Fast Photodegradation of Malachite Green using Nano-ZnO on Ceramic MgAl Carbonate Layered Double Hydroxides Support
Rui-qing Yn,Gui-hu Liu,Qing-feng Wng,Wei Liu,Chun-lin Song.Deprtment of Mterils Engineering,Tizhou University,Tizhou 318000,Chinb.Fulty of Mterils nd Energy,Southwest University,Chongqing 400715,Chin.CAS Key Lb Mt Energy Convers,Deprtment of Mterils Siene nd Engineering,University of Siene nd Tehnology of Chin,Hefei 230026,Chin(Dted:Reeived on My 21,2015;Aepted on August 6,2015)
Fast Photodegradation of Malachite Green using Nano-ZnO on Ceramic MgAl Carbonate Layered Double Hydroxides Support
Rui-qiang Yana∗,Gui-hua Liua,Qing-feng Wanga,Wei Liuc,Chun-lin Songb∗
a.Department of Materials Engineering,Taizhou University,Taizhou 318000,China
b.Faculty of Materials and Energy,Southwest University,Chongqing 400715,China
c.CAS Key Lab Mat Energy Convers,Department of Materials Science and Engineering,University of Science and Technology of China,Hefei 230026,China
(Dated:Received on May 21,2015;Accepted on August 6,2015)
The nanopowders of Mg-Al carbonate layered double hydroxides(MgAl-LDH)were prepared via coprecipitation process.ZnO nanoparticles were homogeneously coated on the ceramic MgAl-LDH surface.After calcination at 500◦C for 4 h,X-ray diffraction and scanning electron microscopy were employed to investigate the crystal structure and morphology,respectively.It was demonstrated that ZnO nanoparticles were successfully prepared on ceramic MgAl-LDH support.The obtained nano-ZnO photocatalyst showed a high photocatalytic degradation of malachite green.The enhanced photocatalytic property can be attributed to both high photocatalytic activity of ZnO and good adsorption behavior of ceramic MgAl-LDH,in which the flaky structure of MgAl-LDH plays an important role.
Degradation,Photocatalyst,ZnO,Coprecipitation
I.INTRODUCTION
Water pollution has been a global problem in the last decades.Dye,e.g.malachite green(MG),is one of the main sources for water pollution.MG is widely used in textile,leather,pharmaceutical,and related industries.It is necessary to remove MG from water prior to the process of water discharge.Many techniques,e.g. membrane separation,adsorption,osmosis,coagulating sedimentation,and photodegradation,have been extensively investigated.Among them,photodegradation,using the photocatalysts,is one of the most economical techniques[1-4].
Recently,semiconductors have attracted a considerable attention as promising photocatalysts for removing organic and inorganic pollutants from water,for example,ZnO has been successfully used in photocatalytic degradation of dyes and pigments[1,2].It was reported that ZnO showed a higher photocatalytic activity to several organic contaminants than TiO2[5,6]. However,ZnO nanoparticles tend to aggregate above 400◦C.Next,aggregation leads to a reduced surface area and a larger crystallite size.To avoid aggregation,an alternative way is to distribute ZnO nanoparticles on adsorbents.This photocatalytic system shows both high adsorbing capacity and high efficiency for the photocatalytic degradation,especially when MG is adsorbed selectively on the adsorbents and supports.
In this work,the enhanced photocatalytic performance of the synthesized ZnO/MgAl-LDH nanocomposite was extensively investigated under UV irradiation.
II.EXPERIMENTS
A.Sample preparation
MgAl-LDH(Mg2+/Al3+=2)was prepared by coprecipitation method[15].A typical synthetic process was as follows∶0.25 mol Mg(NO3)2·6H2O and 0.125 mol Al(NO3)3·9H2O were dissolved in 100 mL distilled water under vigorous stirring.Then,a 125 mL mixed solution of 0.874 mol NaOH and 0.416 mol Na2CO3wasadded.The mixture was then preheated to 80◦C,and suspension was obtained in the beaker.The suspension was stirred at 80◦C for 12 h.Then,the precipitates were collected by filtration,washed three times with distilled water and finally dried overnight at 110◦C. The dried MgAl-LDH powder was further calcined in air at 500◦C for 4 h.
