In vitro interference of cefotaxime at subinhibitory concentrations on biofilm formation by nontypeable Haemophilus influenzae
Sudarat Baothong,Sutthirat Sitthisak,2,Duangkamol Kunthalert,2*
1Department of Microbiology and Parasitology,Faculty of Medical Science,Naresuan University,Phitsanulok 65000,
Thailand
2Centre of Excellence in Medical Biotechnology,Faculty of Medical Science,Naresuan University,Phitsanulok 65000,Thailand
In vitro interference of cefotaxime at subinhibitory concentrations on biofilm formation by nontypeable Haemophilus influenzae
Sudarat Baothong1,Sutthirat Sitthisak1,2,Duangkamol Kunthalert1,2*
1Department of Microbiology and Parasitology,Faculty of Medical Science,Naresuan University,Phitsanulok 65000,
Thailand
2Centre of Excellence in Medical Biotechnology,Faculty of Medical Science,Naresuan University,Phitsanulok 65000,Thailand
ARTICLE INFO
Article history:
Accepted 22 Jan 2016
Available online 26 Jul 2016
Biofilm formation
Cefotaxime
Nontypeable Haemophilus influenzae
Subinhibitory concentration Sub-MIC
Objective:To investigate the in vitro interference of cefotaxime at subinhibitory concentrations[sub-minimal inhibitory concentrations(MIC)]on biofilm formation by nontypeable Haemophilus influenzae(NTHi).
Methods:The interference of subinhibitory concentrations of cefotaxime on biofilm formation of the clinical strong-biofilm forming isolates of NTHi was evaluated by a microtiter plate biofilm formation assay.The effect of sub-MIC cefotaxime on bacterial cell-surface hydrophobicity was determined using a standard microbial adhesion to n-hexadecane test.Additionally,the effects on bacterial adherence to human fibronectin and expression of bacterial adhesins were also investigated.
Results:Subinhibitory concentrations of cefotaxime,both at 0.1×and 0.5×MIC levels,efficiently reduced the NTHi biofilm formation,and this effect was independent of decreasing bacterial viability.Sub-MIC cefotaxime also decreased bacterial cell-surface hydrophobicity and reduced adherence to human fibronectin.Inhibition in the P2 and P6 gene expressions upon exposure to sub-MIC cefotaxime was also noted.
Conclusions:Taken together,our results indicate that sub-MIC cefotaxime interferes with the formation of NTHi biofilm,and this effect is feasibly related to the interference with cell-surface hydrophobicity,fibronectin-binding activity as well as alteration of the P2 and P6 gene expression.The findings of the present study therefore provide a rationale for the use of subinhibitory concentrations of cefotaxime for treatment of NTHi-related diseases.
1.Introduction
Nontypeable Haemophilus influenzae(NTHi)is present as a commensal and opportunistic pathogen that is highly adapted to colonize the human respiratory tract and later progress to cause mucosal infections in children and adults[1-3].This microorganism is responsible for an array of respiratory diseases,includingotitismedia,sinusitis,conjunctivitis,exacerbations of chronic obstructive pulmonary disease,persistent bacterial bronchitis and cystic fibrosis[2-4]. Significant levels of morbidity and mortality as well as socioeconomic burden caused by this microorganism have been of great concern worldwide.Several lines of evidence revealed the presence of NTHi in biofilm communities in the lower and upper airways,and physically in the middle-ear mucosa of experimental chinchilla models of otitis media[5,6]. Bacterial pathogens living in biofilm are resistant to antimicrobials and host immune clearance[5].Compared with the planktonic ones,bacteria within the biofilm state have been shown to be more than 1000-times more resistant to conventional antibiotic treatment and host immune responses[7]. As such,bacterial biofilms are difficult to eradicate,and often
involved with chronic persistent infections[8-10].Currently,the difficult-to-eradicate infection associated with biofilm represents a major challenge in medical practice on a global scale.
