Ab initio Study on Formation Mechanism of Spiro-Si-Heterocyclic Ring Compound Involving Ge from H2Ge=Si:and Formaldehyde
Xiu-hui Lu,Dang-sheng Wang,Jing-jing MingSchool of Chemistry and Chemical Engineering,University of Jinan,Jinan 250022,China(Dated:Received on July 2,2015;Accepted on November 27,2015)
Ab initio Study on Formation Mechanism of Spiro-Si-Heterocyclic Ring Compound Involving Ge from H2Ge=Si:and Formaldehyde
Xiu-hui Lu∗,Dang-sheng Wang,Jing-jing Ming
School of Chemistry and Chemical Engineering,University of Jinan,Jinan 250022,China
(Dated:Received on July 2,2015;Accepted on November 27,2015)
H2Ge=Si∶and its derivatives(X2Ge=Si∶,X=H,Me,F,Cl,Br,Ph,Ar,...)are new species. Its cycloaddition reactions are new area for the study of silylene chemistry.The cycloaddition reaction mechanism of singlet H2Ge=Si∶and formaldehyde has been investigated with the MP2/aug-cc-pVDZ method.From the potential energy profile,it could be predicted that the reaction has one dominant reaction pathway.The reaction rule is that two reactants firstly form a four-membered Ge-heterocyclic ring silylene through the[2+2]cycloaddition reaction. Because of the 3p unoccupied orbital of Si∶atom in the four-membered Ge-heterocyclic ring silylene and the π orbital of formaldehyde forming a π→p donor-acceptor bond,the four-membered Ge-heterocyclic ring silylene further combines with formaldehyde to form an intermediate.Because the Si∶atom in the intermediate undergoes sp3hybridization after transition state,then the intermediate isomerizes to a spiro-Si-heterocyclic ring compound involving Ge via a transition state.The result indicates the laws of cycloaddition reaction between H2Ge=Si∶or its derivatives(X2Ge=Si∶,X=H,Me,F,Cl,Br,Ph,Ar,...)and asymmetric π-bonded compounds are significant for the synthesis of small-ring involving Si and Ge and spiro-Si-heterocyclic ring compounds involving Ge.
H2Ge=Si∶,Four-membered Ge-heterocyclic ring silylene,Spiro-Si-heterocyclic ring compound,Potential energy profile
I.INTRODUCTION
Unsaturated silylene is a kind of important active intermediates.Its cycloaddition reaction can provide a convenient,short synthesis pathway for the synthesis of tensility cyclotella,silapolycyclic compounds,and synthesize the compounds which are difficult to be generated by general methods,and it is also regarded as an effective method in the synthesis of new bonds and heterocyclic compounds with Si.H2C=Si∶is the simplest unsaturated silylene,which was firstly observed experimentally by Leclerq and Dubois in 1979[1].Srinivas et al.[2]have used neutralization-reionization mass spectrometry to show that H2C=Si∶is a viable molecule in the low-pressure gas phase.Theoretical study indicates that the ground state of H2C=Si∶is singlet state.The energy of H2C=Si∶is 84 kcal/mol lower than that of silaacetylide.H2C=Si∶is the lowest energy isomer[3]. We have performed some studies on the cycloaddition reaction of unsaturated silylenes preliminarily[4-8],but these studies are limited to the cycloaddition reactions of H2C=Si∶and its derivatives(X2C=Si∶,X=H,Me,F,Cl,Br,Ph,Ar,...).There are no reports on the cycloaddition reaction of H2Ge=Si∶and its derivatives(X2Ge=Si∶,X=H,Me,F,Cl,Br,Ph,Ar,...)till now,it is a new branch of unsaturated silylene’s cycloaddition reaction.It is quite difficult to investigate mechanisms of cycloaddition reaction directly by experimental methods due to the high activity of H2Ge=Si∶and its derivatives(X2Ge=Si∶,X=H,Me,F,Cl,Br,Ph,Ar,...),therefore,the theoretical study is more practical.To explore the rules of cycloaddition reaction between H2Ge=Si∶(including its derivatives)and the asymmetric π-bonded compounds,H2Ge=Si∶and formaldehyde were selected as model molecules.Using Qiao et al.and Zhao et al.’s research methods[9-11],its mechanism(considering the hydrogen transfer simultaneously)was investigated and analyzed theoretically. The results show that cycloaddition reaction consists of five possible pathways,as shown in Scheme 1.
II.COMPUTATIONAL METHODS
MP2/aug-cc-pVDZ[12]implemented in the Gaussian 03 package is employed to locate all the stationary points along the reaction pathways.Full optimization and vibrational analysis are done for the stationary points on the reaction profile.Zero point energy corrections are included for the energy calculations.In order to explicitly establish the relevant species,the intrinsic reaction coordinate(IRC)[13,14]is also calculated for all the transition states appearing on the cycloadditionenergy surface profile.
Scheme 1 The cycloaddition reaction consists of five possible pathways.

