Melaleuca quinquinervia (Cav.) S.T. Blake (Myrtales: Myrtaceae): Natural alternative for mosquito control
Maureen Leyva, Leidys French-Pacheco, Felipe Quintana, Domingo Montada, Mayda Castex, Ariel Hernandez, María del Carmen Marquetti
1Institute Tropical Medicine ‘Pedro Kouri', Cuba
2Chemical Research Center, Morelos, Mexico
3Center for Integration and Social Welfare, Cuba
Melaleuca quinquinervia (Cav.) S.T. Blake (Myrtales: Myrtaceae): Natural alternative for mosquito control
Maureen Leyva1✉, Leidys French-Pacheco2, Felipe Quintana3, Domingo Montada1, Mayda Castex1, Ariel Hernandez1, María del Carmen Marquetti1
1Institute Tropical Medicine ‘Pedro Kouri', Cuba
2Chemical Research Center, Morelos, Mexico
3Center for Integration and Social Welfare, Cuba
ARTICLE INFO
Article history:
in revised form 23 June 2016
Accepted 19 July 2016
Available online 20 October 2016
Melaleuca quinquinervia
Larvicidal activity
Adulticidal activity
Essential oils
Aedes spp.
Culex quinquefasciatus
Objective: To evaluate an essential oil with larvicide, adulticide and growth inhibitory activity against Aedes aegypti, Aedes albopictus and Culex quinquefasciatus mosquitoes, of medical importance. Methods: Standardized methodology by WHO was used to determine the levels of susceptibility of mosquito larvae exposed to the essential oil. To evaluate the adulticide activity with the essential oil at different doses, bottles were impregnated according to the methodology CDC. To determine the development inhibitory activity of Melaleuca quinquinervia (M. quinquinervia) oil in three mosquito species, third instar larvae were exposed to the LC50and LC90dose (calculated for each population) of M. quinquinervia oil in glass containers with a capacity of 500 mL. After 24 h exposure, the dead larvae were discarded. The mortality of larvae and pupae were recorded on a daily basis. Results: The calculated LC50indicates an order of effectiveness of preferential oil for Culex quinquefasciatus (LC50=0.002 1%), Aedes aegypti (LC50=0.004 7%) and Aedes albopictus (LC50=0.004 9%). Conclusions: The adulticide activity was achieved with impregnated bottles at 40 and 50 mg/mL with the three mosquitoes species. In larvae, a growth inhibition was detected when exposed to sublethal doses. The results indicate that M. quinquinervia is a plant with promising environmentally sustainable source for vector control.
Document heading doi: 10.1016/j.apjtm.2016.07.034
1. Introduction
Culex quinquefasciatus (Cx. quinquefasciatus), Aedes albopictus(Ae. albopictus) and Aedes aegypti (Ae. aegypti) are within the entomological fauna of mosquitoes, vectors responsible for the maintenance and transmission of viruses such as West Nile[1]Dengue[2], Chikungunya[3] and Zika[4] in America region. Increasing population densities, high levels of unemployment, poverty, and lackof political will, among others, are factors that favor the circulation and maintenance of these endemic diseases in communities of developing countries[5].
Unfortunately for many of vector-borne diseases, vaccine candidates are not available, being the chemical control the basic measure to reduce mosquito populations and thus the incidence of disease. This reduction is usually transient without a thorough understanding of ecological aspects of the species responsible for transmission: behavior, habitat preferences and susceptibility to insecticides applied, among others[6-8].
The increased resistance to synthetic insecticides in these vectors of medical importance in Cuba was detected at laboratory level since the late 1990s[7,9]. While it is true that in periods of high infestation, insecticide application is the measure that reduces the incidence of diseases, it is also necessary to study alternativecontrol, with a comprehensive approach to delay or reduce the resistance to synthetic insecticides in field mosquito's population.
Melaleuca quinquinervia (M. quinquinervia) (Cav.) S.T. Blacke(Myrtales: Myrtaceae) is a plant considered for vector control,because of its proven insecticidal activity, being widely distributed,having complementary utilities such as medicinal or food and be environmentally sustainable. This plant is a tree widely distributed in Asian countries and parts of America[10-12].