Generally,effective antimicrobial treatment is expected when the antibiotic concentration is above the minimal inhibitory concentration(MIC).However,after a certain period of time following a dose,antibiotic concentrations become lower than the conventionally determined MIC and this often occurs at the site of infections[11].The subinhibitory concentrations(sub-MICs)of antibiotics,although not able to kill microorganisms,have been shown to alter the chemical and physical cellsurface characteristics and consequently the functionality and expression of bacterial virulence parameters such as adherence,cell-surfacehydrophobicity(CSH),biofilmformationand motility[12,13].Theseevidencesstronglyindicatethe effectiveness of antibiotics at sub-MIC levels and suggest their possible benefits to new treatments for microbial infections,in particular those that are associated with biofilm[14].For NTHi,while vaccine development is a key direction to prevent the infections,this strategy is promisingly in progress.To date,most therapeutic guidelines for NTHi treatment rely only on the existing antibiotics.Considering this,application of such alternative actions of antibiotics where significant virulence properties are altered would be valuable,providing a potential approach to control the infections caused by NTHi biofilm.
This study therefore investigated the in vitro interference of cefotaxime at subinhibitory concentrations on biofilm formation of NTHi.In addition,the effects of sub-MIC cefotaxime against a number of virulence properties expressed by clinical NTHi isolates were also evaluated.
2.Materials and methods
2.1.Bacterial strains and culture conditions
NTHi strains NU11 and NU47 used in this study were originally isolated from the respiratory clinical specimens(sputum and pus)from patients hospitalized at Buddhachinaraj Hospital(Phitsanulok,Thailand).The bacteria were identified according to standard microbiological procedures and PCR-serotyped as described previously[15].All isolates were reconstituted from frozen glycerol stocks and propagated on brain heart infusion(BHI;Oxoid,Basingstoke,England)agar or broth supplemented with nicotinamide adenine dinucleotide(Becton Dickinson,Maryland,USA;10μg/mL)and hemin(Becton Dickinson;10μg/mL)at 37°C under 5%CO2.
2.2.MIC and minimum bactericidal concentration(MBC)determination
MIC was determined by a broth microdilution method according to guidelines from the Clinical and Laboratory Standards Institute[16]using Haemophilus test medium(HTM). Two-fold serial dilutions of cefotaxime(Sigma-Aldrich,St Louis,MO,USA)were prepared in HTM in 96-well microtiter plates(Nunc™,Roskilde,Denmark).An adjusted bacterial inoculum was then added to each well to achieve a final concentration of 5×105CFU/mL.The final concentration of cefotaxime ranged from 0.25 to 512μg/mL.The MIC was defined as the lowest antibiotic concentration that yielded no visible growth after incubation at 37°C in a 5%CO2atmosphere for 24 h.To determine MBC,10μL was aspirated from the wells where there was no visible growth in the MIC experiment and plated onto BHI agar.The plates were incubated at 37°C under 5%CO2for 24 h and the MBC was defined as the lowest antibiotic concentration at which more than 99%of bacteria were killed compared with a non-treated control.
2.3.Biofilm formation assay
The ability of NTHi to form biofilm was determined based on a method described previously[17]with some modifications. Overnight cultures of NTHi were washed,diluted 1:200 in fresh HTM and aliquots(200μL)of the inoculum were added into the wells of 96-well flat-bottom microtiter plates(Nunc™,Roskilde,Denmark).Plates were incubated at 37°C under 5% CO2for 18 h without agitation.Prior to biofilm quantitation,growth was assessed by measuring the optical density(OD)at 600 nm using a microplate reader(Labsystem iEM Reader MF). Biofilms were then quantitated by staining the adherent cells with 1%(w/v)crystal violet solution for 15 min,after which the stained biofilms were washed with deionized water to remove unbound dye.The crystal violet bound to the biofilms was solubilized in 200μL of 95%ethanol and the OD was determined at 540 nm.Biofilm forming index(BFI)was used to express the amounts of biofilm formed by NTHi.This was calculated using the formula(AB-CW)/G,in which AB is the OD of the stained attached microorganisms,CW is the OD of the stained control wells containing microorganisms-free medium only and G is the OD of the cells growth in suspended culture.Semi-quantitative classification of biofilm formation designated as strong,moderate,weak or none was interpreted from the BFI readings[18].All NTHi strains were tested in quadruplicate for each experiment,and the results were reported as two independent experiments.
2.4.Sub-MIC effect of cefotaxime on biofilm formation
Effect of sub-MICs of cefotaxime on NTHi biofilm formation was examined by a microdilution method.Overnight cultures of NTHi adjusted to a final concentration of 5×105CFU/mL were inoculated into the wells of 96-well flat-bottom microtiter plates(Nunc™)and the plates were incubated at 37°C under 5%CO2for 4 h.The medium was discarded and biofilms were then exposed to cefotaxime(Sigma-Aldrich)at the concentrations of 0.1×and 0.5×MICs.Following incubation at 37°C in a 5% CO2atmosphere for 18 h,the medium and unattached cells were decanted and the wells were washed thoroughly with sterile deionized water.Biofilms were quantitated by the biofilm formation assay as described in Section 2.3.Non-treated control consisted of bacteria that were not exposed to the test antibiotic but otherwise treated identically.The percentage of biofilm formed in the presence of different concentrations of the test antibiotic was calculated using equation(OD540of the test/ OD540of non-treated control)×100.