FIG.1 Optimized MP2/aug-cc-pVDZ geometrical parameters and the atomic numbering for the species in cycloaddition reaction(1).Bond lengths are in˚A,bond angles and dihedral angles are in(◦).
III.RESULTS AND DISCUSSION
A.Reaction(1):formation of the three-membered ring product(P1)
Theoretical calculations show that the ground state of H2Ge=Si∶is a singlet state.The geometrical parameters of the intermediate(INT1),transition state(TS1)and product(P1)in reaction(1)of H2Ge=Si∶and formaldehyde are given in Fig.1.The energies are listed in Table I,and the potential energy surface for the cycloaddition reaction is shown in Fig.2.
According to Fig.2,it can be seen that the reaction(1)consists of two steps∶the first step is that the two reactants(R1,R2)firstly form an intermediate(INT1),which is a barrier-free exothermic reaction of 65.8 kJ/mol;in the second step,the intermediate(INT1)isomerizes to a three-membered ring product(P1)through the transition state(TS1)with an energy barrier of 63.1 kJ/mol.
B.Reaction(2):formation of four-membered Ge-heterocyclic ring silylene(P2)and H-transfer products(P2.1 and P2.2)
The geometrical parameters of transition states(TS2,TS2.1,TS2.2)and products(P2,P2.1,P2.2)appearingin reaction(2)between H2Ge=Si∶and formaldehyde are given in Fig.3.The energies are listed in Table I,and the potential energy surface for the cycloaddition reaction is shown in Fig.2.

FIG.2 The potential energy surface of the cycloaddition reactions between H2Ge=Si:and formaldehyde with MP2/aug-ccpVDZ.

TABLE I Zero point energy(ZPE)and relative energies(ER,in kJ/mol)for the species from MP2/aug-cc-pVDZ method.
According to Fig.2,it can be seen that reaction(2)consists of four steps∶the first step is that the two reactants(R1,R2)form an intermediate(INT2),which is a barrier-free exothermic reaction of 72.0 kJ/mol;the second step is that the intermediate(INT2)isomerizes to a four-membered Ge-heterocyclic ring silylene(P2)via the transition state(TS2)with an energy barrier of 1.0 kJ/mol;the next two steps are that the four-membered Ge-heterocyclic ring silylene(P2) undergoes hydrogen transfer via the transition states TS2.1 and TS2.2 with energy barriers of 112.1 and 125.2 kJ/mol,respectively,resulting in the formation of products(P2.1 and P2.2).The hydrogen transfer reactions of P2→P2.1 and P2→P2.2 are prohibited in thermodynamics,because the energies of P2.1 and P2.2 are 40.2 and 79.4 kJ/mol higher than that of P2,and the reaction(2)will end in product P2.Comparing reaction(2)with reaction(1),the energy barrier of TS2 is 62.1 kJ/mol lower than that of TS1,therefore,the reaction(2)is the dominant reaction pathway.