Next,fresh ZnO/MgAl-LDH was obtained by coating nanosized ZnO on MgAl-LDH surface via the following route∶6 g MgAl-LDH powder was dispersed into 13.52 g Zn(CH3COO)2solution,150 mL NaOH solution(0.37 mol/L)was subsequently added into this suspension.The suspension was vigorously stirred at 70◦C for 10 h.The resulting precipitate was washed three times with distilled water and ethanol,respectively,and then dried at 90◦C overnight.After calcination at 500◦C for 4 h in air,ZnO/MgAl-LDH was obtained.As a comparison,ZnO/MgAl-LDH was synthesized via the same method with various ratios of ZnO to MgAl-LDH.For comparison,bare nano-ZnO powder was also prepared via the same method,but no MgAl-LDH was used as absorbent/support.
B.Characterization
Powder X-ray diffraction(XRD)of samples were collected(Bruke AXS D8 Advance),with a scan step of 0.02◦and a scan range of 20◦~70◦.The morphology of the samples were investigated by scanning electron microscopy(SEM∶LEO-1530)with an accelerating voltage of 15 kV.
C.Photocatalytic performance
The photocatalytic activity of ZnO/MgAl-LDH and bare ZnO,in term of decomposition of MG solution,was evaluated under UV light irradiation with a 500 W Hg lamp.Prior to the experiments,a mixture of catalyst and MG solution was loaded into quartz reactor and stirred in the dark at room temperature for 30 min to allow a complete equilibration of the adsorption/desorption of MG on the catalyst and then UV-lamp was turned on.After a 10 or 15 min interval,a 5 mL aliquot was sampled and centrifuged to remove the catalyst particles.The initial and residual amounts of MG in the solution were determined using a UV spectrophotometer at λ=617 nm.
The reaction was preformed for 90 min till almost all the MG was degraded and the degradation efficiency(D)was calculated by∶

where C0and C are the concentrations of initial and after photocatalytic reaction,respectively.

FIG.1 XRD patterns for ZnO/MgAl-LDH with ratio of(a)1:1,(b)2:1,and(c)3:1,also the signal of MgAl-LDH is marked with star.
The influence of ZnO/MgAl-LDH ratio,concentration of photocatalyst,and pH value was investigated.
III.RESULTS AND DISCUSSION
A.Crystal structure
Figure 1 shows the XRD patterns for ZnO/MgAl-LDH with different ratios,XRD of MgAl-LDH is labeled in Fig.1 with star.This result is consistent with the results in Ref.[16].In the XRD patterns,the diffraction peaks could be attributed to the wurtzite structure of ZnO.According to Scherrer formula,the size of nano-ZnO is 24.8,26.7,and 19.5 nm,respectively.This difference may occur because the MgAl-LDH play a vital role in formation of ZnO nuclei and inhibition of ZnO growth during the subsequent calcination[16].
B.Morphology
SEM image(Fig.2)shows that ZnO nanoparticles are successful coated on MgAl-LDH,and the composites still retain a flake superstructure of LDH.It also indicates that ZnO nanoparticles are well distributed on the surface of nanosheets.Flake ZnO is found on ZnO/MgAl-LDH with low ratio(1∶1),and almost spherical nano-ZnO particles is obtained on ZnO/MgAl-LDH with high ratio(2∶1 and 3∶1).Especially,high ratio of ZnO/MgAl-LDH causes that the support of MgAl-LDH is almost covered by ZnO particles,shown in Fig.2(c). However,the support of MgAl-LDH is still visible in Fig.2(b).