2.5.Determination of cell viability at sub-MIC of cefotaxime
The effect of cefotaxime at sub-MIC level on viability of NTHi was determined in 125 mL flasks containing 25 mL of a bacterial culture(5×105CFU/mL,final concentration)andcefotaxime at the concentration of 0.5×MIC.A control was run without the test antibiotic but containing bacterial inoculum at equal cell density.The cultures were grown at 37°C under 5%CO2.At 0,4,8,12 and 16 h,samples were taken and viable counts were determined as follows:the samples were serially diluted in sterile phosphate buffer pH 7.2 and 100μL aliquots were plated onto BHI agar.The plates were incubated at 37°C under 5%CO2for 24 h,followed by enumeration of the CFU.
2.6.CSH assay
The CSH of the bacterial cells treated with 0.1×and 0.5× MIC cefotaxime as well as untreated cells was carried out using a standard microbial adhesion to n-hexadecane test[19].Briefly,4 mL of bacterial suspension prepared in 0.9%NaCl at an OD550of 0.8 was overlaid with 400μL n-hexadecane(Sigma-Aldrich). After 1-min agitation,the phases were allowed to separate for 15 min at room temperature.The OD of the aqueous phase was measured at 550 nm.The results were expressed as the proportion of cells that were excluded from the aqueous phase,determinedbytheequation:{[OD550(originalbacterial suspension)-OD550(aqueous phase)]/OD550(original bacterial suspension)}×100.
2.7.Fibronectin-binding assay
Fibronectin-binding assay was performed using human fibronectin coated 96-well microtiter plates(R&D Systems,Minneapolis,USA).Before the assay,NTHi strains were exposed to 0.1×and 0.5×MIC cefotaxime for 18 h at 37°C in a 5%CO2atmosphere.The bacteria adjusted to a final concentration of 5×105CFU/mL were then added to the fibronectin-coated wells and the plates were incubated at 37°C under 5%CO2for 18 h.After washing with sterile deionized water,bound cells were stained with 1%(w/v)crystal violet for 15 min at room temperature.Plates were washed again with sterile deionized water and allowed to air dry.The dye incorporated by the adherent cells was solubilized in 200μL of 95%ethanol and the OD measured at 540 nm.The OD value obtained from each strain without the test antibiotic was used as the control.
2.8.RNA extraction
Biofilms grown in the presence of 0.5×MIC cefotaxime were undertaken as above.Biofilm cells were harvested and centrifuged at 3500 r/min for 10 min.The bacterial pellet was suspended in Trizol reagent(Invitrogen™,USA)and RNA extraction was performed as per manufacturer protocol.RNA concentration and purity was determined by measuring absorbance at both 260 and 280 nm on a NanoDrop spectrophotometer(NanoDrop Technologies,USA).
2.9.RT-PCR assay
Gene expression was determined by RT-PCR assay.cDNA was obtained using RevertAid™H Minus first strand cDNA synthesis kit(Fermentas)following the manufacturer's instructions.cDNA was synthesized in 20μL reaction mixture using 0.5μg/mL oligo(dT)and 5μg extracted RNA as template. A negative control containing the reaction components without reverse transcriptase was included to ensure no DNA contamination.
The PCR amplification was carried out using the synthesized cDNA as template.Gene specific primers used in this study are listed in Table 1.The 25μL reactions consisted of 1×DreamTaq™Green PCR Master Mix(Fermentas,Canada),1μmol/L each forward primer and reverse primer and 2μL of cDNA.Amplifications were performed in a Gene Q Thermal Cycler(Bioer Technology,China).The PCR conditions consisted of an initial denaturation step of 94°C for 3 min,followed by 35 cycles of denaturation at 94°C for 1 min,annealing at 55°C for 1 min(for P2)or 30 cycles of denaturation at 94°C for 30 s,annealing at 58°C for 30 s,extension at 72°C for 1 min(for P6 and 16S rRNA),and final extension step of 10 min at 72°C.The PCR products were electrophoresed through a 1%a garose gel and visualized with an ultraviolet transilluminator after ethidium bromide staining.The band intensity was determined by ImageJ software.The intensities of the PCR products of the P2 and P6 gene were expressed as ratios to the 16S rRNA control gene.The ratios were then compared between the control(without the test antibiotic)and the test exposed to 0.5×MIC cefotaxime.
