Substituent Effects on Reduction Potentials of Meta-substituted and Para-substituted Benzylideneanilines
Lin-yan Wang,Chao-tun Cao,Chen-zhong Cao∗a.School of Chemistry and Chemical Engineering,Central South University,Changsha 410083,Chinab.School of Chemistry and Chemical Engineering,Hunan University of Science and Technology,Xiangtan 411201,Chinac.Key Laboratory of Theoretical Organic Chemistry and Function Molecule,Ministry of Education,Hunan Provincial University Key Laboratory of QSAR/QSPR,Hunan University of Science and Technology,Xiangtan 411201,China(Dated:Received August 13,2015;Accepted on October 30,2015)
Substituent Effects on Reduction Potentials of Meta-substituted and Para-substituted Benzylideneanilines
Lin-yan Wanga,b,c,Chao-tun Caob,c,Chen-zhong Cao∗a,b,c
a.School of Chemistry and Chemical Engineering,Central South University,Changsha 410083,China
b.School of Chemistry and Chemical Engineering,Hunan University of Science and Technology,Xiangtan 411201,China
c.Key Laboratory of Theoretical Organic Chemistry and Function Molecule,Ministry of Education,Hunan Provincial University Key Laboratory of QSAR/QSPR,Hunan University of Science and Technology,Xiangtan 411201,China
(Dated:Received August 13,2015;Accepted on October 30,2015)
Effects of meta-substituent of 3,4′/4,3′/3,3′-substituted benzylideneanilines (XBAYs) on theelectrochemical reduction potentials (E(Red)) were investigated, in which 49 samples of targetcompounds were synthesized, and their reduction potentials were measured by cyclicvoltammetry. The substituent effects on the E(Red) of target compounds were analyzedand an optimality equation with four parameters (Hammett constant σ of X, Hammettconstant σ of Y, excited-state substituent constant σexCC of X, and the substituent specificcross-interaction effect ΔσexCC2 between X and Y) was obtained. The results show that thefactors affecting the E(Red) of 3,4′/4,3′/3,3′-substituted XBAYs are different from those of4,4′-substituted XBAYs. For 3,4′/4,3′/3,3′-substituted XBAYs, σ(X) and σ(Y) must beemployed, and the contribution of ΔσexCC2 is important and not negligible. Compared with4,4′-substituted XBAYs, X group contributes less to 3,4′/4,3′/3,3′-substituted XBAYs, whileY group contributes more to them. Additionally, it was observed that either para-substitutedXBAYs or meta-substituted XBAYs, the substituent effects of X are larger than those of Yon the E(Red) of substituted XBAYs.
I.INTRODUCTION
In the molecule of benzylideneanilines(abbreviated as XBAYs,XPhCH=NPhYs),CH=N is a bridge linking two aromatic rings,in which one ring carries substituent X and another ring carries substituent Y.Changes of X and Y in XBAY can affect its molecular overall electron distribution and its properties.Therefore,the substituent effects play an important role in the studies of this kind of compounds,and attain great interest in recent years[1-3].In addition,substituent effects are also the focus of quantitative structure property/activity relationship(QSPR/QSAR)[4-6].
Based on the mechanism of electrochemical reduction,the reduction progress of title compounds and their derivatives firstly carried out on the carbon atom of CH=N,and the more positive charge the carbon atom has,the easier the XBAY is to be reduced and the more positive its potential value is[7-10].So, the electrochemical reduction potentials E(Red)values of XBAYs can be determined by the charge of the carbon atoms which is affected by the substituents of X and Y.In previous reports,the substituent effects on the reduction potentials of XBAYs or the analogous compounds were studied[11-13].For example,Celik et al.ever analyzed the effects of para-substituents on the half-wave potentials of several substituted benzaldehyde oximes and acetophenone oximes,and pointed out that their half-wave potentials could be correlated well with Hammett substituent constant σ[12].Their work promotes the studies of the substituent effects on the analogous compounds in the field of electrochemistry[12].The previous studies mostly placed emphasis on the substituent effects by changing the kind of substituents.However,there are few quantitative structure property/activity relationship studies about the substituent effects by changing the position of the substituents.
Recently,Cao et al.analyzed the substituent effects on the E(Red)for 52 samples of 4,4′-disubstituted XBAYs,and obtained a four-parameter equation[14],as shown in Eq.(1),in which σF(X)is the inductive ef-
fect of X, σR(X) and σR(Y) are the conjugative effectsof X and Y respectively, σexCC (X) is the excited-state substituent constant of X

In Ref.[14],the E(Red)values of the 52 samples of 4,4′-disubstituted XBAYs were not corrected by ferrocene. In this work,their E(Red)values were corrected by ferrocene[15],and a modified regression equation was obtained(shown as Eq.(2)).