FIG.3 Optimized MP2/aug-cc-pVDZ geometrical parameters of TS2,TS2.1,TS2.2,P2,P2.1,P2.2 and the atomic numbering for cycloaddition reaction(2).Bond lengths are in˚A,bond angles and dihedral are in(◦).

FIG.4 Optimized MP2/aug-cc-pVDZ geometrical parameters of INT3,TS3,P3 and the atomic numbering for cycloaddition reaction(3).Bond lengths are in˚A,bond angles and dihedral angles are in(◦).
C.Reaction(3):formation of a spiro-Si-heterocyclic ring compound involving Ge(P3)
In reaction(3),the four-membered Ge-heterocyclic ring silylene(P2)further reacts with formaldehyde(R2)to form a spiro-Si-heterocyclic ring compound involving Ge(P3).The geometrical parameters of intermediate(INT3),transition state(TS3)and product(P3)which appear in reaction(3)are given in Fig.4.The energies are listed in Table I,and the potential energy surface for the cycloaddition reaction is shown in Fig.2.
According to Fig.2,it can be seen that the process of the reaction(3)is as follows∶first,on the basis of P2 formed in the reaction(2),P2 further reacts with formaldehyde to form an intermediate(INT3),which is also a barrier-free process with an exothermic process of 44.0 kJ/mol;then,the intermediate(INT3)isomerizes to a spiro-Si-heterocyclic ring compound involving Ge(P3)via the transition state(TS3)with a energy barrier of 83.4 kJ/mol.
D.Reaction(4):formation of four-membered Ge-heterocyclic ring silylene(INT4)and its isomer(P4),H-transfer product(P4.1)
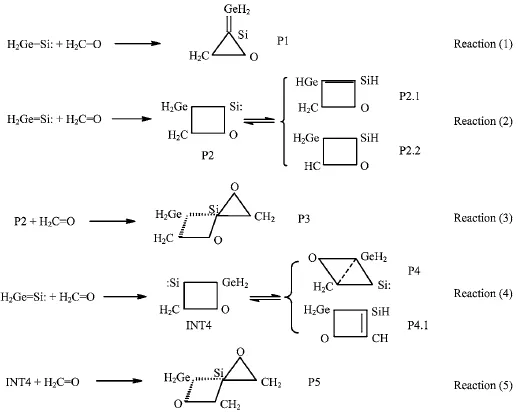
The geometrical parameters of the intermediate(INT4),transition states(TS4,TS4.1)and products(P4,P4.1)which appear in reaction(4)between H2Ge=Si∶and formaldehyde are given in Fig.5.The energies are listed in Table I,and the potential energy surface for the cycloaddition reaction is shown in Fig.2.
According to Fig.2,it can be seen that reaction(4)consists of three steps∶in the first step,the two reactants(R1,R2)form a four-membered Ge-heterocyclic ring silylene(INT4),which is a barrier-free exothermic reaction of 173.4 kJ/mol;in the second step,the INT4 isomerizes to a four-membered ring product(P4)through the transition state(TS4)with energy barrier of 30.4 kJ/mol;in the third step,the INT4 undergoes hydrogen transfer via transition states(TS4.1)with energy barrier of 43.3 kJ/mol,resulting in the formation of product(P4.1).INT4→P4 and INT4→P4.1 are pro-hibited in thermodynamics,because the energies of P4 and P4.1 are 26.7 and 11.8 kJ/mol higher than that of INT4,and the reaction(4)will end in product INT4.
According to Fig.2,Fig.3,and Fig.5 and statistical thermodynamics formula∶

it can be seen that INT2 and INT4 are isomerides,R1+R2→INT2 and R1+R2→INT4 are two parallel reactions,the equilibrium distributions of INT2 and INT4 are PT(INT2)and PT(INT4),respectively.