2.10.Statistical analysis
Data are expressed as mean±SEM.All data were statistically analyzed by Student's t-test using SPSS version 11.5(SPSS,Chicago,IL,USA).The differences were considered statistically significant for P<0.05.
3.Results
3.1.MIC and MBC
The MICs of cefotaxime were 48 and 64μg/mL while the MBCs were 160 and 320μg/mL for NTHi strains NU11 and NU47,respectively.It is noted that the MBC values were 3-5 times higher than their corresponding MIC values.

Table 1 Primers used in PCR amplifications.
3.2.Biofilm formation by NTHi
The ability of clinical NTHi isolates NU11 and NU47 to form biofilm was determined on the static condition and expressed as BFI.The BFI values of NU11 and NU47 were 1.41±1.17(biofilm OD,0.463;growth OD,0.328)and 3.08±1.29(biofilm OD,1.917;growth OD,0.622),respectively.According to the semi-quantitative classification of biofilm formation,any bacteria with BFI values≥1.10 are defined as strong-biofilm producers[18].The results indicated that the clinical NTHi isolates in this study were capable of forming biofilms and that all were strong-biofilm producing strains.
3.3.Effect of sub-MICs of cefotaxime on NTHi biofilm formation
The effect of sub-MIC cefotaxime on biofilm formation of the clinical NTHi strains NU11 and NU47 is shown in Figure 1.Modification on biofilm formation was obviously observed upon the addition of sub-MIC cefotaxime.The amounts of biofilm formation for NU11 and NU47 significantly lowered(P<0.01)in the presence of 0.1×MIC of cefotaxime than that of the non-treated control(without the test antibiotic),with the inhibition levels of 44.78%and 57.73%,respectively. The biofilm reduction level of up to 80%(NU11)was significantly(P<0.01)detected when exposed to 0.5×MIC of cefotaxime.It was also noted that,although varying in degrees,the inhibitory effects were observed in both of the clinical NTHi strains studied.
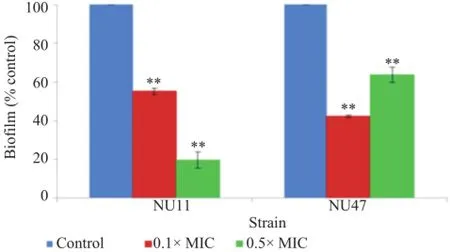
Figure 1.Effect of sub-MICs of cefotaxime on biofilm formation of NTHi strains NU11 and NU47.
3.4.Effect of sub-MIC cefotaxime on cell viability
As shown in Figure 2,all studied NTHi cultures,although slightly delayed in growth rate,continued to grow after addition of 0.5×MIC cefotaxime and the total bacterial counts appeared to be similar to those of the non-treated control cultures after prolonged exposure to the test antibiotic.The results suggested that cefotaxime at sub-MIC levels has no bactericidal.
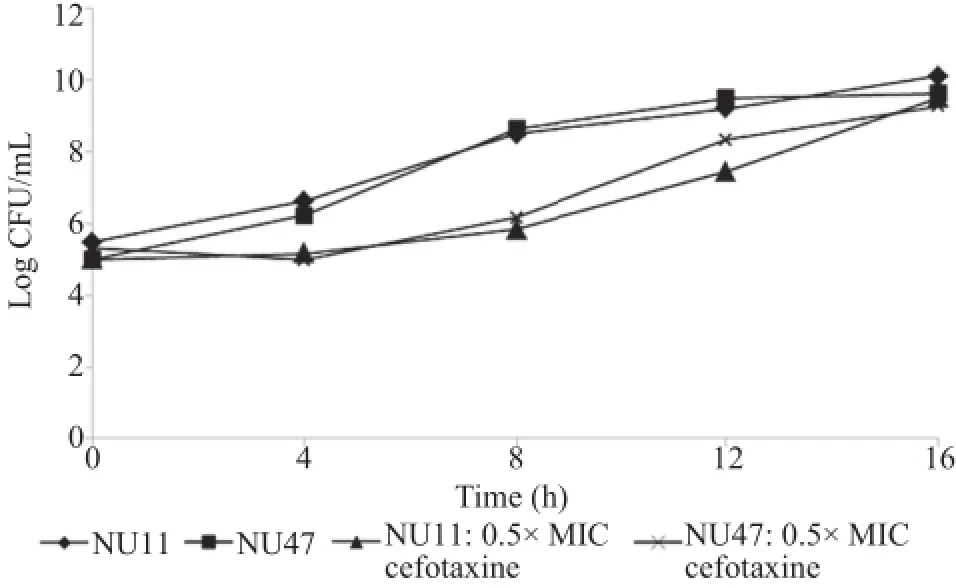
Figure 2.Effects of sub-MIC of cefotaxime on viability of NTHi.
