UFMylation:A Unique&Fashionable Modification for Life
Ying WeiXingzhi Xu*b
Beijing Key Laboratory of DNA Damage Response and College of Life Sciences,Capital Normal University,Beijing 100048,China
REVIEW
UFMylation:A Unique&Fashionable Modification for Life
Ying Weia,Xingzhi Xu*,b
Beijing Key Laboratory of DNA Damage Response and College of Life Sciences,Capital Normal University,Beijing 100048,China
Available online 20 May 2016
Handled by Zhao-Qi Wang
KEYWORDS
Ubiquitin-fold modifier 1;
Ufmylation;
Endoplasmic reticulum
stress;
Cancer;
Post-translation modification;
Ubiquitin-like proteins
AbstractUbiquitin-fold modifier 1(UFM1)is one of the newly-identified ubiquitin-like proteins. Similar to ubiquitin,UFM1 is conjugated to its target proteins by a three-step enzymatic reaction. The UFM1-activating enzyme,ubiquitin-like modifier-activating enzyme 5(UBA5),serves as the E1 to activate UFM1;UFM1-conjugating enzyme 1(UFC1)acts as the E2 to transfer the activated UFM1 to the active site of the E2;and the UFM1-specific ligase 1(UFL1)acts as the E3 to recognize its substrate,transfer,and ligate the UFM1 from E2 to the substrate.This process is called ufmylation.UFM1 chains can be cleaved from its target proteins by UFM1-specific proteases(UfSPs),suggesting that the ufmylation modification is reversible.UFM1 cascade is conserved among nearly all of the eukaryotic organisms,but not in yeast,and associated with several cellular activities including the endoplasmic reticulum stress response and hematopoiesis.Furthermore,the UFM1 cascade is closely related to a series of human diseases.In this review,we summarize the molecular details of this reversible modification process,the recent progress of its functional studies,as well as its implication in tumorigenesis and potential therapeutic targets for cancer.
Introduction
Protein post-translational modifications(PTM)refer to covalent and generally enzymatic modification of proteins during or after protein biosynthesis.Up to now,more than 200 types of PTMs have been reported,including addition of functional groups(e.g.,phosphorylation,methylation,and acetylation),conjugation of other proteins or peptides(e.g.,ubiquitination),chemical modification of amino acid(AA)residues(e.g.,deamidation),and structural changes(e.g.,disulfide bridges)[1,2].A protein can be modified with one type of PTM or varieties of combinations of PTM types.Moreover,one type of PTM may contain one(e.g.,phosphorylation),a few(e.g.,methylation),and up to many(e.g.,ubiquitination and glycosylation)subtypes.Therefore,PTMs expand exponentially cellular protein varieties and increase a cellular proteome to more than one million different proteins to cope with the complex and fine-tuned cellular activities,such as proteinprotein interactions,cell cycle control,cell differentiation,gene transcription,signaltransduction,DNAdamagerepair,apoptosis,endocytosis,and receptor trafficking[3-5].
Ubiquitination is one of the most common PTMs,next to glycosylation and phosphorylation[6].Ubiquitin(Ub)is conjugated to its target proteins by an elaborate enzymatic reaction chain,which consists of an activating enzyme E1,a conjugating enzyme E2,and a ligating enzyme E3[6].The human genome encodes one E1,dozens of E2,and hundreds of E3.Besides,the Ub chain can be cleaved from its target proteins by deubiquitinating enzymes(DUBs),suggesting that ubiquitination is reversible[7].A target protein is modified covalently with a single moiety of Ub(monoubiquitination)through its C-terminal glycine(Gly)residue and a lysine(Lys,K)residue within the target protein,or subsequently with multiple moieties of Ub through its C-terminal Gly residue and a Lys residue or the 1st methionine(Met)residue within the pre-added Ub moiety.There are seven Lys residues(K6,K11,K27,K29,K33,K48,and K63)and the initial Met residue in the Ub polypeptide.Thus,there are at least 8 different linkage types for polyubiquitination chains.Yet,a polyubiquitination chain could be a mixture of more than one linkage type,making this modification more complex and divergent. Different linkage types of ubiquitination modification confer a target with different biological functions[8].Among them,it is well known that the K48-linked poly-ubiquitination modification targets proteins for degradation through the 26S proteasome,while the K63 linkage poly-ubiquitination regulates protein activity and protein-protein interaction networks[9,10].