After its introduction in Cuba, this plant has become an invasive specie in the wetlands of the Ciénaga of Zapata, where has caused losses to the botanical biodiversity as a result of its high reproductive potential and its ability to withstand long dry periods[13].
Despite its adverse effects on the ecosystem, its essential oil and various extracts show a potential as antiprotozoal[14], antimalarial[15],bactericide, fungicide[16] and insect repellent[17].
Because of the importance that requires the search for natural alternatives for vector control, the objective was to determine the insecticidal activity of essential oil of (M. quinquinervia) on the vector species Ae. albopictus, Cx. quinquefasciatus and Ae. aegypti.
2. Materials and methods
2.1. Mosquito populations in the study
Population Fraga 2012: Ae. albopictus specie collected at larval stage in Reparto Juan de Dios Fraga in the municipality of La Lisa,Havana Cuba in 2012.
Population Regla 2013: Cx. quinquefasciatus specie collected in larval and pupal stage in the municipality Regla, Havana, Cuba, in 2013.
Population Rockefeller: Ae. aegypti, laboratory reference strain susceptible to insecticides, supplied by the Center for Disease Control and Prevention (CDC), San Juan, Puerto Rico, 1996.
Population Marianao 2013: Ae. aegypti specie strain collected in larval and pupal stages in 2013, during an intensive phase of vector control in the municipality of Marianao, Havana, Cuba.
The mosquito colonies were stabilized in the department insectarium Vector Control Institute of Tropical Medicine ‘Pedro Kouri' Cuba, following the methodology of the Manual Technical Indications Insectarium[18] available on http://blue/bvs1/monografias/ manual.pdf.
2.2. Bioassays to determine larvicidal activity of essential oils
Standardized methodology by WHO was used to determine the levels of susceptibility of mosquito larvae exposed to the essential oil[19].
The stock solutions were prepared in absolute ethanol. One ml of each concentration was added in a volume of 99 mL of water. A total of 125 larvae instar third or early fourth instar of Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus, for each concentration were added. Each concentration had a control. Four replicates were done. Mortality was determined after 24 h and lethal concentrations (LC50and LC90) were calculated using the Probit test implemented in SPSS(version 11 for Windows).
2.3. Bioassays to determine the development inhibitory activities
Third instar larvae of three mosquito species were exposed to the LC50and LC90dose (calculated for each population) of M. quinquinervia oil in glass containers with a capacity of 500 mL. For each species, 150 larvae in 500 mL of water were used as control. After 24 h of exposure, the dead larvae were discarded and the survivors were added fishmeal as food. They remained in the water exposure until they reached the pupa state.
The mortality of larvae and pupae were recorded on a daily basis. The surviving pupae were separated by sex in separate vials until adult emergence. For the analysis of data normality the Kolmogorov-Smirnov tests and Shapiro-Wilk were used. Multifactor ANOVA was applied to the analysis of daily mortality of each state for all species(Statistica 7). Tukey post hoc test was used to identify differences between dose and immature stages.
2.4. Bioassays to determine adulticidal activity by impregnating bottles at different concentrations
To evaluate the adulticide activity with the essential oil at different doses, bottles were impregnated according to the methodology proposed[20]. Glass bottles of 250 mL capacity with frosted glass cover were used. The bottles were impregnated with 1 mL of each concentration of the oil, rotating them in every way until the acetone used as a solvent was evaporated. The bottles were covered with aluminum foil and kept uncovered overnight. Subsequently, they were capped until used. For each evaluated concentration, one control and four replicates were used. Fifteen females aged three days without blood feeding of each species were exposed. Every 5 min for 1 h mosquitoes knocked down were recorded.
Data of the doses that produced mosquito knockdown were analyzed with Probit test implemented in SPSS (version 11 for Windows).
3. Results
The M. quinquinervia essential oil showed larvicidal activity at the concentrations evaluated in three mosquito species (Table 1). The oil was more effective in Cx. quinquefascitus followed by Ae. aegypti and Ae. albopictus according to the LC50values calculated.