3.5.Effect of sub-MICs of cefotaxime on CSH
The effect of sub-MICs of cefotaxime on CSH was assessed based on the bacterial adherence to hydrocarbon n-hexadecane. As shown in Figure 3,CSH in the test strains was affected in varying capacity when treated with sub-MICs of cefotaxime. Decrease in CSH of the test strains,in comparison to the nontreated control,was obviously demonstrated when exposed to 0.5×MIC cefotaxime.The reduction levels appeared to be 80.51%and 50.68%for NU11 and NU47,respectively.

Figure 3.Effect of sub-MICs of cefotaxime on cell surface hydrophobicity of NTHi strains NU11 and NU47.
3.6.Effect of sub-MICs of cefotaxime on fibronectinbinding activity
The ability of NTHi after the exposure to sub-MICs of cefotaxime to bind to host extracellular matrix fibronectin was investigated using a microtiter plate adherence assay.As shown in Figure 4,the fibronectin-binding activity of NTHi strains NU11 and NU47 was significantly reduced(P<0.01)after the exposure to 0.1×MIC of cefotaxime as compared with that of non-treated control.The reduction levels for NU11 and NU47 were 56.75%and 46.43%,respectively.Significant decrease in fibronectin-binding activity was also observed when the testNTHi strains were exposed to 0.5×MIC of cefotaxime,with the reduction levels of 60.58%and 44.92%for NU11 and NU47,respectively.
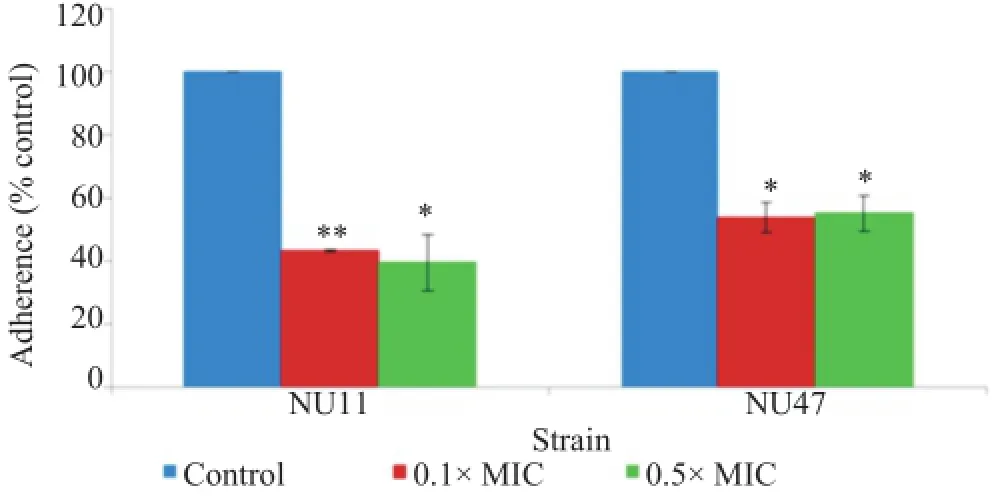
Figure 4.Effect of sub-MICs of cefotaxime on NTHi strains NU11 and NU47 binding to human fibronectin.
3.7.Effect of sub-MICs of cefotaxime on expression of P2 and P6 genes
To further investigate whether sub-MIC cefotaxime altered the expression of NTHi adhesin genes,semi-quantitative RTPCR was performed.Figure 5 shows that in the absence of the test antibiotic the NTHi strains in this study,although differed in degrees,expressed both the P2 and P6 genes.Expression of the P2 gene in relation to 16S rRNA was inhibited after the NTHi culture was exposed to 0.5×MIC cefotaxime(Figure 5A),compared to the culture without the test antibiotic.Significant down-regulation(P<0.01)was also detected for the P6 gene after the exposure to 0.5×MIC of cefotaxime,compared to nontreated control(Figure 5B).

