Methanol extract of Codium fragile inhibits tumor necrosis factorα-induced matrix metalloproteinase-9 and invasiveness of MDAMB-231 cells by suppressing nuclear factor-κB activation
Matharage Gayani Dilshara, Rajapaksha Gedara Prasad Tharanga Jayasooriya, Chang-Hee Kang,, Yung-Hyun Choi, Gi-Young Kim*Department of Marine Life Sciences, Jeju National University, Jeju-si 64, Republic of KoreaNakdonggang National Institute of Biological Resource, Sangju-si, Gyeongsangbuk-do 74, Republic of KoreaDepartment of Biochemistry, College of Oriental Medicine, Dong-Eui University, Busan 4740, Republic of Korea
ARTICLE INFO ABSTRACT
Article history:
Received in revised form 16 March 2016 Accepted 15 April 2016
Available online 20 June 2016
Methanol extract of Codium fragile inhibits tumor necrosis factorα-induced matrix metalloproteinase-9 and invasiveness of MDAMB-231 cells by suppressing nuclear factor-κB activation
Matharage Gayani Dilshara1, Rajapaksha Gedara Prasad Tharanga Jayasooriya1, Chang-Hee Kang1,2, Yung-Hyun Choi3, Gi-Young Kim1*1Department of Marine Life Sciences, Jeju National University, Jeju-si 63243, Republic of Korea
2Nakdonggang National Institute of Biological Resource, Sangju-si, Gyeongsangbuk-do 37242, Republic of Korea
3Department of Biochemistry, College of Oriental Medicine, Dong-Eui University, Busan 47340, Republic of Korea
ARTICLE INFO ABSTRACT
Article history:
Received in revised form 16 March 2016 Accepted 15 April 2016
Available online 20 June 2016
Codium fragile
Invasion
Nuclear factor-κB
Matrix metalloproteinase-9 Tumor necrosis factor-
Objective: To evaluate whether the methanol extract of Codium fragile (MECF) regulates tumor necrosis factor-α (TNF-α)-induced invasion of human breast cancer MDA-MB-231 cells by suppressing matrix metalloproteinase-9 (MMP-9). Methods: Reverse transcriptionpolymerase chain reaction (RT-PCR) and western blot analysis were performed to analyze the expression of MMP-9 and nuclear factor-κB (NF-κB) subunits, p65 and p50, and Iκ B in MDA-MB-231 cells. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide(MTT) assay was used for cell viability. MMP-9 activity and invasion were measured by gelatin zymography and a matrigel invasion assay, respectively. NF-κB activity was measured by an electrophoretic mobility shift assay and luciferase activity. Results: MECF had no effects on cell viability up to a concentration of 100 μg/mL in human breast cancer MDA-MB-231 cells regardless of the presence of TNF-α. MDA-MB-231 cells that were stimulated with TNF-α showed a marked increase of invasion compared to the untreated control, whereas pretreatment with MECF downregulated the TNF-α-induced invasion of MDA-MB-231 cells. Additionally, zymography, western blot analysis, and reverse transcriptase-polymerase chain reaction (RT-PCR) confirmed that MECF decreased TNF-α-induced MMP-9 expression and activity which is a key regulator for cancer invasion. According to an electrophoretic morbidity shift assay, pretreatment with MECF in MDA-MB-231 cells significantly decreased the TNF-α-induced DNA-binding activity of nuclear factor-κB (NF-κB), which is an important transcription factor for regulating cancer invasion-related genes such as MMP-9. Furthermore, treatment with MECF sustained the expression of p65 and p50 in response to TNF-α in the cytosolic compartment. The luciferase assay demonstrated that MECF attenuated TNF-α-induced NF-κB luciferase activity. Conclusion: MECF exhibited its antiinvasive capability by downregulating TNF-α-induced MMP-9 expression, resulting from the suppression of NF-κB activity in the human breast cancer cell line MDA-MB-231.