In assessing the adulticide activity, in the Rockefeller population a 100% knockdown after 30 min was obtained when using the dose of40 mg/mL. In populations of Marianao 2013, Regla 2013 and Fraga 2012, an increase in dose to 50 mg/mL was required to achieve the knockdown of 100% of the population in 30 minutes. The response of the three field populations was homogeneous in front of this oil despite the slight increase in the dose to achieve its toxic effect(Figure1).

Table 1Larvicidal activity of M. quinquinervia in populations of Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus used in the study.
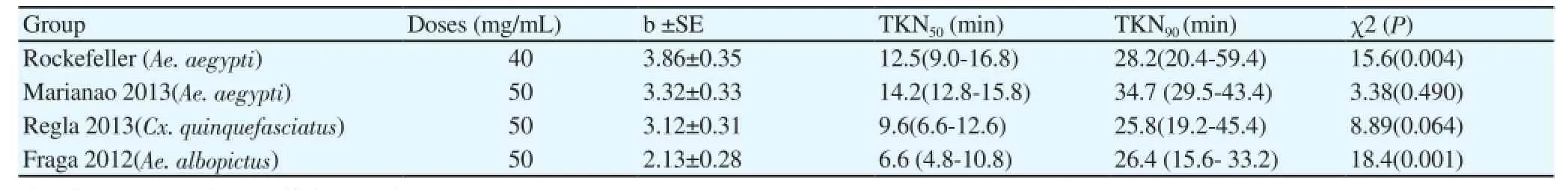
Table 2Knockdown times obtained in populations of Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus used in the study, by impregnating bottles with M. quinquinervia oil.
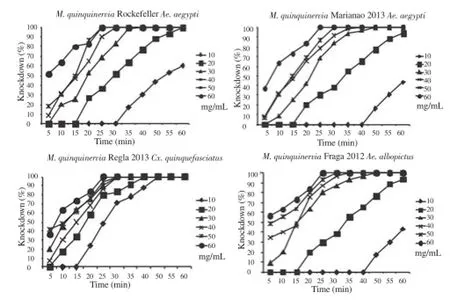
Figure 1. Knockdown percentage obtained during one hour of exposure to different concentrations of M. quinquinervia oil in Ae. aegypti, Cx. quinquefasciatus and Ae. albopictus.
In Table 2 are shown knockdown time (TKN) calculated doses of 40 mg/mL for Rockefeller and 50 mg/mL in the rest of the evaluated populations. The times obtained (TKN50) suggest that the oil acts relatively quickly after exposure in any of the three species tested.
With respect to the inhibitory activity of development, significant difference between individuals exposed to each lethal concentration and the control (F=4.829 7, P=0.008 03) was found, which makes evident the toxic effect of M. quinquinervia oil in mosquito larvae of the three species studied.
The analysis of mortality among immature stages showed significant difference between larvae and pupae, and between pupae and adults in all the mosquito species (F=6.853 0, P=0.000 02). The greatest lethal effect occurred in Ae. albopictus, followed by Cx. quinquefasciatus and Ae. aegypti (Figure 2). Only 4% of the surviving pupae of three mosquito species reached the adult stage. Male mosquitoes emerged exceeded 3 times the number of females. Total N of individuals emerged was insufficient to study the effect of oil M. quinquinervia on fertility.
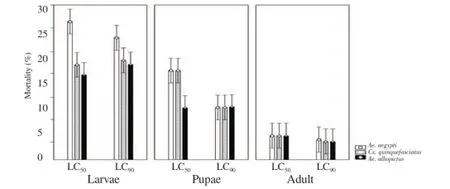
Figure 2. Mortality obtained by immature stages of mosquitoes Ae. albopictus, Cx. quinquefasciatus and Ae. aegypti species CL50and CL90exposed to doses M. quinquinervia oil.
Graphic obtained by a Multifactor Anova (F=2.089 3, P=0.0795 4). Error bars represent confidence intervals.