Figure 5.The relative mRNA levels of the adhesion P2(A)and P6(B)genes to 16S rRNA gene after exposure to 0.5×MIC of cefotaxime.
4.Discussion
This study reports the in vitro interference of cefotaxime at subinhibitory concentrations on biofilm formation of NTHi.The results herein demonstrated that subinhibitory concentrations of cefotaxime efficiently decreased the formation of NTHi biofilms. This biofilm inhibitory activity was evidenced both at 0.1×and 0.5×MIC levels and in all the NTHi strains studied.The NTHi used in this study were isolated from clinical specimens and were all verified to be strong biofilm producers.Our results therefore suggested that the biofilm inhibitory activity of sub-MIC cefotaxime occurred among various strong biofilm-forming clinical isolates of NTHi,even with the very strong biofilm-producing strain(NU47).These findings indicate the potent biofilm inhibitory activity of sub-MIC cefotaxime and suggest its potential use for treatment of biofilm-related NTHi infections.
The biofilm inhibitory activity in this study was observed after the addition of sub-MIC cefotaxime to the initial NTHi biofilms(4 h),and that biofilm inhibition is independent of decreasing bacterial viability,it is possible that sub-MIC cefotaxime inhibited NTHi biofilm formation by interfering the early step of bacterial adhesion.The initial adherence and colonization to surfaces is the first crucial step for establishment of infection and the following biofilm formation.Our findings are thus of clinical importance because cefotaxime at sub-MIC levels may act as an anti-adherent and anti-biofilm agent,and its presence would prevent establishment of infection and subsequently inhibit the biofilm formation.Interfering the requisite virulence properties(e.g.bacterial adherence)directly,rather than the vital cellular growth or viability provides an attractive alternative for limiting or reducing the severity of infections[23].Since anti-biofilm activity does not affect the vital bacterial viability,cefotaxime at sub-MIC levels will possibly exert limited selective pressure on NTHi and therefore may avoid development of resistant bacteria.
Enormous literature so far has provided evidence that a number of pathogenic bacteria depend on the hydrophobic interactions for successful adherence to and colonization on host cells[24,25].A positive correlation between hydrophobicity and both levels of bacterial adhesiveness and biofilm formation has been described[25-27],thus emphasizing significance of hydrophobicity for bacterial infections.In this study,decreased cell-surface hydrophobicity was obviously seen after the test NTHi were exposed to sub-MIC cefotaxime.Consistently,significant reduction on the ability to bind to fibronectin was also observed after treated the NTHi strains with cefotaxime at sub-MIC levels.Fibronectin is an extracellular matrix protein of host cells,and interactions with extracellularmatrixfibronectinhavebeenreported to beone ofthe successfuladherencestrategiesemployedbyNTHi[28].Ourresults indicated the effectiveness of sub-MIC cefotaxime in reducing the critical step in bacterial adherence to host cells.Thus,these findings further strengthened the potential use of sub-MIC cefotaxime for interrupting the establishment of infection and the subsequent stage of NTHi biofilm development.
In order to provide additional insight into the mechanisms by which sub-MIC cefotaxime decreased bacterial adherence and inhibited biofilm formation,alteration in gene expression of bacterial components conferring cell-surface interaction as well as biofilm development was determined.The outer membrane proteins P2 and P6 are Haemophilus influenzae surface structures that play a significant role in the initial binding of bacteria to host cells[29].Previous studies also revealed that these two proteins are expressed during growth as a biofilm[17].
Recently,biofilm-specific proteins presented in immature biofilms were investigated,and the outer membrane protein P2 was one of the two proteins identified[30].The fact that inhibition in both P2 and P6 gene expressions upon the exposure to sub-MIC cefotaxime was noted in this study,cefotaxime at sub-MIC levels might interrupt the expression of P2 and P6 genes,thereby reducing expression of NTHi adhesins,and subsequently leading to the decreased bacterial adherence and the inhibition NTHi biofilm formation.