1. Introduction
Metastasis and invasion are key factors that determine the malignant behavior of cancer cells. In particular, extracellular proteinases are essential for the metastasis and invasion processes that degrade the components of the extracellular matrix (ECM) and facilitate the disconnection of intercellular adhesions and separation of single cells from solid tumor tissue to metastatic colonies at distant sites[1]. Matrix metalloproteinases (MMPs) are important zinc-dependent endopeptidases responsible for the degradation of major components of ECM, including type IV and V gelatin,and collagen. Thus, its role is closely related to the metastasis and invasion of cancer cells[2]. Among the MMP family, which consists
Tumor necrosis factor alpha (TNF-α) plays a key role in tumorigenesis by enhancing the growth of cancer and its survival,invasion, and metastasis, resulting in a direct network of MMP-9 production[6,7]. After TNF-α binds to its receptor, adapter molecules are recruited to the conformationally-changed receptor, allowing the initiation of the intracellular signaling pathways including nuclear factor-κB (NF-κB)[8]. In particular, NF-κB is not only well known to contribute to cancer invasion and resistance to chemotherapy, but also to be constitutively activated in various cancer[9,10]. Until cells receive an appropriate stimulus, NF-κ B complexes are sequestered by binding with the inhibitor kappa B (IκB) in the cytoplasm, forming a transcriptionally inactive stage[11]. In response to various stimuli such as TNF-α, IκB is phosphorylated and degraded, resulting in the liberation of NF-κB,which allows NF-κB to accumulate in the nucleus and thus activates the expression of target genes such as MMP-9[12,13]. Therefore, the inhibition of NF-κB can be considered as an adjuvant approach to suppress MMP-9 expression and thus prevent cancer invasion and metastasis.
Marine macroalgae are an inexhaustible source of unique natural products with pharmacological activities, which produce a vast array of bioactive secondary metabolites[14]. The secondary metabolites from marine macroalgae were recently demonstrated to possess anti-tumor and anti-inflammatory capabilities[15]. Codium fragile(C. fragile) is a green macroalgae belonging to family Codiaceae,which has demonstrated anti-cancer activity against various cancers. Previously, Noda et al., reported that the C. fragile extract exhibited a 43.4% cancer growth inhibition rate in mice[16]. Furthermore,clerosterol from C. fragile enhanced cytotoxicity in human melanoma cells[17]. Additionally, aqueous extracts of C. fragile exhibited strong cytotoxic activities against the CT26 mouse colon carcinoma cell line and carotenoids from C. fragile induced apoptosis in human leukemia cells[18,19]. However, no study has investigated the effect of C. fragile on MMP-9 expression and activity or invasion and metastasis in TNF-α- stimulated cancer cells.
In the present study, we examined effects of the methanol extract of C. fragile (MECF) on MMP-9 expression and activity in TNF-α -stimulated MDA-MB-231 human breast cancer cells. Our results indicate that MECF suppressed TNF-α-induced MMP-9 expression and activity by downregulating the NF-κB pathway, leading to the inhibition of MDA-MB-231 invasion.
2. Materials and methods
2.1. Preparation of C. fragile
MECF was purchased from Jeju HI-Tech Industry Development Institute (Jeju, Republic of Korea). In brief, C. fragile was collected along the Jeju Island coast (Republic of Korea). Fresh C. fragile was washed three times with tap water to remove salt, epiphyte, and sand on the surface of the samples before storage at -20 ℃. The frozen samples were lyophilized and homogenized using a grinder before extraction. The dried powder was extracted with 80% methanol and evaporated in the vacuum. The extract was filtered through 0.45 μm filter and the filtrate was freeze-dried (yield, approximately 4.1 g)and stored at 4 ℃. The dried filtered through 0.22 μm filter before use.
2.2. Antibodies and reagents
Antibodies against p50, p65, IκB, β-actin, and nucleolin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against MMP-9 was obtained from Cell Signaling(Beverly, MA). Peroxidase-labeled donkey anti-rabbit and sheep anti-mouse immunoglobulin, and TNF-α were purchased from KOMA Biotechnology (Seoul, Republic of Korea). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (St. Louis, MO). RPMI 1640 medium, fetal bovine serum (FBS), and antibiotics mixture were purchased from WelGENE (Daegu, Republic of Korea).