So,INT4 is the main distribution.
FIG.5 Optimized MP2/aug-cc-pVDZ geometrical parameters of INT4,TS4,TS4.1,P4,P4.1 and the atomic numbering for the species in cycloaddition reaction(4).Bond lengths are in˚A,bond angles and dihedral angles are in(◦).

FIG.6 Optimized MP2/aug-cc-pVDZ geometrical parameters of INT5,TS5,P5 and the atomic numbering for cycloaddition reaction(5).Bond lengths are in˚A,bond angles and dihedral are in(◦).
E.Reaction(5):formation of spiro-Si-heterocyclic ring compound involving Ge(P5)
In reaction(5),the four-membered Ge-heterocyclic ring silylene(INT4)further reacts with formaldehyde(R2)to form a spiro-Si-heterocyclic ring compound involving Ge(P5).The geometrical parameters of intermediate(INT5),transition state(TS5)and product(P5)which appear in reaction(5)are given in Fig.6. The energies are listed in Table I,and the potential energy surface for the cycloaddition reaction is shown in Fig.2.
According to Fig.2,it can be seen that the process of reaction(5)as follows∶on the basis of the two reactants(R1 and R2)to form a four-membered Ge-heterocyclic ring silylene(INT4),it further reacts with formaldehyde(R2)to form an intermediate(INT5),which is a barrier-free exothermic reaction of 0.8 kJ/mol.And then intermediate(INT5)isomerizes to a spiro-Si-heterocyclic ring compound involving Ge(P5)via a transition state(TS5)with energy barrier of 6.5 kJ/mol.
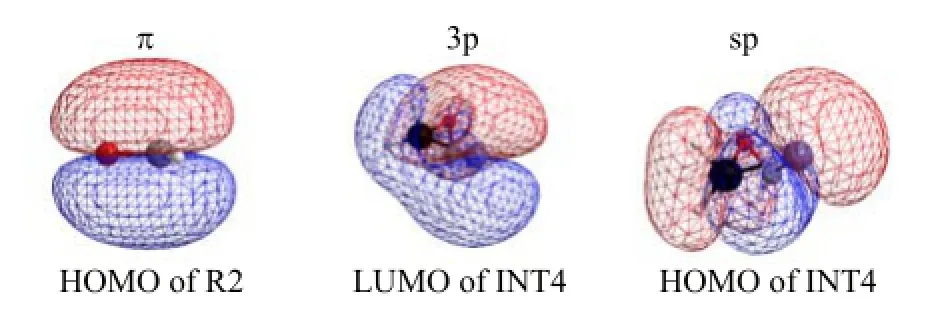
FIG.7 The frontier molecular orbital of R2 and INT4.
F.Theoretical analysis and explanation of the dominant reaction channel
According to the above analysis,reaction(5)should be the dominant reaction channel of the cycloaddition reaction between singlet H2Ge=Si∶and formaldehyde as follows∶