C.Photocatalytic properties
Figure 3 shows the results of degradation of MG by bare ZnO,MgAl-LDH,and ZnO/MgAl-LDH.It can be seen that the degradation of MG increases slowly withthe reaction time for ZnO and MgAl-LDH,while it increases rapidly for ZnO/MgAl-LDH.The results indicate that the photocatalytic degradation of ZnO/MgAl-LDH is much higher than that of bare ZnO and MgAl-LDH.The higher photocatalytic performance can be ascribed as follows∶Firstly,the size of ZnO nanoparticle coated on MgAl-LDH is in the nano-scale,ensuring its higher photocatalytic ability;Secondly,ZnO nanoparticles are dispersed to a large extent on the surface of MgAl-LDH nanoflakes,thus,a larger surface area is obtained;Thirdly,MgAl-LDH provides a high adsorption capability,which is very important to photocatalytic reactions.Because ZnO/MgAl-LDH combines the steps of adsorption and photodecomposition(see in Fig.4),it degrades MG very rapidly.This result complies with the literature report[17],in which superior photocatalytic efficiency of nanosized titania on a mesoporous matrix was demonstrated.

FIG.2 SEM images of ZnO/MgAl-LDH with ratio of(a)1:1,(b)2:1,and(c)3:1.
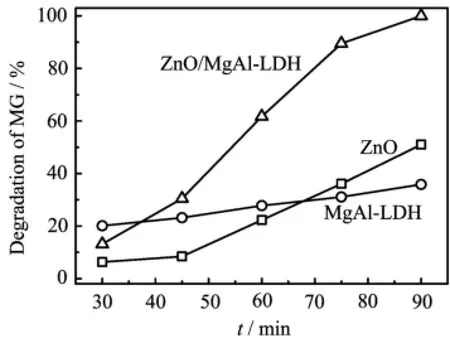
FIG.3 Time dependence of degradation of 50 mg/L MG by ZnO,MgAl-LDH,and ZnO/MgAl-LDH(2:1)with a concentration of 0.7 g/L,initial pH=5.
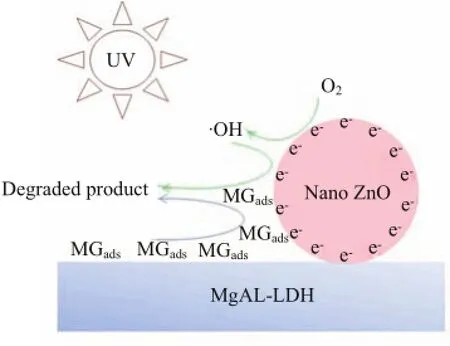
FIG.4 Possible mechanism of photodegradation process of MG on ZnO/MgAl-LDH,where the steps of adsorption and photodegradation is combined.
Figure 5 shows the degradation process with different mole ratio of ZnO to MgAl-LDH.When the reaction time increases,the degradation of MG also increases. It is worth noting that almost all the MG is degraded from water after 90 min.The degradation efficiency of ZnO/MgAl-LDH(2∶1)is the highest.The phenomena are also implied in Fig.2,because ZnO/MgAl-LDH combines adsorption and photodecomposition.MG can be adsorbed on the support of MgAl-LDH with ZnO to MgAl-LDH ratio of 1∶1,but less ZnO on MgAl-LDH support is acted as photocalysts,causing low degradation efficiency.On the other hand,ZnO/MgAl-LDH of 3∶1 can provide more ZnO particles as photocatalyst,however,MG is hardly absorbed on MgAl-LDH,because almost all the surface of MgAl-LDH is covered by ZnO particles.
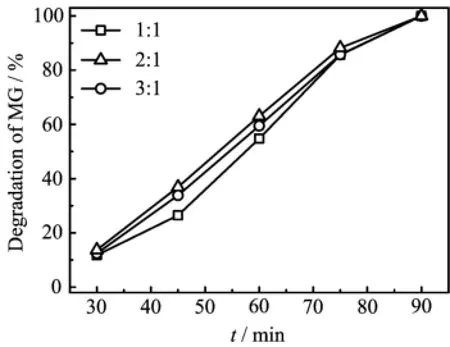
FIG.5 Time dependence of degradation of 50 mg/L MG by 0.7 g/L ZnO/MgAl-LDH with different ratios of 1:1,2:1,and 3:1,initial pH=5.