4. Discussion
It is understood as environmental sustainability: the exploitation of a biological system below its limit renewal without affecting adjacent diversity and ecosystem productivity[21]. A plant can be regarded as candidate for vector control, if in addition to its proven insecticidal activity, presents environmentally sustainable qualities. M. quinquinervia stands out among the 100 most harmful to the ecosystem and of greatest concern to botanical species in Cuba. It invades about 40 000 hectares in the swamps of Ciénaga of Zapata and Ciénaga of Majaguillar both in the province of Matanzas[22]. A form of exploitation of this renewable resource is to obtain its essential oil, which decrease the damage generated by their excessivegrowth[13].
The intensive search for alternative methods of vector controlling,and in this case, of plants with insecticidal activity have been focused mostly in plants where the essential oils and extracts have medicinal bioactivity or condiments utility[23-25]. The pesticide bioactivity depends largely on the botanical specie, extraction method, insects used and their susceptibility to synthetic insecticides[26,27].
WHO has not established diagnostic dose for the determination of the larvicidal activity of natural products. Authors like Komalamisra et al., 2005 suggest that a natural product with CL50≤50 mg/ L is active and if the LC50is between 50 mg/L and 100 mg/L is moderately active[28]. Moreover Ravi-Kiran et al., 2006[29] suggest that compounds with a CL50≤100 mg/L present a significant larvicidal activity. In all our studies we have LC50values below 50 mg/L, so the M. quinquinervia oil is active and has significant larvicidal activity for the species Culex and Aedes spp.
In numerous studies, insecticide action of plants is supported besides the bioassays, by enzymatic studies in the insects and chromatographic analysis that supporting the majority compound of the oils[30-33]. Authors recommended that because the mechanisms of action of secondary metabolites in many plants are different(inhibition of acetylcholinesterase, interrupting channels Na and K, blocking octopamine receptors) and similar to those used by synthetic insecticides in insects[34,35].
M. quinquenervia produces different chemotypes, mainly based on the proportion of monoterpenes and sesquiterpenes 1.8 cineol and viridiflorol[10-12]. In the chemical characterization of the essential oil used in our studies, it was determined monoterpenes containing 1.8 cineol, α-pinene, β-pinene, α-terpineol, limonene and hydroxylated sesquiterpenoid viridiflorol, as majority compounds, all in a superior composition to 1%[36]. Several authors attribute this presence of metabolites in the essential oil of M. quinquinervia, to the insecticide action found in oils from other plants[23,37].
There is no consensus on whether to attribute the insecticidal activity to the major components of oil, or one in particular. Certain metabolites isolated, produce an agonist effect when evaluated on their own, while others show a synergistic effect when combined with other components of oil[37,38].
In studies by Giatropoulus et al. 2012[39] with a strain of Ae. albopictus, the α and β- pinene were higher when they were CL50calculated for isolates and compared with CL50three citrus oils which were isolated. This result demonstrated the synergistic role of components within an essence. Kim et al,. 2008[40] found significant larvicide and adulticide activity of 1.8 cineol, compared with Culex pipiens, and Zahram et al 2011 at doses of 500 mg/mL detected larvicidal activity against this species and had not yet elapsed effective adulticide activity after 48 h of exposure[41]. Noleto-Diaz et al.(2015) although it doesn't evaluate isolated metabolites, of the five plants used in their study, Eugenia piauhiensis presents the lower CL50value and the monoterpenes 1.8 cineol, α-pinene, β -pinene, α-terpineol and viridiflorol were the majority compounds in its essence[42].
In any case, due to the criteria variability of specialists in the field,complementary studies with isolated metabolites should be made. However, the results obtained show the insecticidal activity of metabolites present in the oil.
Bio-responses to phytochemicals may differ between larvae and adults because the adult insect is physiologically stronger, what could justify the increase in adults CL50evaluated. There are papers in which the method of the impregnated bottles (CDC methodology)is used to evaluate the adulticide activity of plant oils. Articles that evaluate this type of activity are made by impregnating papers with solutions of essential oils or isolated metabolites but most without a standardized methodology[43,44]. The methodology of the bottles is a cheap, simple and easily applicable method under laboratory conditions and terrain.