A number of small Ub-like proteins(UBLs),such as small Ub-like modifier(SUMO)[11],neural precursor cell-expressed and developmentally-downregulated 8(NEDD8)[12],and interferon-stimulated gene 15(ISG15)[13],have been subsequently identified.Although most UBLs have no obvious sequence identity to Ub,they all share a similar tertiary structure.Like Ub,these UBLs can be covalently conjugated to their target proteins through a series of enzymatic reactions analogous to ubiquitylation,endowing them with different biological functions[14].The UBLs are divided into two subfamilies.Type-1 UBLs,such as SUMO,NEDD8,and UCRP/ ISG15,are ligated to target proteins in a process similar to ubiquitylation.Type-2 UBLs,which are also known as Ub domain proteins(UDPs),have a similar structure to Ub and are embedded in different large proteins[13].These include Rad23,elongin B,and parkin[14].
Ubiquitin fold modifier 1(UFM1)is one of the newlyidentified type 1 UBLs[15].Like ubiquitination,UFM1 is conjugated to a target protein by a three-step enzymatic reaction involving E1,the UFM1-activating enzyme(ubiquitin-like modifier-activatingenzyme5;UBA5);E2,theUFM1-conjugating enzyme 1(UFC1);and E3,the UFM1-specific ligase 1(UFL1).Usually,UFM1 is present in a precursor form(Pro-UFM1).CleavagebytheUFM1-specificproteases(UfSPs)exposes the conserved Gly residue on its C-terminus and this residue is essential for its subsequent conjugating reactions.Thereafter,with the help of ATP,the mature form of UFM1 is activated by UBA5,forming a high energy thioester bond with the Cystine(Cys)250 of UBA5.Next,UFC1 interacts with the Ub-fold domain of UBA5,and the activated UFM1 is transferred to the UFC1,forming a similar thioester linkage with Cys116 in UFC1.Finally,with the help of UFL1,UFM1 is conjugated to its target proteins,such as UFM1-binding protein 1(UfBP1)[16]and activating signal cointegrator 1(ASC1)[17](Figure 1).The specific UfSPs can also cleave UFM1 from its target proteins,suggesting that ufmylation is reversible[18,19](Figure 1).UFM1 cascade is conserved among nearly all the eukaryotic organisms,except yeast[18,20],implying the essential role of UFM1 modification in cellular activities.Recent studies have uncovered that UFM1 modification is tightly related to the endoplasmic reticulum(ER)stress[21,22],hematopoiesis[23-25],fatty acid metabolism[26],and G-protein coupled receptor(GPCR)biogenesis[27].Furthermore,the abnormal UFM1 cascade is reported to be associated with a number of human diseases,including cancer,ischemic heart diseases,diabetes,atherosclerosis,hip dysplasia,and schizophrenia[17,18].
The human genome encodes a single ufmylation cascade(UBA5-UFC1-UFL1);however,its physiological substrates are far from clear.In this review,we will introduce the human ufmylation cascade,its known physiological substrates,and its involvement in a few cellular activities.We will summarize the abnormalities of the ufmylation cascade in different tumors.A perspective of ufmylation in tumorigenesis and cancer therapy will be discussed as well.
Key players of the ufmylation process
Key players of the ufmylation process include UFM1,UBA5,UFC1,UFL1,and UfSP2[18].
UFM1,also known as C13orf20,consists of 85 AA residues with a calculated molecular mass of 9.1 kDa.Although human UFM1 only has 16%sequence identity with Ub,the global fold of human UFM1 is similar to that of Ub in their tertiary structures[15].While Ub and many UBLs have the conserved C-terminal di-Gly(Gly-Gly)residues,the mature UFM1 only has a single Gly residue at its C-terminus followed by a Ser-Cys dipeptide.Present in a precursor form in human cells,UFM1 is processed by the two UfSPs,namely,UfSP1 and UfSP2,to produce mature UFM1[19].Immunocytochemical analysis revealed that UFM1 is localized both in the nucleus and the cytoplasm[15].UFM1 is conserved in metazoan and plants,but not found in yeast,suggesting its specific roles in multicellular organisms.
UBA5,also known as UBE1DC1,is the only E1 enzyme for ufmylation encoded by the human genome[15,28].UBA5 encodes a polypeptide of 404 AA residues with a calculated molecular weight of 44.7 kDa.Typically,a Ub-activating enzyme E1 comprises the first and second catalytic cysteine half-domains(FCCH and SCCH,respectively),adenylation domain,and C-terminal Ub-fold domain[29].However,UBA5 does not possess all these characteristic domains;instead,it only has an adenylation domain where the catalytic Cys250 is located and a Ub-fold domain[30].Thus,UBA5 is significantly smaller than other Ub-associated(UBA)family members.UBA5 is mainly detected in the cytoplasm.Interestingly,a typical E1 works as a dimer to activate its specific Ub-like proteins.While the monomeric UBA5 is able to activate UFM1 in vitro[15,30],whether UBA5 works as a dimer or monomer in vivo is awaiting further investigation.