2.3. Cell culture and viability
Human breast cancer cell line MDA-MB-231 was obtained from the American Type Culture Collection (Manassas, VA). The cells were cultured at 37 ℃ in a 5% CO2-humidified incubator and maintained in RPMI 1640 medium containing 10% heatinactivated FBS and 1% antibiotics mixture. The cells were seeded at 5×104cells/mL, incubated, and then treated with the indicated concentrations of MECF in the presence or absence of TNF-α (20 ng/mL). Following 24-h incubation, the viability was measured by MTT assay.
2.4. Invasion assay
Invasion assay was performed into 24-well chambers (Corning,Corning, NY) coated with matrigel in serum-free RPMI 1640 medium. Precoated filters (6.5 mm in diameter and 8 μm pore size)were rehydrated and the cells (5×104cells/mL) in medium with or without MECF in the presence of TNF-α (20 ng/mL) were seeded into the upper part of each chamber. Following 24-h incubation,non-migrated cells were removed using cotton swabs and migrated cells were fixed and stained with 0.125% Coomassie Blue in a methanol:acetic acid: distilled water mixture (15:10:45, v/v/v). Invading cells were counted under a light microscope.
2.5. Gelatin zymography assay
Cultured MDA-MB-231 cells were collected and washed three times with serum-free RPMI 1640 medium. The cells were seed at 3×105cells/mL in serum-free RPMI 1640 medium and treated with MECF in the presence of TNF-α (20 ng/mL). The medium was collected and electrophoresed on a polyacrylamide gel containing 0.1% (w/v) gelatin as a substrate. The gel was washed at room temperature for 10 min, 3 times with 2.5% Triton X-100 washing buffer, and subsequently incubated at 37 ℃ for 24 h in reaction buffer (0.2% Brij, 35.5 mM CaCl2, 1 mM NaCl, and 50 mM Tris,pH7.4). The gel was stained for 1 h with 0.25% Coomassie Blue in 10% acetic acid/40% methanol and then destained with 10% acetic acid/40% methanol for 1 h. Areas of protease activity appeared as clear bands.
2.6. RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted using Easy-Blue reagent (iNtRON Biotechnology, Sungnam, Korea). RNA concentration and purity were measured by absorbance at 260 nm/280 nm. Genes of interest were amplified from cDNA that was synthesized from 1 μg total RNA using MMLV reverse transcriptase (Bioneer Corp., Daejeon,Korea). Primers for glyceraldenhyde-3-phosphate dehydrogenase(GAPDH) (sense 5'-CCA CCC ATG GCA AAT TCC ATG CA-3' and antisense 5'-TCT AGA CGG CAG GTC AGG TCC ACC-3') and MMP-9 (sense 5'-CCT GGA GAC CTG AGA ACC AAT CT-3' and antisense 5'-CCA CCC GAG TGT AAC CAT AGC-3') were used. PCR was performed at 94 ℃ for 5 min followed 27 cycles of 94 ℃for 30 sec, annealing temperature (GAPDH at 62 ℃ and MMP-9 at 59 ℃) for 30 sec, 72 ℃ for 30 sec followed via final extension at 72 ℃ for 5 min. Following amplification, PCR products were separated 1% agarose gels and visualized using ethidium bromide fluorescence.
2.7. Western blot analysis
Total protein extracts were prepared by a PRO-PREP protein extraction kit (iNtRON Biotechnology). Briefly, MDA-MB-231 cells were harvested, washed twice with ice-cold PBS, and gently lysed for 30 min in 100 μL ice-cold PRO-PREP lysis buffer. The lysates were centrifuged at 14 000 g at 4 ℃ for 10 min. Supernatants were collected and protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). The samples were stored at -80 ℃ or immediately used for Western blot analysis. The proteins were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Proteins were detected by an enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL).
2.8. Electrophoretic mobility shift assay (EMSA)
The cytoplasmic and nuclear proteins were prepared using the NEPER nuclear and cytoplasmic extraction reagents (Pierce, Rockford,IL). DNA-binding activity of NF-κB was performed using nuclear extracts. Synthetic complementary NF-κB-binding oligonucleotides(5'-AGT TGA GGG GAC TTT CCC AGG C-3'; Santa Cruz Biotechnology) were biotinylated using biotin 3'-end DNA labeling kit (Pierce) and annealed for 30 min at 37 ℃ according to the manufacturer's instructions. Labeled oligonucleotides, 10 μg nuclear protein extracts, 100 ng poly[deoxyinosinic-deoxycytidylic] acid,and LightShiftTM chemiluminescent EMSA kit mixture were then incubated for 30 min at room temperature. The samples were loaded onto native 4% polyacrylamide gels pre-electrophoresed for 1 h in 0.5 Tris-borate/EDTA (TBE) buffer and transferred onto a positively charged nylon membrane (HybondTM-N+) in 0.5 TBE buffer at 100 V for 1 h on ice. Transferred DNAs were cross-linked on the membrane at 120 mJ/cm2 and detected using horseradish peroxidase-conjugated streptavidin according to the manufacturer's instructions.