In conclusion,the present study demonstrated for the first time that cefotaxime at subinhibitory concentrations inhibited biofilm formation of the clinical strong-biofilm forming NTHi isolates.Such anti-biofilm effects were possibly related to interference with cell-surface hydrophobicity,fibronectin-binding activity as well as alteration of the P2 and P6 gene expression.Since other bacterial adhesins or other nonadhesin components or even other mechanisms by which sub-MIC cefotaxime interfered with NTHi biofilm development may be involved and should not be excluded,further studies remain to be investigated.Nevertheless,our data provide a rationale for the use of subinhibitory concentrations of cefotaxime in diseases involving NTHi biofilms.Future work is required to confirm our findings in vivo with an appropriate animal model.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was supported by the National Research Council of Thailand through the Annual Research Fund of Naresuan University(Grant No.R2557B011).
References
[1]Swards WE.Nontypeable Haemophilus influenzae biofilms:role in chronic airway infections.Front Cell Infect Microbiol2012;25(2):97.
[2]Agrawal A,Murphy TF.Haemophilus influenzae infections in the H.influenzae type b conjugate vaccine era.J Clin Microbiol 2011;49(11):3728-32.
[3]Garmendia J,Martí-Lliteras P,Moleres J,Puig C,Bengoechea JA. Genotypic and phenotypic diversity in the noncapsulated Haemophilus influenzae:adaptation and pathogenesis in the human airways.Int Microbiol 2012;15(4):159-72.
[4]Van Eldere J,Slack MP,Ladhani S,Cripps AW.Non-typeable Haemophilus influenzae,an under-recognised pathogen.Lancet Infect Dis 2014;14(12):1281-92.
[5]de la Fuente-N´uñez C,Reffuveille F,Fern´andez L,Hancock RE. Bacterial biofilm development as a multicellular adaptation:antibiotic resistance and new therapeutic strategies.Curr Opin Microbiol 2013;16(5):580-9.
[6]Ehrlich GD,Veeh R,Wang X,Costerton JW,Hayes JD,Hu FZ,et al.Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media.JAMA 2002;287(13):1710-5.
[7]R¨omling U,Balsalobre C.Biofilm infections,their resilience to therapy and innovative treatment strategies.J Intern Med 2012;272(6):541-61.
[8]Hamilos DL.Host-microbial interactions in patients with chronic rhinosinusitis.J Allergy Clin Immunol 2014;133:640-53.
[9]Chen L,Wen YM.The role of bacterial biofilm in persistent infections and control strategies.Int J Oral Sci 2011;3(2):66-73.
[10]Kyd JM,McGrath J,Krishnamurthy A.Mechanisms of bacterial resistance to antibiotics in infections of COPD patients.Curr Drug Targets 2011;12(4):521-30.
[11]Mandell LA,Afnan M.Mechanisms of interaction among subinhibitory concentrations of antibiotics,human polymorphonuclear neutrophils,and Gram-negative bacilli.Antimicrob Agents Chemther 1991;35(7):1291-7.
[12]de Andrade JP,de Macˆedo Farias L,Ferreira JF,Bruna-Romero O,da Gl´oria de Souza D,de Carvalho MA,et al.Sub-inhibitory concentration of piperacillin-tazobactam may be related to virulence properties of filamentous Escherichia coli.Curr Microbiol 2016;72(1):19-28.
[13]Cirioni O,Silvestri C,Ghiselli R,Kamysz W,Minardi D,Castelli P,et al.In vitro and in vivo effects of sub-MICs of pexiganan and imipenem on Pseudomonas aeruginosa adhesion and biofilm development.Infez Med 2013;21(4):287-95.
[14]Ratcliff WC,Denison RF.Alternative actions for antibiotics.Science 2011;332(6029):547-8.
[15]Kunthalert D,Baothong S,Khetkam P,ChokchaisiriS,Suksamrarn A.A chalcone with potent inhibiting activity against biofilm formation by nontypeable Haemophilus influenzae. Microbiol Immunol 2014;58(10):581-9.
[16]Clinical and Laboratory Standards Institute.Performance standards for antimicrobial susceptibility testing,document M100-S17. Wayne,PA:Clinical and Laboratory Standards Institute;2007.[Online]Available from:http://microbiolab-bg.com/wp-content/ uploads/2015/05/CLSI.pdf[Accessed on 1st November,2015]
[17]Murphy TF,Kirkham C.Biofilm formation by nontypeable Haemophilus influenzae:strain variability,outer membrane antigen expression and role of pili.BMC Microbiol 2002;2:7.
[18]Naves P,del Prado G,Huelves L,Gracia M,Ruiz V,Blanco J,et al. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent.J Appl Microbiol 2008;105:585-90.