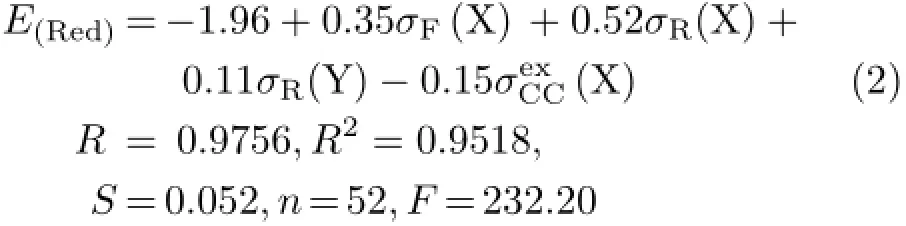
Then we want to know if Eq.(2)will still be applicable in the case of X and/or Y changed from para-position to meta-position.If not,what are the difference and the reasons?To answer above questions,49 samples of 3,4′/4,3′/3,3′-substituted benzylideneanilines(as shown in Fig.1)were synthesized and the substituent effects on their E(Red)values were analyzed,and meaningful results were obtained.
II.EXPERIMENTS
A.Preparation of XBAYs
The substituted benzylideneanilines were all synthesized with the solvent-free method according to Fig.1[24,25].They were purified with anhydrous alcohol,and were confirmed with1H NMR and13C NMR.The NMR spectra were recorded with Bruker AV 500 MHz in CDCl3at room temperature at an approximate concentration.The detailed data of the synthesized compounds are available in the supplementary materials.
B.Measurment of redox potentials
The electrochemical experiments were carried out by cyclic voltammetry(CV)and using a CS300 electrochemical apparatus in deaerated acetonitrile under nitrogen atmosphere at 298 K.n-Bu4NPF6(0.1 mol/L)in acetonitrile was employed as the supporting electrolyte. A standard three-electrode cell consists of a glassy carbon disk as work electrode,a platinum wire as a counter electrode,and 0.01 mol/L AgNO3/Ag(in 0.1 mol/L n-Bu4NPF6/acetonitrile)as reference electrode.The scanning speed was 50 mV/s.Ferrocene was taken as an external reference.For example,Fig.2 is the cyclic voltammetric curve of m-FBANMe2-p.

FIG.1 Target compounds used in this work.
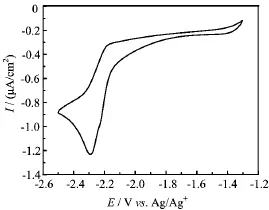
FIG.2 Cyclic voltammetric curve of m-FBANMe2-p.
III.RESULTS AND DISCUSSION
Firstly,the parameters in Eq.(2)were assumed to be applicable for correlating with the E(Red)of thesubstituted XBAYs.So the correlation between the experimental E(Red)values of Table I with the parameters σF(X),σR(X),σR(Y)and(X)was carried out,and Eq.(3)was obtained.
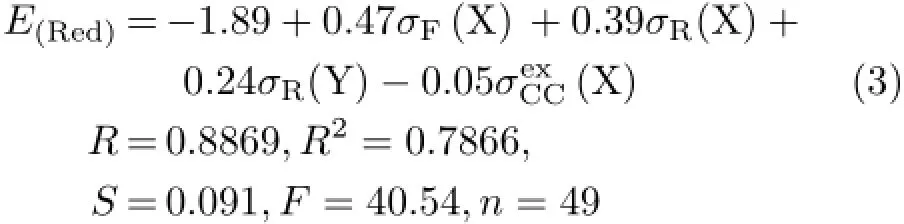
As seen from Eq.(3),its correlation result is not good enough and worse than that of Eq.(2).The coefficients of the parameters in Eq.(2)were different from those of corresponding parameters in Eq.(3),as well as their intercepts.It implies that the factors affecting the E(Red)ofsubstituted XBAYs may be different from those of para-substituted XBAYs.Therefore,we used the parameters listed in Table I to make regression analysis against the E(Red)values of the 49 samples once again,and obtained the optimality equation(Eq.(4)).