Figure 6 illustrates the degradation of MG with different concentrations of ZnO/MgAl-LDH(2∶1).It can be seen that the degradation efficiency of MG increases when ZnO/MgAl-LDH increases from 0.7 g/L to 1.3 g/L,because the active radicals,e.g.·OH and O2·,increase with the concentration of photocatalyst.However,the degradation of MG begins to decline quickly when the concentration increases to 1.7 g/L.The phe-nomena is mostly related to shielding effect,because ZnO/MgAl-LDH powder in the water also scatters UV light,causing UV light can not penetrate the suspension,less photon contributes to the photocatalytic reaction[2].Thus,degradation decreases at high concentration of photocatalyst.
Figure 7 shows the influence of pH value of MG solution on the performance of photocatalysts.The photocatalysts provides a high degradation efficiency at high pH values.The reasonable explanation could be related to adsorption and regeneration of active radicals∶Firstly,as pH value increases,the surface of ZnO/MgAl-LDH can be negatively charged,and the active sites available for adsorption of cationic dye,i.e.,MG,increase,and more MG is absorbed[2].Secondly,the amount of OH increases with pH value,more OH radicals is greatly helpful for the process of photodegradation.
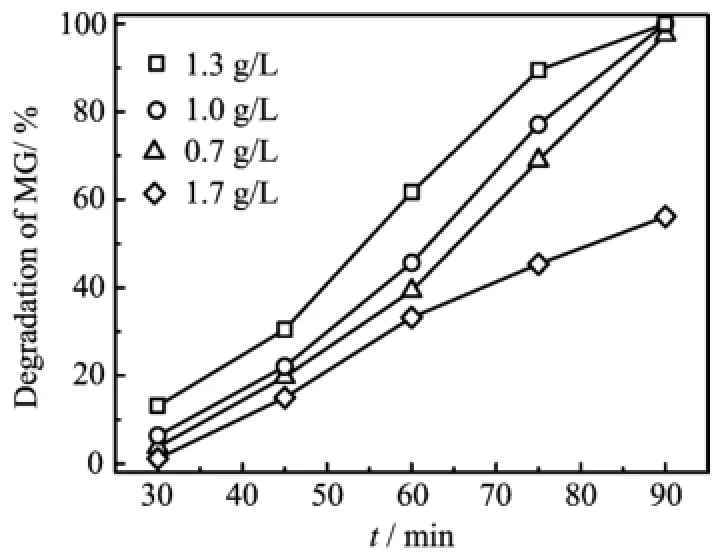
FIG.6 Effect of catalyst loading on photodegration efficiency of ZnO/MgAl-LDH(2:1)in 50 mg/L MG solution with an initial pH=5.
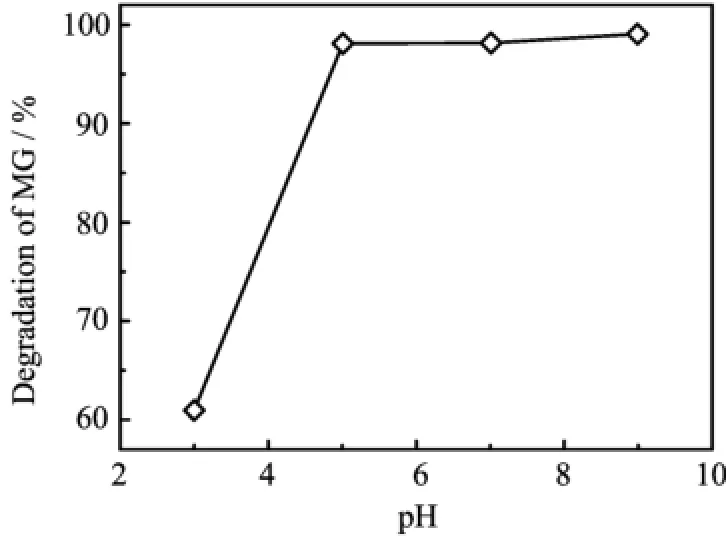
FIG.7 Effect of the pH value on photodegration efficiency of 0.7 g/L ZnO/MgAl-LDH(2:1)in 50 mg/L MG solution after irradiating for 90 min.The pH value was adjusted using HCl or NaOH solution.