In terms of adulticide activity of essential oils against mosquitoes,there are very few articles that allow comparison of results. In the Rockefeller population, with a dose of 40 mg/mL, the 100% knockdown of exposed females was obtained. The dose used in our work for the rest of the population (50 mg/mL =5%) is in the range of those used in other studies, e.g. experiments conducted with aereosoles of Melaleuca cajeputi[45]. The slight dose increase may be related to the fact that three of the populations studied were collected in a period of high pesticide application and were resistant to some groups of insecticides[46]. Therefore, they are likely to have increased levels of detoxifying enzymes and antioxidant mechanisms, which could influence the increase in dose. This phenomenon of crossed response has been already described in many papers[47]. The possible implication of the mechanisms of metabolic action on those made up with the essential oil of M. quinquinervia should be studied with more detail, given the possibility of using this promising candidate for vector control. A variety of formulations with this oil could be used for controlling of field populations who do not show any specific type of enzyme activity, as other authors suggest[47].
With respect to the inhibitory activity of development, oil M. quinquinervia has a toxic effect on larvae exposed to cumulative sublethal doses, as reflected in the high mortality found in this immature stage, in dead or deformed pupae observed and inhibition of emergence of male were adhered to exuvias.
These results may be due to the disruption of the hormonal balance caused by some secondary metabolites in insects exposed to sublethal doses[48,49].
Molecular studies should be performed on possible sites of action and target organs. Most of the plants, which are inferred to have insecticidal activity against mosquitoes, have at least larvicidal activity, but few studies cover a wide bioactivity (larvicide,adulticide, inhibiting development and repellent) on the same plant. Our results allow recommending the use of M. quinquinervia oil for vector mosquito control. In this way it manages to give utility to aninvasive plant of wetlands in the western part of our country and propose an alternative to control mosquito populations, contributing to an environmentally sustainable pest management.
Conflict of interest statement
The authors declare that they have no conflict interest.
Acknowledgments
This study was supported by program ‘Determinants health risks and disease prevention in vulnerable groups' of Ministry of Science,Technology and Environment. Proyect 1601078 ‘Insecticidal activity of essential oils as a natural alternative for mosquito control' of the Institute of Tropical Medicine ‘Pedro Kouri'.
Reference
[1] Diaz LA, Qualia A, Flores FS, Contiagiani MS. Virus West Nile en Argentina: un agente infeccioso emergente que plantea nuevos desafíos. Hornero 2011; 26(1): 5-28.
[2] Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg 2011; 84: 200-207.
[3] Corrales E, Troyo A, Calderón O. Chikunguya: un virus que nos acecha. Act Méd Costarricense 2015; 57(1): 7-15.
[4] Fauci AS, Morens DM. Zika virus in the Americas-yet another arbovirus threat. N Eng J Med 2016; 37(4): 601-604
[5] Kourí G, Pelegrino JL, Munster BM, Guzmán GM. Sociedad, economía,inequidades y dengue. Rev Cubana Med Trop 2007; 59(3). Aviliable from: http://scielo.sld.cu/pdf/mtr/v59n3/mtr01307.pdf.
[6] Marquetti MC, Leyva M, Bisset JA, García A. Recipientes asociados a la infestación por Aedes aegypti en el municipio La Lisa. Rev Cubana Med Trop 2009; 61(3): 232-238.
[7] Bisset JA, Rodríguez MM, Moya M, Ricardo Y, Montada D, Gato R, et al. Efectividad de formulaciones de insecticidas para el control de adultos de Aedes aegypti en La Habana, Cuba. Rev Cubana Med Trop 2011; 63(2): 166-170.
[8] Bisset JA, Rodríguez MM, Hernández H, Valdéz V, Fuentes I, Hurtado D. Resistencia a insecticidas y sus mecanismos bioquímicos en Aedes aegypti del municipio Boyeros en los años 2010 y 2012. Rev Cubana Med Trop 2016; 68(1). Aviliable from: http://www.revmedtropical.sld.cu/index.php/ medtropical/article/view/129/113
[9] Rodríguez MM, Bisset JA, Ricardo Y, Pérez O, Montada D, Figueredo D et al. Resistencia a insecticidas organofosforados en Aedes aegypti(Diptera: Culicidae) de Santiago de Cuba, 1997-2009. Rev Cubana Med Trop 2010; 62(3): 217-223.