2.9. Luciferase activity assay
NF-κB reporter construct was purchased from Clonetech (Palo Alto, CA). In brief, MDA-MB-231 cells were seeded onto 6-well plates at a density of 3×105cells/mL and incubated overnight. The cells were transfected with NF-κB reporter construct by Lipofectamine method. After 6-h incubation, the cells were washed and cultured in RPMI 1640 containing 10% FBS with MECF (100 μg/mL) in the presence or absence of TNF-α (20 ng/mL). The cells were lysed with lysis buffer (2 mM DTT, 150 mM NaCl, 20 mM Tris-HCl, and 1 % Triton X-100) at 24 h. The lysate was mixed with luciferase activity reagent, and luminescence was measured using a GLOMAX luminometer (Promega, Madison, WI) and intensity of luminescence was obtained using an image analyzer.
2.10. Statistical analysis
The images were visualized with Chemi-Smart 2000(VilberLourmat, Marine, Cedex, France). Images were captured using Chemi-Capt (VilberLourmat) and transported into Photoshop. All bands were shown a representative obtained in three independent experiments and quantified by Scion Imaging software (http://www. scioncorp.com). Statistical analyses were conducted using SigmaPlot software (version 12.0). Values were presented as mean ± standard error (S.E.). Significant differences between the groups were determined using the unpaired one-way ANOVA test by Bonferroni's test. Statistical significance was regarded at P< 0.05.
3. Results
3.1. MECF inhibits TNF-α-induced cell invasion
In order to measure whether MECF influences cell viability, we treated MDA-MB-231 cells with various concentrations of MECFin the presence or absence of TNF-α for 24 h and then performed an MTT assay. Exposure up to 100 μg/mL MECF had no effect on cell viability in the presence or absence of TNF-α (Figure 1A). Furthermore, to examine the inhibitory effects of MECF on the invasion of MDA-MB-231 cells, we performed an invasion assay using matrigel-coated transwells. TNF-α-stimulated MDA-MB-231 cells showed a marked increase of invasion compared to that of the untreated control; however, pretreatment with MECF downregulated the TNF-α-induced invasion of the cells (Figure 1B). Therefore,these results indicate that MECF inhibited TNF-α-induced invasion in MDA-MB-231 cells without any direct cytotoxicity.