IV.CONCLUSION
Nanaocomposites of MgAl-LDH and nano-ZnO were successfully prepared via a wet chemical method.On the MgAl-LDH support,ZnO nanopowder was well dispersed.It is demonstrated that nano-ZnO photocatalyst on the MgAl-LDH support ZnO/MgAl-LDH provides an enhanced photocatalytic properties in the process of photadegradation of MG in the water.This could be attributed to the combination of MG adsorption on MgAl-LDH support and photocatalytic recation on the nano-ZnO surface.
V.ACKNOWLEDGMENTS
The work is supported by the Key Disciplines of Applied Chemistry of Zhejiang Province(Taizhou University),the National Natural Science Foundation of China(No.51403149),and the Fundamental Research Funds for the Central Universities,China(No.SWU113045 and No.XDJK2015C002).
[1]H.Y.Wu,Q.S.Xie,L.An,P.Jin,D.L.Peng,C.J. Huang,and H.L.Wan,Mater.Lett.139,393(2015).
[2]L.Saikia,M.Saikia,B.Malakar,D.K.Dutta,and P. Sengupta,Appl.Catal.A 490,42(2015).
[3]S.Saha,J.M.Wang,and A.Pal,Sep.Purif.Technol. 89,147(2012).
[4]V.Eskizeybek,F.Sari,H.Gulce,A.Gulce,and A. Avci,Appl.Catal.B.119,197(2012).
[5]J.H.Sun,S.Y.Dong,Y.K.Wang,and S.P.Sun,J. Hazard.Mater.172,1520(2009).
[6]J.G.Yu and X.X.Yu,Environ.Sci.Technol.42,4902(2008).
[7]D.Tichit,M.N.Bennani,F.Figueras,R.Tessier,and J.Kervennal,Appl.Clay.Sci.13,401(1998).
[8]J.S.Valente,M.Sanchez-Cantu,E.Lima,and F. Figueras,Chem.Mater.21,5809(2009).
[9]Y.Z.Chen,C.W.Liaw,and L.I.Lee,Appl.Catal.A. 177,1(1999).
[10]J.T.Feng,Y.J.Lin,D.G.Evans,X.Duan,and D.Q. Li,J.Catal.266,351(2009).
[11]R.D.Hetterley,R.Mackey,J.T.A.Jones,Y.Z. Khimyak,A.M.Fogg,and I.V.Kozhevnikov,J.Catal. 258,250(2008).
[12]M.D.Martinez-Ortiz,D.Tichit,P.Gonzalez,and B. Coq,J.Mol.Catal.A.201,199(2003).
[13]F.Prinetto,M.Manzoli,G.Ghiotti,M.D.M.Ortiz,D.Tichit,and B.Coq,J.Catal.222,238(2004).
[14]V.Rives,F.M.Labajos,R.Trujillano,E.Romeo,C. Royo,and A.Monzon,Appl.Clay.Sci.13,363(1998).
[15]W.T.Reichle,Solid State Ionics 22,135(1986).
[16]Y.Zhi,Y.G.Li,Q.H.Zhang,and H.Z.Wang,Langmuir 26,15546(2010).
[17]X.Z.Yang,Y.Dongjiang,H.Y.Zhu,J.W.Liu,W. N.Martins,R.Frost,L.Daniel,and Y.N.Shen,J. Phys.Chem.C 113,8243(2009).
∗Authors to whom correspondence should be addressed.E-mail:yanrq@tzc.edu.cn,chunlinsong@swu.edu.cn,Tel.:+86-1888332-7083,+86-13757697720
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年2期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年2期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Incorporation of Reactive Corrosion Inhibitor in Waterborne Acrylic Polyurethane Coatings and Evaluation of its Corrosion Performance
- Response Ability to External Signal Enhanced by Biological Spatial Configuration in Coupled Hindmarsh-Rose Neural System
- Synthesis and Surface Activity of Heterogemini Imidazolium Surfactants
- Substituent Effects on Reduction Potentials of Meta-substituted and Para-substituted Benzylideneanilines
- Preparation of Nitrogen-Doped Carbon Catalyst to Oxygen Reduction Reaction and Influence of Protective Gas Flowing on Its Activity
- Structural and Magnetic Properties of Chemically Synthesized Pd-Modified NiFe2O4Nanoparticles