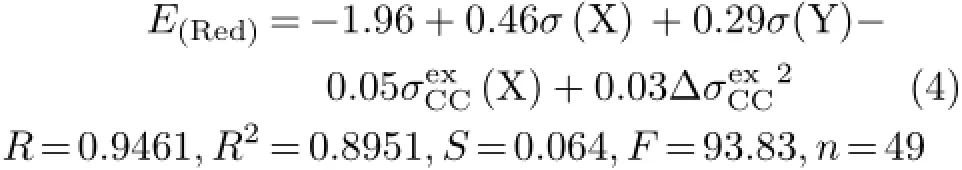
In Eq.(4),σ(X)=σF(X)+σR(X),σ(Y)=σF(Y)+σR(Y),andrelation of Eq.(4)is much better than that of Eq.(3),and its standard deviation S is down to 0.064 from 0.091 of Eq.(3).And the number of variables employed in Eq.(4)is equal to that in Eq.(3).Furthermore,the intercept of Eq.(4)is equal to that of Eq.(2). It can be explained as follows∶in case X and Y groups all are H atom,both ofsubstituted XBAYs andsubstituted XBAYs all returned to the parent molecule,benzylideneaniline(HBAH).Thus,the intercepts of Eq.(4)and Eq.(2)should be equal to each other,which express the reduction potential of HBAH.

TABLE I The corrected E(Red)values of 49 samples of 3,4′/4,3′/3,3′-substituted benzylideneanilines.
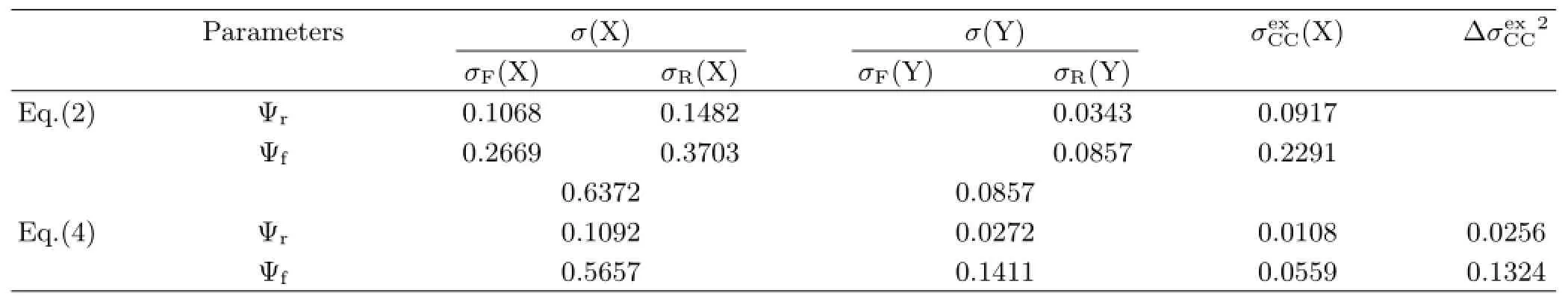
TABLE II The relative and fraction contribution(Ψrand Ψf)of parameters in Eq.(2)and Eq.(4).
In addition,the plot of the E(Red)calcd.values calculated by Eq.(4)vs.the experimental E(Red)expt.values forsubstituted XBAYs of Table I was made and shown in Fig.3.
Comparing Eq.(4)and Eq.(2),it can be seen that the factors affecting the E(Red)ofsubstituted XBAYs are different from those ofsubstituted XBAYs.Here the relative importance of parameters in each equation is investigated from the relative contributions)or fraction contributions(ψf)of the corresponding parameters to E(Red)[20,21].
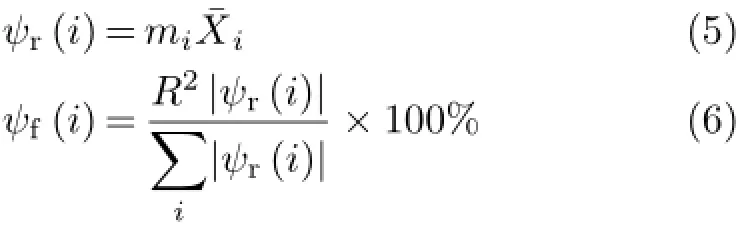
The miandare the coefficient and the averagevalue of the ith parameter in each equation, and the Ris the correlation coefficient of each equation. The sumis over the parameters in the equations. The contributionresults for the corresponding parameters of theequations are all shown in Table II.
It should be paid attention that the effects of X group on the E(Red)are larger than that of Y group in bothsubstituted XBAYs andsubstituted XBAYs.The reasons may be as follows∶the distance between X and carbon atom of CH=N is closer than that between Y and the carbon atom,and the aniline ring with Y group is twisted out of the C-C=N-C plane by 41◦-55◦exhibited by crystal structures of XBAY molecules[22,23],which may hinder the transmission of the conjugative effect some what from Y to the CH=N.

FIG.3 Plot of E(Red)calcd.values calculated by Eq.(4)vs. E(Red)expt.values for target compounds.
