7): 989-1000.
[8] Wang Z, Zhang H, Yuan W, Gong W, Tang H, Liu B, et al. Antifungal nortriterpene and triterpene glycosides from the sea cucumber Apostichopus japonicus Selenka. Food Chem 2012; 132(1): 295-300.
[9] Liu X, Sun Z, Zhang M, Meng X, Xia X, Yuan W, et al. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr Polym 2012; 90(4): 1664-1670.
[10] Al Marzouqi N, Iratni R, Nemmar A, Arafat K, Ahmed Al Sultan M,Yasin J, et al. Frondoside A inhibits human breast cancer cell survival,migration, invasion and the growth of breast tumor xenografts. Eur J Pharmacol 2011; 668(1-2): 25-34.
[11] Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods-a review. Mar Drugs 2011;9(10) 1761-1805.
[12] Wu F-J, Xue Y, Liu X-F, Xue C-H, Wang J-F, Du L, Takahashi K, et al. The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem Int 2014; 64: 9-17.
[13] Althunibat OY, Ridzwan BH, Taher M, Daud JM, Jauhari Arief Ichwan S, Qaralleh H, et al. Antioxidant and cytotoxic properties of two sea cucumbers, Holothuria edulis lesson and Stichopus horrens Selenka. Acta Biol Hung 2013; 64(1): 10-20.
[14] Mour o PAS, Pereira MS, Pav o MSG, Mulloy B, Tollefsen DM, Mowinckel MC, et al. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. J Biol Chem 1996;271(39): 23973-23984.
[15] Gao N, Wu M, Liu S, Lian W, Li ZZhao J. Preparation and characterization of O-acylated fucosylated chondroitin sulfate from sea cucumber. Mar Drugs 2012; 10(8): 1647-1661
[16] Fedorov SN, Ermakova SP, Zvyagintseva TNStonik VA. Anticancer and cancer preventive properties of marine polysaccharides: some results and prospects. Mar Drugs 2013; 11(12): 4876-4901
[17] Lee M-O, Oh H-G, Park S-H, Lee H-A, Sul J-D, Song J, et al. Skin Whitening Effects of Sanguisorba officinalis and Stichopus japonicus. Lab Anim Res 2010; 26(2): 127-132.
[18] Kim S, Woo S, Yun H, Yum S, Choi E, Do JR, et al. Total phenolic contents and biological activities of Korean seaweed extracts. Food Sci Biotechnol 2005; 14: 798-802.
[19] Kim JH, Byun JC, Bandl AKR, Hyun CG, Lee NH. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J Med Plant Res 2009; 3(11): 914-920.
[20] Van de Loosdrecht AA, Nennie E, Ossenkoppele GJ, Beelen RH,Langenhuijsen MM. Cell mediated cytotoxicity against U 937 cells by human monocytes and macrophages in a modified colorimetric MTT assay. A methodological study, J Immunol Methods 1991; 141(1): 15-22.
[21] Suh SS, Seo HH, Lee JH, Hwang J, Park M, Moh SH et al. Anticorrugation activity of micosporine-like amino acid mixtures from Chlamydomonas sp. J Korea Acad Indust Soci 2014; 15(8): 5393-5399.
[22] Suh SS, Hwang J, Park M, Park HS, Lee TK. Phenol Content,Antioxidant and Tyrosinase Inhibitory Activity of Mangrove Plants in Micronesia. Asian Pac J Trop Med 2014; 7(7): 531-535.
[23] Azmi N, Hashim P, Hashim DM, Halimoon N, Majid MN. Anti-elastase,anti-tyrosinase and matrix metalloproteinase-1 inhibitory activity of earthworm extracts as potential new anti-aging agent. Asian Pac J Trop Biomed 2014; 4(Suppl 1): S348-S352.
[24] Rafiquzzaman SM, Kim EY, Kim YR, Nam TJ, Kong IS. Antioxidant activity of glycoprotein purified from Undaria pinnatifida measured by an in vitro digestion model. Int J Biol Macromol 2013; 62: 265-272.
10 April 2016
#These authors contributed equally to this work.
So Jung Kim, Gyeongbuk Institute for Marine Bio-Industry, Uljin 36315, Republic of Korea.
So Yun Park, South Sea Environment Research Department, Korea Institute of Ocean Science and Technology, Geoje 53201, Republic of Korea
Dr. Taek-Kyun Lee, South Sea Environment Research Department, Korea Institute of Ocean Science and Technology, Geoje 53201,Republic of Korea.
Tel.: +82-55-639-8630
Fax: +82-55-639-8509
E-Mail: tklee@kiost.ac.kr
 Asian Pacific Journal of Tropical Medicine2016年10期
Asian Pacific Journal of Tropical Medicine2016年10期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of emodin on mobility signal transduction system of gallbladder smooth muscle in Guinea pig with cholelithiasis
- Effect and mechanism of Irbesartan on occurrence of ventricular arrhythmias in rats with myocardial ischemia through connexin43 (cx43)
- Arginine kinase in Toxocara canis: Exon-intron organization, functional analysis of site-directed mutants and evaluation of putative enzyme inhibitors
- Who should be checked for hepatitis C virus infection in endemic areas?
- Epidemiology of the outbreak, vectors and reservoirs of cutaneous leishmaniasis in Mali: A systematic review and meta-analysis
- Melaleuca quinquinervia (Cav.) S.T. Blake (Myrtales: Myrtaceae): Natural alternative for mosquito control