A canonical E1 activates a UBL through a three-step mechanism.In the first step,E1 binds ATP and UBL,forming a UBL-adenylate intermediate.Second,the conserved Cys residue in the catalytic cysteine domain reacts with the UBL-adenylate to form an E1-UBL thioester.Third,the E1-UBL thioester catalyzes another round of UBL adenylation withATP,forming a ternary complex[29].In contrast,the activation of UFM1 by UBA5 is via a two-step mechanism.First,mature UFM1 forms a noncovalent complex with UBA5. Then,with the help of ATP,UFM1 is conjugated to UBA5 at Cys250 in the catalytic domain via a thioester bond,forming a binary complex[30].It has been demonstrated that UBA5 is specific for UFM1 activation[23],although there is a report showing that UBA5 activates SUMO2 in the nucleus[31].
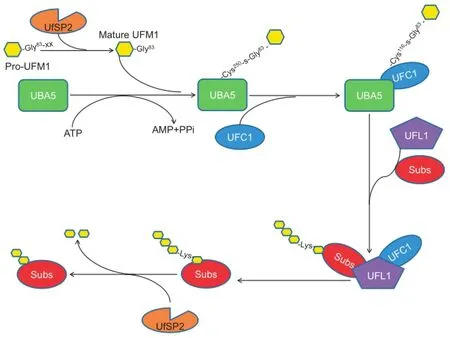
Figure 1 The human ufmylation pathway
Significantly,Uba5 knockout mice die in utero due to severe anemia resulting from defective development in the megakaryocyte and erythroid progenitors[23].The anemia phenotype of the Uba5-deficient mouse embryos can be rescued by transgenic expression of Uba5 in the erythroid lineage,together with prolonged survival,although Uba5 is dispensable for the erythropoietin production.These results reveal the important role of the ufmylation process in erythroid differentiation[23].
UFC1,also known as HSPC155,consists of 167 AA residues with a calculated molecular weight of 19.4 kDa.UFC1 is predominantly localized in the nucleus and partially in the cytoplasm[15].Despite lacking obvious sequence homology with other E2 enzymes,UFC1 does have a relatively conserved catalytic core domain,which is conserved in all E2 enzymes[15].In the catalytic core domain,there are approximately 10 AA residues surrounding the active site Cys116,forming a flexible loop that is highly solvent-accessible.Once UFC1 binds to the C-terminal domain of UBA5,the activated UFM1istransferredtotheCys116ofUFC1bya trans-esterification reaction[18,20].
The neuronal cell adhesion molecule(NCAM)plays important roles in the neurodevelopment and in synaptic plasticity in the adult brain[32].NCAM140,an isoform of NCAM,was shown to interact with UFC1 through its cytoplasmic domain and co-localize with UFC1 on the surface of B35 neuroblastoma cells[32].Besides,overexpression of UFM1 resulted in the increased NCAM140 endocytosis[32].Therefore,the UFM1 cascade may play an essential role in trafficking of cell surface molecules.
UFL1,also known as Maxer,regulator of C53/LZAP and DDRGK1(RCAD),novel LZAP-binding protein(NLBP),and KIAA0776,consists of 794 AA residues with a calculated molecular weight of 89.5 kDa.UFL1 is mainly located in the endoplasmic ER[16].It possesses a transmembrane domain and a nuclear localization signal(NLS),which is functional only when the transmembrane domain is deleted[16].The N-terminal region of UFL1 is highly conserved across different species and is important for the transfer of UFM1 from UFC1 to its target proteins[16].Surprisingly,UFL1 does not possess any typical domains conserved in E3 ligases,such as the homologous to the E6AP carboxyl terminus domain(HECT),the really interesting new gene(RING)finger domain,or U-box[15].For instance,an active cysteine site for transthiolation reaction within N terminus is typically identified in HECT type E3 ligases;however,UFL1 lacks this cysteine site[16].Thus,it is believed that UFL1 may be a scaffold type E3-like RING finger E3 protein that can recruit E2 enzymes and target proteins.
Recent investigations have demonstrated that Ufl1 knockout mice die in utero due to severe anemia[24].Knocking down of Ufl1 resulted in increased ER stress and unfolded protein response(UPR)in bone marrow cells.Furthermore,loss of UFL1 compromised autophagic degradation,while elevating mitochondrial mass and reactive oxygen species,p53 activation,DNA damage response,and death of hematopoietic stem cells(HSCs).Thus,UFL1 is indispensable for embryonic development,erythroid differentiation,and HSC survival[24].