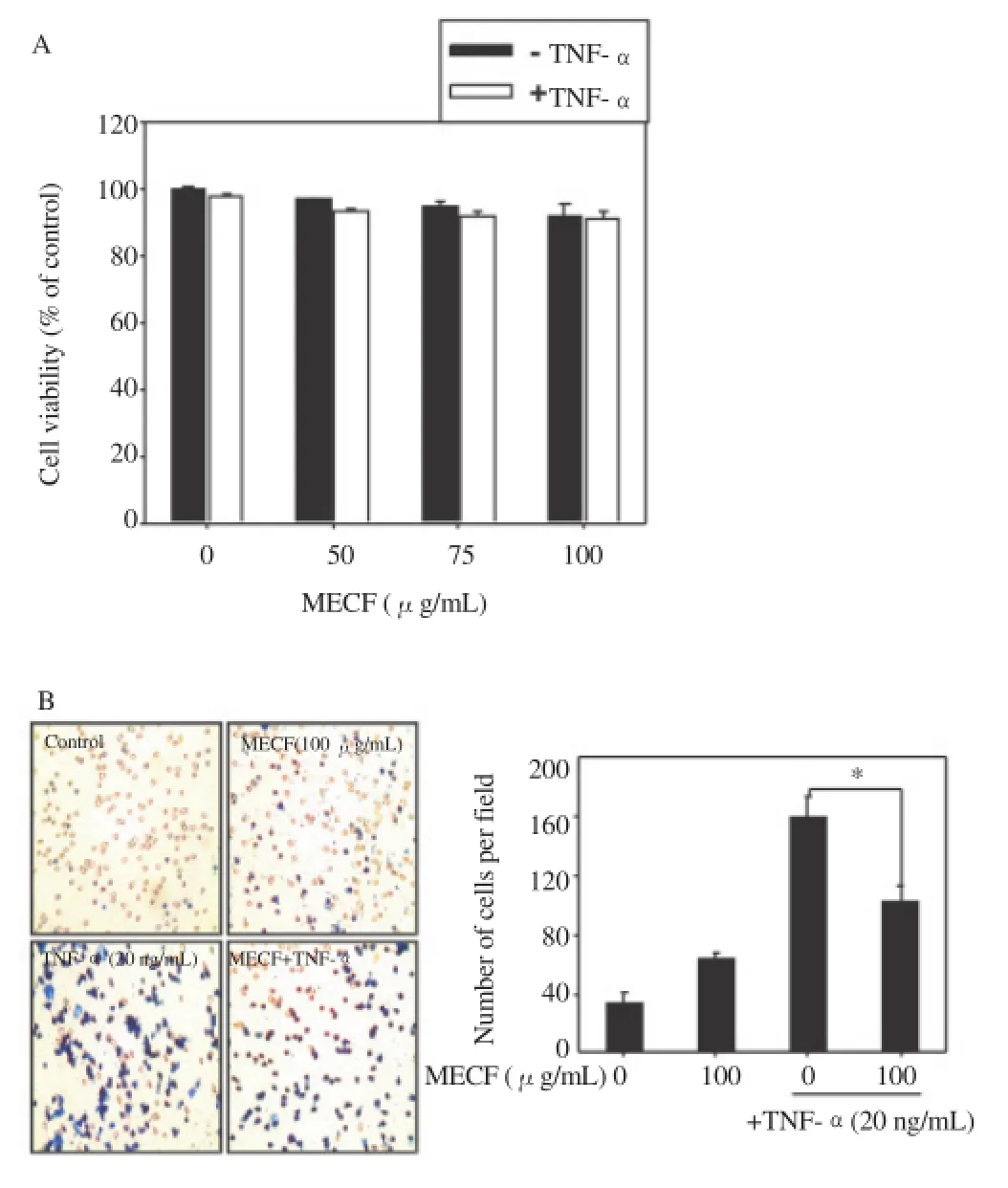
Figure 1. Effects of MECF on the viability and invasion activity of MDAMB-231 cells.
(A) MDA-MB-231 cells (1×105cells/mL) were cultured in 24-well plates and treated with the indicated concentrations (0 - 100 μg/mL) of MECF in the presence or absence 20 ng/mL TNF-α for 24 h. Cell viability was determined by an MTT assay. (B) For the invasion assay, the upper compartments of transwells were coated with matrigel. MDA-MB-231 cells were cultured in serum-free media for 3 h before treatment with MECF (100 μg/mL) in the presence or absence of TNF-α (20 ng/mL) for 24 h. The cells that passed through the matrigel to the membrane were stained using 0.125% Coomassie blue in ethanol.
3.2. MECF downregulates TNF-α-induced MMP-9 activity and expression
MMP-9 is a key enzyme for the invasion and metastasis of cancer cells. Subsequently, in order to evaluate whether MECF regulates MMP-9 activity and expression, we performed zymography, a Western blot analysis, and RT-PCR. According to the zymography data, TNF-α-treated MDA-MB-231 cells significantly increased the gelatin-degrading activity of MMP-9 whereas MECF-pretreated cells decreased the TNF-α-induced MMP-9 activity in a concentrationdependent manner (Figure 2A). In addition, the Western blot analysis revealed that stimulation with TNF-α resulted in a remarkable upregulation of MMP-9 expression in the protein compared to that of the untreated control; however, pretreatment with MECF inhibited TNF-α-induced MMP-9 expression (Figure 2B). In accordance with the suppression of MMP-9 activity and protein expression of MECF, the RT-PCR analysis also showed that MECF significantly downregulated TNF-α-induced MMP-9 expression in MDA-MB-231 cells (Figure 2C). These data suggests that MECF inhibited MMP-9 expression and activity, leading to the suppression of invasion of MDA-MB-231 cells.