[10] Trilles BL, Bombarda I, Bouraima-Madjebi S, Raharivelomanana P,Bianchi JP, Gaydou EM. Ocurrence of various chemotypes in naiouli(Melaleuca quinquinervia (Cav) S.T. Blake) essential oil from New Caledonia. Flav Frag J 2006; 21: 677-682.
[11] Wheeler GS, Pratt PD, Giblin-Davis RM, Ordung KM. Intraespecific variation of Melaleuca quinquinervia leaf oils in its naturalized range in Florida, the Caribbean and Hawaii. Biochem Systc Ecology 2007; 35: 489-500.
[12] Silva CJ. Morfoanatomia foliar e composição química dos oleos essências de sete espécies de Melaleuca L. (Myrtacea) cultivadas em Brasil. [master's thesis]. Universidad Federal de Viçosa, Brasil; 2007.
[13] Quintana F, Navarro P, Gonzáles I. Melaleuca quinquinervia Cav(cayeput): Utilización económica y control. Manual técnico informativo. Grupo Agricultura y Naturaleza de la Organización e Integración para el Bienestar Social. 2014; Available from: www.oibs.cu.
[14] Rodríguez-Pérez M, Martínez JM, Rivero LR, Álvarez HMH, Valdez AFC, Rodríguez DA, et al. Evaluación de la actividad antimalárica de algunas plantas utilizadas en la medicina tradicional cubana. Rev Cienc Farm Básica Aplic 2006; 27(3): 197-205.
[15] Fernández-Calienes A, Mendiola J, Scull R, Vermeersch M, Cos P,Maes L. In vitro anti-microbial activity of the Cuban medicinal plants Simarouba glauca DC, Melaleuca leucadendron L. and Artemisia absinthium L. Mem Inst Oswaldo Cruz 2008; 103(6): 615-618.
[16] Guevara- Pérez E, Cabrera- Dorta T, Peña- Ruiz T, Fernández- Rodríguez CJ, Quintana-Guevara I, Fernández-Rodríguez E. Efecto antimicrobiano de hojas de Melaleuca leucadendron L, que crece en la Ciénaga de Zapata. Rev Méd Elect 2010; 32(4). Aviliable from: http://scielo.sld.cu/ pdf/rme/v32n4/spu04410.pdf
[17] Leyva M, Castex M, Montada D, Quintana D, Lezcano D, Marquetti MC, et al. Actividad repelente de formulaciones del aceite esencial de Melaleuca quinquenervia (Cav.) S.T. Blake (Myrtales: Myrtaceae) en mosquitos. Anales de Biología 2012; 34: 47-56.
[18] Pérez O, Bisset JA, Leyva M, Rodríguez J, Fuentes O, García I, et al. Manual de Indicaciones Técnicas para Insectarios. La Habana: Editorial Ciencias Médicas; 2004, p. 16-53.
[19] WHO. Instructions for determining the susceptibility or resistance of mosquito larvae Aedes to insecticides. Geneva: WHO/VBC/81.807; 1981. p. 1-6.
[20] CDC. Guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay. 1st edition. Centers for Disease Control and Prevention; 2010. Aviliable from: http://www.cdc.gov/malaria.
[21] Brundtland Report. 20 March 1987. ONU. Aviliable from: http://www. cfr.org/economic-development/report-world-commission-environmentdevelopment-our-common-future-brundtland-report/p26349.
[22] Oviedo R, Gonzalez L. Lista nacional de las plantas Invasoras y potencialmente invasoras en la República de Cuba. Bissea 2015; 9(2): 90
[23] Noleto Diaz C, Fernandez D. Essential oils and their compounds as Aedes aegypti L. (Díptera Culicidae) larvicides: review. Parasitol Res 2013. doi. 10.1007/s00436-013-3687-6.