The processing of the C-terminal region of Pro-UFM1,as well as releasing of UFM1 from its target proteins,is mediated by two UfSPs,UfSP1 and UfSP2[19].Human UfSP1 consists of 217 AA residues with a calculated molecular weight of 23.4 kDa,while human UfSP2(C4orf20)consists of 469 AA residues with a calculated molecular weight of 53.2 kDa. UfSP2 differs from UfSP1 due to the presence of an extended N-terminal domain,which has a unique structure and may play a role in recognizing the specific substrate during the deconjugation process[19].UfSP2 is present in nearly all multicellular organisms,while UfSP1 is not found in plants and nematodes and is usually expressed weaker than UfSP2[19]. Although UfSP1 and UfSP2 have no obvious sequence homology with the known Ub-like protein specific proteases(ULPs)and DUBs,they exhibit typical features of cysteine proteases,including possession of a conserved catalytic triad Cys-His-Asp and sensitivity of catalytic inhibition by sulfhydrylblocking agents[19].Thus,the UfSPs may constitute a new subfamily of the cysteine protease.
UfSP2 may also play a role in the maturation of GPCRs on the ER membrane in a UFM1-independent manner in Caenorhabditis elegans.The odorant response abnormal protein(ODR-8;analogous to human UfSP2)-containing complex in the ER promotes GPCR folding,maturation,and export from ER[27].ODR-8 is reported to have a distinct neuronal expression profile in C.elegans,while other UFM1 pathway members are mainly expressed in the intestine[33].Besides,a mutation of UfSP2 was reported in a case of the Beukes familial hip dysplasia[34],suggesting that the deufmylation process gets involved in human pathogenesis as well.
Key physiological substrates of ufmylation
The physiological targets of the UFM1 conjugation system remain largely undefined.Only a handful of ufmylation targets have been identified so far,including UFM1-binding protein 1(UfBP1)and activating signal cointegrator 1(ASC1).
UfBP1,also known as C20orf116,Dashurin,or DDRGK domain containing 1(DDRGK1),consists of 314 AA residues with a calculated molecular weight of 35.6 kDa.UfBP1 is the first identified substrate of ufmylation[16].UfBP1 contains a transmembrane helix(AA residues 4-21),a NLS sequence(AA residues 64-68),a proteasome-COP9-initiation factor(PCI)domain(AA residues 228-272),and a DDRGK domain(AA residues 253-267).The ufmylation of UfBP1 takes place at the K267 in the PCI domain[16].The PCI domain is bestknown for its role in mediating protein-protein interaction and in the formation of several multiprotein complexes,such as the 26S proteasome,the COP9 signalosome(CSN),and the eukaryotic translation initiation factor 3(eIF3)complex[35].UfBP1 is predominantly localized in the ER,and its N-terminal signal domain is important for its localization in the cytosolic side of the ER membrane.The ER-localized UfBP1 can recruit UfSP2,and interact with UFL1 and C53(also known as Cdk5rap3,one of the UFM1 substrates),to form a large multi-protein complex[16].Defect in the N-terminal signal sequence of UfBP1 results in nuclear localization[16].Overexpression of UfBP1 was associated with ER proliferation and neogenesis[36],whereas knockout of UfBP1 caused an elevated ER stress and activation of UPR[25].
Moreover,UfBP1 can interact with UFM1 and UFL1,as well as the substrates of ufmylation,such as ASC1 and C53,forming a big complex.UfBP1 can promote the ufmylation of ASC1[17].Consistent with this phenomenon,knockdown of UfBP1 abolished the ufmylation of ASC1,and overexpression of the UfBP1(K267R)mutant,which is deficient in the UfBP1 ufmylation,also abolished the ASC1 ufmylation[17].Germ-line deletion of UfBP1 resulted in defective erythroid development and embryonic lethality,while somatic ablation of UfBP1 impaired adult hematopoiesis,leading to pancytopenia and animal death[25].Thus,UfBP1 is not only a substrate of ufmylation,but the ufmylated UfBP1 may serve as a cofactor for the ufmylation of other substrates.
ASC1 is a transcriptional coactivator of estrogen receptor-α(ERα)as well as other nuclear receptors(e.g.,C53,androgen receptor,and thyroid hormone receptor)[37].ASC1 possesses a zinc finger(ZF)domain,which is thought to be a binding site for nuclear receptors and transcriptional coactivators such as p300 and steroid receptor coactivator1(SRC1)[37].Thus,ASC1 may serve as a platform protein to recruit the necessary componentsfornuclearreceptor-mediatedtranscription. Besides,ASC1 is thought to be a tumor suppressor,as it can activate p53,induce apoptosis,and suppress NF-κB signaling[17].Knockdown of ASC1 reduced expression of erythroid transcription factors,but did not elevate basal ER stress[25].