[19]Mattos-Guaraldi AL,Formiga LC,Andrade AF.Cell surface hydrophobicity of sucrose fermenting and nonfermenting Corynebacterium diphtheriae strains evaluated by different methods.Curr Microbiol 1999;38(1):37-42.
[20]Hiltke TJ,Sethi S,Murphy TF.Sequence stability of the gene encoding outer membrane protein P2 of nontypeable Haemophilus influenzae in the human respiratory tract.J Infect Dis 2002;185(5):627-31.
[21]StrålinK,B¨ackmanA,HolmbergH,FredlundH,Olc´enP.Designof a multiplex PCR for Streptococcus pneumoniae,Haemophilus influenzae,Mycoplasma pneumoniae and Chlamydophila pneumoniae to be used on sputum samples.APMIS 2005;113(2):99-111.
[22]Abdeldaim GM,Strålin K,Kirsebom LA,Olc´en P,Blomberg J,Herrmann B.Detection of Haemophilus influenzae in respiratory secretions from pneumonia patients by quantitative real-time polymerase chain reaction.Diagn Microbiol Infect Dis 2009;64(4):366-73.
[23]Klemm P,Vejborg RM,Hancock V.Prevention of bacterial adhesion.Appl Microbiol Biotechnol 2010;88(2):451-9.
[24]Krasowska A,Sigler K.How microorganisms use hydrophobicity and what does this mean for human needs?Front Cell Infect Microbiol 2014;4:112.
[25]Gomes LC,Silva LN,Simões M,Melo LF,Mergulhão FJ.Escherichia coli adhesion,biofilm development and antibiotic susceptibility on biomedical materials.J Biomed Mater Res A 2015;103:1414-23.
[26]Bruzaud J,Tarrade J,Coudreuse A,Canette A,Herry JM,Taffin de Givenchy E,et al.Flagella but not type IV pili are involved in the initial adhesion of Pseudomonas aeruginosa PAO1 to hydrophobic or superhydrophobic surfaces.Colloids Surf B Biointerfaces 2015;131:59-66.
[27]Souza MC,dos Santos LS,Sousa LP,Faria YV,Ramos JN,Sabbadini PS,et al.Biofilm formation and fibrinogen and fibronectin binding activities by Corynebacterium pseudodiphtheriticum invasive strains.Antonie Van Leeuwenhoek 2015;107(6):1387-99.
[28]Su YC,Mukherjee O,Singh B,Hallgren O,Westergren-Thorsson G,Hood D,et al.Haemophilus influenzae P4 interacts with extracellular matrix proteins promoting adhesion and serum resistance.J Infect Dis 2015;213(2):314-23.
[29]Erwin AL,Smith AL.Nontypeable Haemophilus influenzae:understanding virulence and commensal behavior.Trends Microbiol 2007;15(8):355-62.
[30]Wu S,Baum MM,Kerwin J,Guerrero D,Webster S,Schaudinn C,et al.Biofilm-specific extracellular matrix proteins of nontypeable Haemophilus influenzae.Pathog Dis 2014;72(3):143-60.
16 Nov 2015
in revised form 14 Dec 2015
Duangkamol Kunthalert,Department of Microbiology and Parasitology,Faculty of Medical Science,Naresuan University,Phitsanulok 65000,Thailand.
Tel:+66 5596 4626
Fax:+66 5596 4770
E-mails:kunthalertd@yahoo.com,duangkamolk@nu.ac.th
Foundation Project:Supported by the National Research Council of Thailand through the Annual Research Fund of Naresuan University(Grant No.R2557B011).
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creative commons.org/licenses/by-nc-nd/4.0/).
 Asian Pacific Journal of Tropical Biomedicine2016年9期
Asian Pacific Journal of Tropical Biomedicine2016年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Milk losses due to bovine tropical theileriosis(Theileria annulata infection)in Algeria
- Vasoprotective effects of rice bran water extract on rats fed with high-fat diet
- Purification,characterization and antiproliferative activity of L-asparaginase from Aspergillus oryzae CCT 3940 with no glutaminase activity
- Anticancer effects of saponin and saponin-phospholipid complex of Panax notoginseng grown in Vietnam
- Free radical scavenging activity of three different flowers-Hibiscus rosa-sinensis,Quisqualis indica and Senna surattensis
- Tuberculosis in the mines of Zambia:A case for intervention