[24] George D, Finn R, Graham K, Sparango O. Present and future potentialof plant-derived products to control arthropods of veterinary and medical significance. Parasit Vectors 2014; 7: 28
[25] Granados-Echegoyen C, Pérez-Pacheco R, Alonso-Hernández N,Vásquez-López A, Lagunez-Rivera L, Rojas-Olivos A. Chemical characterization and mosquito larvicidal activity of essential oil from leaves of Persea americana Mill (Lauraceae) against Culex quinquefasciatus (Say). Asian Pac J Trop Dis 2015; 5(6): 463-467.
[26] Innocent E, Hassanali ,Kisinza W, Mutalemwa P, Magesa S, Kayombo. E Anti-mosquito plants as an alternative or incremental method for malaria vector control among rural communities of Bagamoyo District, Tanzania. J Ethnob Ethnom 2014; 10: 56.
[27] Perumalsamy H, Jin JM, Kim J, Kadarkarai M, Young-Joon A. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasit Vectors 2015; 8: 237.
[28] Komalamisra N, Trongtokit Y, Rongsriyam Y, Apiwathnarson C. Screening for larvicidal activity in some Thai plants against four mosquitoes vector species. S Asian J Trop Med Public Health 2005;36(2): 1412-1422.
[29] Ravi-Kiran S, Bhavani P, Sita -Devi BR, Rajeswara R, Janardahan K. Composition and larvicidal activity of leaves an steam essential oils of Chloroxylon swietenia DC against Aedes aegypti and Anopheles stephensis. Biores Techn 2006; 97(18): 2481-2484.
[30] Elango G, Rahuman A, Kamaraj C, Bagavan A, Zahir A. Adult emergence inhibition and adulticidal activity of leaf crude extracts against Japanese encephalitis vector, Culex quinquefascitus. J King Saud Univ Sci 2012; 24: 73-80.
[31] Dua V, Kumar A, Pandey A, Kumar S. Insecticidal and genotoxic activity of Psoralea corylifolia Linn (Fabaceae) against Culex quinquefasciatus say 1823. Parasit Vectors 2013; 6: 30.
[32] Smith S, Zambrano D, Mendez-Sanchez S, Rodriguez-Sanabria F,Stashenko E, Duque JE. Essential oils with insecticidal activity against larvae of Aedes aegypti (Diptera: Culicidae). Parasitol Res 2014; 113: 2647-2654. doi: 10.10007/s00436-014-3917-6.
[33] Gemeda N, Mokonnene W, Lemma H, Tadele A, Urga K, Addis G, et al. Insecticidal activity of some traditionally used Ethiopian medicinal plants against sheep ked Melophags ovinus. J Parasitol Res 2014; 2014: 978537. doi: 10.1155/2014/978537.
[34] Rattan RS. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot 2010; 29: 913-920.
[35] El-Wakeil N. Botanical pesticides and their mode de action. Gesunde Pflanzen 2013; 65: 125-149.
[36] Morales Rico CL, Marrero-Delange D, González-Canavaciolo VL,Quintana F, Camejo I. Composición química del aceite esencial de las partes aéreas de Melaleuca quinquinervia. Rev CENIC Cienc Quím 2012;43: 1-2.
[37] Koutsaviti K, Giatropoulus A, Piatrokili D, Paachristos D, Michaelakis A,Tzakou O. Greek Pinus essential oils: larvicidal activity and repellency against Aedes albopictus (Diptera : Culicidae). Parasitol Res 2014; 114(2): 583-592. doi: 10.1007/s00436-014-4220-2
[38] Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol Res 2015; 14: 3835-3853.
[39] Giatropoulus A, Papachristos D, Kimbaris A, Koliopoulus G, Polissiou M, Emmanouel N, et al. Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their componentes enantiomeric distribution. Parasitol Res 2012; 111(6): 2253-2263. doi: 10.1007/s00436-012-3074-8.
[40] Kim NJ, Byun SG, Cho JE, Chung K, Anh YJ. Larvicidal activity of essential oils extracted from commonly used herbs in Lebanon against the seaside mosquito Ochlerotatus caspius. Bio Technnol 2008; 99: 763-768.