ASC1 is a novel UFM1 target protein[16].It was recently demonstrated that the polyufmylation of ASC1 promotes breast cancer development[17].The ASC1 ufmylation requires all factors of the UFM1 cascade,including UFM1,UBA5(E1),UFC1(E2),UFL1(E3),and UfBP1.Absence of any of these factors will abrogate the ASC1 ufmylation.K324,K325,K334,and K367 of ASC1 all serve as the acceptor sites for UFM1 modification,whereas only the K69 of UFM1 is involved in the poly-UFM1 chain formation on ASC1[17]. In the absence of estrogen,ASC1 in the ERα-positive breast cancer cells undergoes polyufmylation,which is quickly reversed by the bound UfSP2.In the presence of estrogen,the ligand-bound dimeric ERα displaces UfSP2 from binding toASC1,allowingaccumulationofASC1ufmylation. Polyufmylated ASC1 may serve as a scaffold protein to recruit p300,SRC1,and ASC1 itself to the promoter of ERα target genes for transcriptional activation,thus leading to excessive cell proliferation and ultimately tumor formation[38].
Key cellular processes regulated by ufmylation
Based on the physiological targets of ufmylation identified so far,we have gained some insight of ufmylation in ER homeostasis,development and differentiation of blood progenitors. In addition,the ufmylation may be involved in GPCR maturation in C.elegans as mentioned above.
ER is an organelle essential for the protein processing,folding,and trafficking,as well as lipid biosynthesis and calcium homeostasis[39,40].Thus,ER homeostasis is essential for protein maturation[39].Disturbance of the ER homeostasis will result in the ER stress response and the activation of UPR[39].The UfBP1,UfSP2,UFL1,and UFM1 can form a large protein complex at the cytosolic side of the ER membrane,and the UFM1 modification system was reported to play an important role in the ER function[21,22].
The pathology of both type 2 diabetes and ischemic heart disease is related to the ER stress response[21].The expression of UFM1 cascade is up-regulated in the pancreatic islets of Langerhans and some other secretory tissues[21].With the treatment of glycosylation inhibitor tunicamycin(TM),or ER Ca2+ATPase inhibitor thapsigargin(TG)for ER stress induction,increasedexpressionofUFM1,UfBP1,and UFL1 was observed in the mouse INS-1E cells[41].When vesicle trafficking was inhibited by brefeldin A(BFA,an inhibitor of vesicle trafficking),the transcript levels of UFM1 system increased,which was not observed in mouse embryonic fibroblast cells(MEFs)with knocking down of X-box binding protein 1(Xbp1),a transcription factor of UPR[22].The ufmylation-related genes all contain an XBP1-binding element(UPRE)in their promoter regions[22].Thus,the UFM1 system may participate in vesicle trafficking,loss of which will lead to an increased protein load in the ER and subsequently increase the ER stress[22].
It was reported that loss-of-function of ufmylation resulted in an increased apoptosis of pancreatic beta cells[41].Knockdown of UFM1 increased the apoptosis of the mouse macrophage cell line RAW264.7 cells[42].However,in C.elegans,the UFM1 conjugation is high when the protein load is low and vice versa[41].Additionally,the UFM1 modification can also participate in other stress response,like oxidative,heat,and pathogen stresses[43].In Leishmania donovani,the UFM1 cascade is located in the mitochondria,and can interact with mitochondrial trifunctional protein(MTP)to affect the beta oxidation of long-chain acyl-CoA esters[26,44].Loss of UFM1 results in the decreased amount of acyl-CoA and reduces the survival of amastigotes in human macrophage cells[26,44].Thus,UFM1 modification may play a protective role in maintaining the ER homeostasis and avoid the ER stressinduced apoptosis.
Germ-line deletion of Uba5,Ufl1,or Ufbp1 in mice all led to embryonic lethality and impaired hematopoietic development(erythroid linage development in particular)[23-25]. Additionally,Ufl1 knockout in mice compromised HSC survival,and tamoxifen-induced somatic knockout of Ufl1 in mice resulted in death due to severe anemia[23-25].Both Ufl1 knockout and Ufbp1 knockout mice exhibited increased ER stress and activation of UPR[24,25],further confirming ufmylation in ER homeostasis.It has been proposed that the ufmylation pathway targets ASC1 for ufmylation,stimulates expression of erythroid transcription factors,and thus ensures erythroid development.In addition,the pathway also targets a yet unknown factor for ufmylation,maintaining ER homeostasis,and thus promoting HSC survival[25].
The ufmylation pathway in human diseases
Abnormalities in the ufmylation pathway have been found to associate with varieties of human diseases,including ischemic heart diseases[21],diabetes[21],atherosclerosis[42],hip dysplasia[34],schizophrenia[45],and cancer[17].