IV.CONCLUSION
An optimality equation (Eq.(4)) with four parameterswas obtained for the electrochemical reduction potentialsE(Red)of 49 samples ofsubstitutedXBAYs. The results indicated that the factors affectingthe E(Red)ofsubstituted XBAYs aredifferent from those of′-substituted XBAYs. As regardsthe E(Red)ofsubstituted XBAYs,the contributions of σFandσR>of X(or Y)are equal>to each other, so the parametersσFandσRwere employed, and the contribution ofis also important and not negligible.Compared w i t h-substituted XBAYs, X group contributes lessto the E(Red)ofsubstituted XBAYs, whileY group contributes more. Finally, X group contributesmore than Y group to the E(Red)of substituted XBAYs wherever they are in para-position or meta-position.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.21272063),the Scientific Research Fund of Hunan Provincial Education Department(No.14C0466),and the Natural Science Foundation of Hunan(No.14JJ3112).
[1]A.Kawasaki,J.Chem.Soc.Perkin Trans.2.2,223(1990).
[2]K.Neuvonen,F.F¨ul¨op,H.Neuvonen,A.Koch,E. Kleinpeter,and K.Pihlaja,J.Org.Chem.68,2151(2003).
[3]H.Neuvonen,K.Neuvonen,and F.F¨ul¨op,J.Org. Chem.71,3141(2006).
[4]B.Hemmateenejad and M.Sanchooli,J.Chemometrics. 21,96(2007).
[5]M.Karelson,V.S.Lobanov,and A.R.Katritzky,Chem.Rev.96,1027(1996).
[6]J.J.Sullivan,A.D.Jones,and K.K.Tanji,J.Chem. Inf.Comp.Sci.40,1113(2000).
[7]X.Q.Zhu,Y.Tan,and C.T.Cao,J.Phys.Chem.B 114,2058(2010).
[8]A.J.Fry and R.G.Reed,J.Am.Chem.Soc.91,6448(1969).
[9]C.P.Andrieux and J.M.Saveant,J.Electroanal. Chem.33,453(1971).
[10]K.Polat,M.U¸car,M.L.Aksu,and H.¨Unver,Can.J. Chem.82,1150(2004).
[11]V.A.Sauro and M.S.Workentin,J.Org.Chem.66,831(2001).
[12]H.Celik,G.Ekmekci,J.Ludv´ıK,J.P´ıCha,and P.Zuman,J.Phys.Chem.B 110,6785(2006).
[13]X.Q.Zhu,Q.Y.Liu,Q.Chen,and L.R.Mei,J.Org. Chem.75,789(2010).
[14]C.Z.Cao,Y.K.Bi,and C.T.Cao,Chin.J.Org.Chem. 35,1302(2015).
[15]N.G.Connelly and W.E.Geiger,Chem.Rev.96,877(1996).
[16]C.Hansch,A.Leo,and R.W.Taft,Chem.Rev.91,165(1991).
[17]C.Z.Cao,G.F.Chen,and Z.Q.Yin,J.Phys.Org. Chem.21,808(2008).
[18]C.Z.Cao,B.Sheng,and G.F.Chen,J.Phys.Org. Chem.25,1315(2012).
[19]C.Z.Cao and Y.X.Wu,Sci.China Chem.56,883(2013).
[20]D.E.Needham,I.C.Wei,and P.G.Seybold,J.Am. Chem.Soc.110,4186(1988).
[21]F.P.Liu,Y.Z.Liang,C.Z.Cao,and N.Zhou,Talanta 72,1307(2007).
[22]H.B.B¨urgi and J.D.Dunitz,Helv.Chim.Acta.53,1747(1970).
[23]H.B.B¨urgi and J.D.Dunitz,Helv.Chim.Acta.54,1255(1971).
[24]C.Z.Cao,B.T.Lu,and G.F.Chen,J.Phys.Org. Chem.24,335(2011).
[25]J.Schmeyers,F.Toda,J.Boy,and G.Kaupp,J.Chem. Soc.Perkin Trans.2 4,989(1998).
∗Author to whom correspondence should be addressed.E-mail:czcao@hnust.edu.cn
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年2期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年2期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Incorporation of Reactive Corrosion Inhibitor in Waterborne Acrylic Polyurethane Coatings and Evaluation of its Corrosion Performance
- Response Ability to External Signal Enhanced by Biological Spatial Configuration in Coupled Hindmarsh-Rose Neural System
- Synthesis and Surface Activity of Heterogemini Imidazolium Surfactants
- Preparation of Nitrogen-Doped Carbon Catalyst to Oxygen Reduction Reaction and Influence of Protective Gas Flowing on Its Activity
- Structural and Magnetic Properties of Chemically Synthesized Pd-Modified NiFe2O4Nanoparticles
- Fast Photodegradation of Malachite Green using Nano-ZnO on Ceramic MgAl Carbonate Layered Double Hydroxides Support