[41] Zaharan HEDM, Abdelgaleil S. Insecticidal and development inhibitory properties of monoterpenes on Culex pipiens (Diptera: Culicidae). J Asia Pac Entomol 2011; 14: 46-51.
[42] Noleto C, Lima LP, da Franca KA, Aranha MC, Dos Santos C, Medocca de Amaral FM, et al. Chemical Composition and larvicidal activity of essential oils extracted from brazilian legal amazon plants against Aedes aegypti L. (Diptera: Culicidae). Evid-Bas Complem Alternative Med 2015;2015: 490765. doi: 10.1155/2015/490765.
[43] Da Silva AC, Lagos K, Maia FC, Vilmar L, Tadei W, Pohlit AM. Adulticidal activity of dillapiol and semisynthetic derivatives of dillapiol against Aedes aegypti (L). J Mosquito Res 2012; 2(1): 1-7.
[44] Cárdenas E, Riveros I, Lugo L. Efecto insecticida de cuatro aceites esenciales sobre adultos de Aedes aegypti y Anopheles albimanus en condiciones experimentales. Entomotrópica 2013; 28(1): 1-10.
[45] Bakar A , Sulaiman S , Mat Ali Omar. Evaluation of Melaleuca cajuputi(Family: Myrtaceae) essential oil in aerosol spray cans against dengue vectors in low cost Housing Flats. J Arthropod Borne Dis 2012; 6(1): 28-35.
[46] Leyva M, French L, Marquetti MC, Montada D, Santos D, Hernandez A, et al. Insecticidal activity of modified turpentine oil in Culex quinquefasciatus and Aedes albopictus (Diptera: Culicidae). Rev Cubana Med Trop 2015; 67(3). Available from: http://scielo.sld.cu/scielo. php?script=sci_arttext&pid=S0375-07602015000300004&lng=en&nrm =iso&tlng=es.
[47] Cordeiro A, Napoleão T , Viana E, de Lima N , Andrade L, Fontes de Oliveira CM, et al. Effect of Moringa oleifera lectins on survival and enzyme activities of Aedes aegypti larvae susceptible and resistant to organophosphate. Parasitol Res 2013; 113(1): 175-184. doi 10.1007/ s00436-013-3640-8.
[48] Salazar J, Torres P, Serrato B, Dominguez M, Alarcón J, Céspedes C. Insect Growth Regulator (IGR) effects of Eucalyptus citriodora Hook(Myrtaceae). Bol Lat Caribe Plant Med Arom 2015; 14(5): 403-422.
[49] Céspedes C, Molina SC, Muñoz E, Lamilla C, Alarcon J, Palacios SM,et al. The insecticidal, molting disruption and insect growth inhibitory activity of extracts from Condalia microphylla Cav. (Rhamnaceae). Ind Crops and Prod 2013; 42: 78-86.
22 May 2016
✉First and corresponding author: Maureen Leyva, Institute Tropical Medicine ‘Pedro Kouri' Autopista Novia del Mediodía km 6 1/2, La Lisa PO Box 601, Marianao 13,La Habana 11400, Cuba.
Tel: (53)72553626
Fax: 53-7-2046051; 53-7-2020633
E-mail: maureen@ipk.sld.cu
 Asian Pacific Journal of Tropical Medicine2016年10期
Asian Pacific Journal of Tropical Medicine2016年10期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of emodin on mobility signal transduction system of gallbladder smooth muscle in Guinea pig with cholelithiasis
- Skin whitening and anti-corrugation activities of glycoprotein fractions from liquid extracts of boiled sea cucumber
- Effect and mechanism of Irbesartan on occurrence of ventricular arrhythmias in rats with myocardial ischemia through connexin43 (cx43)
- Arginine kinase in Toxocara canis: Exon-intron organization, functional analysis of site-directed mutants and evaluation of putative enzyme inhibitors
- Who should be checked for hepatitis C virus infection in endemic areas?
- Epidemiology of the outbreak, vectors and reservoirs of cutaneous leishmaniasis in Mali: A systematic review and meta-analysis