In the reaction,the frontier molecular orbitals of R2 and INT4 are shown in Fig.7.According to Fig.7,the frontier molecular orbitals of R2 and INT4 can be expressed in Fig.8.The mechanism of reaction(5)can be explained with the frontier molecular orbital diagrams(Fig.8)and Fig.1,Fig.5,and Fig.6.According to Fig.1 and Fig.5,as H2Ge=Si∶(R1)initially interacts with formaldehyde(R2),the[2+2]cycloaddition of the bonding π-orbitals first results in a four-membered Geheterocyclic ring silylene(INT4).
Because INT4 is an active intermediate,so INT4 may further react with formaldehyde(R2)to form a spiro-Si-heterocyclic ring compound involving Ge(P5).The mechanism of the reaction can be explained with Fig.6 and Fig.8.When INT4 interacts with formaldehyde(R2),the 3p unoccupied orbital of the Si∶atom in INT4 will insert the p orbital of formaldehyde from oxygen side,Then the shift of π-electrons to the p unoccupied orbital gives a π→p donor-acceptor bond,leading to the formation of intermediate(INT5).As the reaction goes on,because∠C1SiO2(INT5∶112.3◦,TS5∶120.2◦)increases gradually,∠C2O2Si(INT5∶137.8◦,TS5∶114.2◦)decrease gradually,and the length of C2-O2 bond(INT5∶1.380˚A,TS5∶1.408˚A)elongates gradually.Finally the Si atom in INT5 hybridizes to sp3hybrid orbital after the transition state(TS5),forming the stabler spiro-Si-heterocyclic ring compound involving Ge(P5).
IV.CONCLUSION
On the basis of the potential energy surface profile the cycloaddition reaction between singlet H2Ge=Si∶and formaldehyde obtained with the MP2/aug-cc-pVDZ method can be predicted.This reaction has one dominant channel.It consists of three steps∶the first step is that the two reactants(R1,R2)form a fourmembered Ge-heterocyclic ring silylene(INT4)through reaction(4),which is a barrier-free exothermic reaction of 173.4 kJ/mol;the second step is that INT4 further reacts with formaldehyde(R2)to form an intermediate(INT5),which is also a barrier-free exothermic reaction of 0.8 kJ/mol;the third step is that intermediate(INT5)isomerizes to a spiro-Si-heterocyclic ring compound involving Ge(P5)via a transition state(TS5)with an energy barrier of 6.5 kJ/mol.

FIG.8 A schematic interaction diagram for the frontier orbitals of INT4 and H2C=O(R2).
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.51102114).
[1]H.Leclercq and I.Dubois,J.Mol.Spectrosc.76,39(1979).
[2]R.Srinivas,D.Sulzle,and H.Schwarz,J.Am.Chem. Soc.113,52(1991).
[3]R.Damrauer,C.H.DePuy,S.E.Barlow,and S. Gronert,J.Am.Chem.Soc.110,2005(1988).
[4]X.H.Lu,H.B.Yu,and W.R.Wu,New J.Chem.29,332(2005).
[5]X.H.Lu,H.B.Yu,Y.H.Xu,P.P.Xiang,and Y.D. Liu,J.Mol.Struct.Theochem.770,185(2006).
[6]X.H.Lu,H.B.Yu,Y.H.Xu,P.P.Xiang,and X.Che,Mol.Phys.105,1961(2007).
[7]X.H.Lu,X.Che,H.B.Yu,P.P.Xiang,and Y.H.Xu,J.Mol.Struct.Theochem.821,53(2007).
[8]X.H.Lu,H.B.Yu,X.Che,and P.P.Xiang,Int.J. Quant.Chem.108,1114(2008).
[9]Y.Qiao and K.L.Han,Org.Biomol.Chem.10,7689(2012).
[10]Y.Qiao and T.S.Chu,J.Org.Chem.76,3086(2011).
[11]G.J.Zhao and K.L.Han,Acc.Chem.Res.45,404(2012).
[12]L.A.Curtis,K.Raghavachari,and J.A.Pople,J. Chem.Phys.98,1293(1993).
[13]K.Fukui,J.Phys.Chem.74,4161(1970).
[14]K.Ishida,K.Morokuma,and A.Komornicki,J.Chem. Phys.66,2153(1977).
∗Author to whom correspondence should be addressed.E-mail:lxh@ujn.edu.cn
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年2期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年2期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Incorporation of Reactive Corrosion Inhibitor in Waterborne Acrylic Polyurethane Coatings and Evaluation of its Corrosion Performance
- Response Ability to External Signal Enhanced by Biological Spatial Configuration in Coupled Hindmarsh-Rose Neural System
- Synthesis and Surface Activity of Heterogemini Imidazolium Surfactants
- Substituent Effects on Reduction Potentials of Meta-substituted and Para-substituted Benzylideneanilines
- Preparation of Nitrogen-Doped Carbon Catalyst to Oxygen Reduction Reaction and Influence of Protective Gas Flowing on Its Activity
- Structural and Magnetic Properties of Chemically Synthesized Pd-Modified NiFe2O4Nanoparticles