Table 1 Abnormality of the ufmylation-related genes in different cancers
The ufmylation of ASC1 is important for the transactivation of ERα and thus breast cancer development[17].In addition,UfBP1 knockdown compromises cell proliferation and invasion in human osteosarcoma U2OS cells[46].However,both UFL1 and UfBP1 have been reported to suppress the NF-κB signaling pathway[46].Thus,the ufmylation pathway may promote or suppress tumorigenesis through targeting different substrates.Indeed,in The Cancer Genome Atlas(TCGA)cancer database(www.cbioportal.com),for lung squamous cell carcinoma,lung adenocarcinoma,breast invasive carcinoma,ovarian serous cystadenocarcinoma,uterine corpus endometrioid carcinoma,esophageal carcinoma,liver hepatocellular carcinoma,bladder urothelial carcinoma,and sarcoma,at least one of the genes encoding the ufmylation factors(UBA5,UFC1,and UFL1)were amplified,while the gene encoding UfSP2,the only known deufmylation factor,sometimes was deleted,in more than 3%of patient cases(Table 1). However,despite the amplification of UFC1 in pancreatic adenocarcinoma and amplification of both UFC1 and UFL1 in diffuse large B-cell lymphoma,high percentage of UFL1 deletion and UFL1/UFM1 deletion was also detected in pancreatic adenocarcinoma and diffuse large B-cell lymphoma,respectively(Table 1).It is of note that high percentage of UFL1/ UFM1 deletion was detected in prostate adenocarcinoma aswell(Table 1).These molecular epidemiological datasets further support the observation that the ufmylation process may have opposite effects on tumorigenesis.Given that only one E3 for ufmylation has been identified so far,we believe that more E3 ligases await exploration.
Conclusions
The UFM1 modification is a brand-new and very attractive Ub-like modification.With the very limited knowledge of ufmylation targets,we are far from appreciation of its molecular impact on cellular activities and etiology of human diseases,as well as its implication as therapeutic targets. We are very keen to understand(1)what the additional E3 ligases for ufmylation are;(2)what the additional ufmylation substrates are;(3)in addition to ER stress response and hematopoietic development,what other cellular activities the reversible ufmylation pathway regulates;(4)what the molecular mechanisms of the opposite effects on tumorigenesis are;(5)which ufmylation factor(s)is(are)an ideal biomarker and therapeutic target for certain types of cancer. Hence,the sleeping beauty of ufmylation is waiting for the awakening.
Competing interests
The authors have declared no competing interests.
Acknowledgments
We thank other members of the Xu lab for help.The research inXu’slabwassupportedbytheNationalNatural Science Foundation of China(NSFC;Grant Nos.31530016 and 31461143012),the National Basic Research Program ofChina(973Program;GrantNos.2013CB911002 and2015CB910601),andtheScientificResearchBase Development Program of the Beijing Municipal Commission of Education,China to XX.
References
[1]Hsu YC,Hsieh YH,Liao CC,Chong LW,Lee CY,Yu YL. Targeting post-translational modifications of histones for cancer therapy.Cell Mol Biol(Noisy-le-grand)2015;61:69-84.
[2]Hitosugi T,Chen J.Post-translational modifications and the Warburg effect.Oncogene 2014;33:427985.
[3]Chen ZJ,Sun LJ.Nonproteolytic functions of ubiquitin in cell signaling.Mol Cell 2009;33:275-86.
[4]Mukhopadhyay D,Riezman H.Proteasome-independent functionsofubiquitininendocytosisandsignaling.Science 2007;315:201-5.
[5]Tompa P,Davey NE,Gibson TJ,Babu MM.A million peptide motifs for the molecular biologist.Mol Cell 2014;55:161-9.
[6]Herhaus L,Dikic I.Expanding the ubiquitin code through posttranslational modification.EMBO Rep 2015;16:1071-83.
[7]Sahtoe DD,Sixma TK.Layers of DUB regulation.Trends Biochem Sci 2015;40:456-67.
[8]Hershko A,Ciechanover A.The ubiquitin system.Annu Rev Biochem 1998;67:425-79.
[9]Pickart CM,Eddins MJ.Ubiquitin:structures,functions,mechanisms.Biochim Biophys Acta 2004;1695:55-72.
[10]Finley D.Recognition and processing of ubiquitin-protein conjugates by the proteasome.Annu Rev Biochem 2009;78:477-513.
[11]Wang Y,Ladunga I,Miller AR,Horken KM,Plucinak T,Weeks DP,et al.The small ubiquitin-like modifier(SUMO)and SUMO-conjugatingsystemofChlamydomonasreinhardtii.Genetics 2008;179:177-92.
[12]Chung D,Dellaire G.The Role of the COP9 signalosome and neddylation in DNA damage signaling and repair.Biomolecules 2015;5:2388-416.
[13]Vertegaal AC.Uncovering ubiquitin and ubiquitin-like signaling networks.Chem Rev 2011;111:7923-40.
[14]Jentsch S,Pyrowolakis G.Ubiquitin and its kin:how close are the family ties?Trends Cell Biol 2000;10:335-42.
[15]Komatsu M,Chiba T,Tatsumi K,Iemura S,Tanida I,Okazaki N,et al.A novel protein-conjugating system for Ufm1,a ubiquitin-fold modifier.EMBO J 2004;23:1977-86.
[16]Tatsumi K,Sou YS,Tada N,Nakamura E,Iemura S,Natsume T,et al.A novel type of E3 ligase for the Ufm1 conjugation system.J Biol Chem 2010;285:5417-27.
[17]Yoo HM,Kang SH,Kim JY,Lee JE,Seong MW,Lee SW,et al.Modification of ASC1 by UFM1 is crucial for ERalpha transactivationandbreastcancerdevelopment.MolCell 2014;56:261-74.
[18]Daniel J,Liebau E.The ufm1 cascade.Cells 2014;3:627-38.
[19]Kang SH,Kim GR,Seong M,Baek SH,Seol JH,Bang OS,et al. Two novel ubiquitin-fold modifier 1(Ufm1)-specific proteases,UfSP1 and UfSP2.J Biol Chem 2007;282:5256-62.
[20]Liu G,Forouhar F,Eletsky A,Atreya HS,Aramini JM,Xiao R,etal.NMRandX-raystructuresofhumanE2-like ubiquitin-fold modifier conjugating enzyme 1(UFC1)reveal structural and functional conservation in the metazoan UFM1-UBA5-UFC1 ubiquitination pathway.J Struct Funct Genomics 2009;10:127-36.
[21]Azfer A,Niu J,Rogers LM,Adamski FM,Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease.Am J Physiol Heart Circ Physiol 2006;291:H1411-20.
[22]Zhang Y,Zhang M,Wu J,Lei G,Li H.Transcriptional regulation of the Ufm1 conjugation system in response to disturbance of the endoplasmic reticulum homeostasis and inhibition of vesicle trafficking.PLoS One 2012;7:e48587.
[23]Tatsumi K,Yamamoto-Mukai H,Shimizu R,Waguri S,Sou YS,Sakamoto A,et al.The Ufm1-activating enzyme Uba5 is indispensable for erythroid differentiation in mice.Nat Commun 2011;2:181.
[24]Zhang M,Zhu X,Zhang Y,Cai Y,Chen J,Sivaprakasam S,et al. RCAD/Ufl1,a Ufm1 E3 ligase,is essential for hematopoietic stem cell function and murine hematopoiesis.Cell Death Differ 2015;22:1922-34.
[25]Cai Y,Pi W,Sivaprakasam S,Zhu X,Zhang M,Chen J,et al. UFBP1,a key component of the Ufm1 conjugation system,is essential for ufmylation-mediated regulation of erythroid development.PLoS Genet 2015;11:e1005643.
[26]Gannavaram S,Connelly PS,Daniels MP,Duncan R,Salotra P,Nakhasi HL.Deletion of mitochondrial associated ubiquitin fold modifier protein Ufm1 in Leishmania donovani results in loss of beta-oxidation of fatty acids and blocks cell division in the amastigote stage.Mol Microbiol 2012;86:187-98.
[27]Chen C,Itakura E,Weber KP,Hegde RS,de Bono M.An ER complexofODR-4andODR-8/Ufm1specificprotease2 promotes GPCR maturation by a Ufm1-independent mechanism. PLoS Genet 2014;10:e1004082.
[28]Dou T,Gu S,Liu J,Chen F,Zeng L,Guo L,et al.Isolation and characterization of ubiquitin-activating enzyme E1-domain containing 1,UBE1DC1.Mol Biol Rep 2005;32:265-71.
[29]Schulman BA,Harper JW.Ubiquitin-like protein activation by E1 enzymes:the apex for downstream signalling pathways.Nat Rev Mol Cell Biol 2009;10:319-31.
[30]Bacik JP,Walker JR,Ali M,Schimmer AD,Dhe-Paganon S. Crystal structure of the human ubiquitin-activating enzyme 5(UBA5)bound to ATP:mechanistic insights into a minimalistic E1 enzyme.J Biol Chem 2010;285:20273-80.
[31]Zheng M,Gu X,Zheng D,Yang Z,Li F,Zhao J,et al. UBE1DC1,an ubiquitin-activating enzyme,activates two different ubiquitin-like proteins.J Cell Biochem 2008;104:2324-34.
[32]Homrich M,Wobst H,Laurini C,Sabrowski J,Schmitz B,Diestel S.Cytoplasmic domain of NCAM140 interacts with ubiquitin-fold modifier-conjugatingenzyme-1(Ufc1).ExpCellRes 2014;324:192-9.
[33]Gannavaram S,Davey S,Lakhal-Naouar I,Duncan R,Nakhasi HL.Deletion of ubiquitin fold modifier protein Ufm1 processing peptidase Ufsp in L.donovani abolishes Ufm1 processing and alters pathogenesis.PLoS Negl Trop Dis 2014;8:e2707.
[34]Watson CM,Crinnion LA,Gleghorn L,Newman WG,Ramesar R,Beighton P,et al.Identification of a mutation in the ubiquitinfold modifier 1-specific peptidase 2 gene,UFSP2,in an extended South African family with Beukes hip dysplasia.S Afr Med J 2015;105:558-63.
[35]Pick E,Hofmann K,Glickman MH.PCI complexes:beyond the proteasome,CSN,and eIF3 troika.Mol Cell 2009;35:260-4.
[36]Wu J,Lei G,Mei M,Tang Y,Li H.A novel C53/LZAP-interacting protein regulates stability of C53/LZAP and DDRGK domain-containing protein 1(DDRGK1)and modulates NF-kappaB signaling.J Biol Chem 2010;285:15126-36.
[37]Xu J,Wu RC,O’Malley BW.Normal and cancer-related functions of the p160 steroid receptor co-activator(SRC)family. Nat Rev Cancer 2009;9:615-30.
[38]Yoo HM,Park JH,Jeon YJ,Chung CH.Ubiquitin-fold modifier 1 acts as a positive regulator of breast cancer.Front Endocrinol(Lausanne)2015;6:36.
[39]Schroder M,Kaufman RJ.The mammalian unfolded protein response.Annu Rev Biochem 2005;74:739-89.
[40]Hotamisligil GS.Endoplasmic reticulum stress and the inflammatory basis of metabolic disease.Cell 2010;140:900-17.
[41]Lemaire K,Moura RF,Granvik M,Igoillo-Esteve M,Hohmeier HE,Hendrickx N,et al.Ubiquitin fold modifier 1(UFM1)and its target UFBP1 protect pancreatic beta cells from ER stressinduced apoptosis.PLoS One 2011;6:e18517.
[42]Hu X,Pang Q,Shen Q,Liu H,He J,Wang J,et al.Ubiquitin-fold modifier 1 inhibits apoptosis by suppressing the endoplasmic reticulum stress response in Raw264.7 cells.Int J Mol Med 2014;33:1539-46.
[43]Hertel P,Daniel J,Stegehake D,Vaupel H,Kailayangiri S,Gruel C,et al.The ubiquitin-fold modifier 1(Ufm1)cascade of Caenorhabditis elegans.J Biol Chem 2013;288:10661-71.
[44]Liu H,Li J,Tillman B,French BA,French SW.Ufmylation and FATylation pathways are downregulated in human alcoholic and nonalcoholic steatohepatitis,and mice fed DDC,where Mallory-Denk bodies(MDBs)form.Exp Mol Pathol 2014;97:81-8.
[45]Rubio MD,Wood K,Haroutunian V,Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systemsinschizophrenia.Neuropsychopharmacology 2013;38:1910-20.
[46]Xi P,Ding D,Zhou J,Wang M,Cong YS.DDRGK1 regulates NF-κB activity by modulating IκBα stability.PLoS One 2013;8: e64231.
19 February 2016;revised 15 April 2016;accepted 22 April 2016
*Corresponding author.
E-mail:Xingzhi_Xu@mail.cnu.edu.cn(Xu X).aORCID:0000-0003-4320-7784.bORCID:0000-0002-4459-2082.
Peer review under responsibility of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
http://dx.doi.org/10.1016/j.gpb.2016.04.001
1672-0229ⓒ2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
This is an open access article under the CC BY license(http://creativecommons.org/licenses/by/4.0/).
 Genomics,Proteomics & Bioinformatics2016年3期
Genomics,Proteomics & Bioinformatics2016年3期
- Genomics,Proteomics & Bioinformatics的其它文章
- DNA End Resection:Facts and Mechanisms
- Functions of PARylation in DNA Damage Repair Pathways
- New Edges of RNA Adenosine Methylation Modifications
- Topoisomerase I in Human Disease Pathogenesis and Treatments
- Connecting Malfunctioning Glial Cells and Brain Degenerative Disorders
- DNA Damage Response in Hematopoietic Stem Cell Ageing