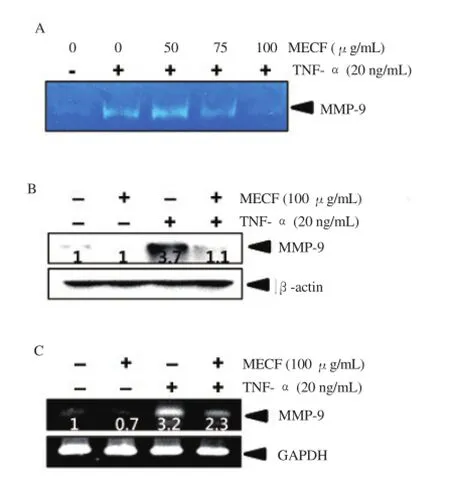
Figure 2. Effects of MECF on TNF-α induced MMP-9 expression and activity in MDA-MB-231 cells.
(A) MDA-MB-231 cells were treated with the indicated concentrations(0 - 100 μg/mL) of MECF for 1 h before treatment with 20 ng/mL TNF-α in the absence of FBS for 24 h. The conditioned medium was collected from cultures and subjected to gelatin zymography. (B) Equal amounts of cell lysates were resolved on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and probed with antibodies against MMP-9. (C)MDA-MB-231 cells were incubated with the indicated concentrations (0 -100 μg/mL) of MECF for 1 h before treatment with TNF-α (20 ng/mL) for 6 h. Total RNA was isolated, and RT-PCR analysis of MMP-9 was performed. GAPDH and β-actin were used as internal controls for RT-PCR and Western blot analysis, respectively.
3.3. MECF suppresses TNF-α-induced NF-κB
To further examine whether MECF suppresses MMP-9 expression by inhibiting NF-κB, we determined the specific DNA-binding activity of NF-κB. According to EMSA data, the TNF-α-induced DNA-binding activity of NF-κB at 30 min was 5-fold to that of the untreated control; however, pretreatment with MECF significantlydecreased TNF-α-induced NF-κB activity (Figure 3A). Consistent with EMSA data, the Western blot analysis also showed that treatment with TNF-α increased p65 and p50 levels in the nuclear compartment, whereas MECF attenuated p65 and p50 levels in the nuclear extract (Figure 3B). In a parallel experiment, TNF-α decreased p65, p50, and IκB expression in the cytosol which suggests that TNF-α induces nuclear translocation of p65 and p50 by degrading IκB. However, treatment with MECF sustained p65,p50, and IκB in response to TNF-α in the cytosolic compartment. Moreover, we examined NF-κB promoter activity using transiently transfected MDA-MB-231 cells with a luciferase reporter vector consisting of NF-κB binding sites. The TNF-α-stimulated MDAMB-231 cells showed approximately a 7-fold increases in NF-κB luciferase activity when compared to the untreated control (Figure 3C). Interestingly, pretreatment with MECF decreased TNF-α -induced NF-κB luciferase activity. Taken together, these results indicated that MECF attenuated TNF-α-induced NF-κB activity,resulting in MMP-9 gene expression.

Figure 3. Effect of MECF on TNF-α-induced NF-κB activity in MDAMB-231 cells. MDA-MB-231 cells were treated with MECF (100 μg/mL) in the presence of TNF-α (20 ng/mL) and nuclear and cytosolic extracts were extracted.
(A) The nuclear extracts were prepared after 30 min, and NF-κB binding to its DNA promoter region in the extract was measured using an EMSA.(B) Levels of p65, p50, and IκB in the nuclear (top) and cytosolic (bottom)extracts were determined by Western blot analysis at 30 min. β-Actin and nucleolin were used as cytosolic and nuclear internal controls for the Western blot analysis. (C) MDA-MB-231 cells were transfected with the NF-κB promoter-containing reporter vector and treated with MECF (100 μg/mL) in the presence of TNF-α (20 ng/mL). Luciferase activity was measured by a luminometer. (*P< 0.05 vs. TNF-α-treated group).
4. Discussion
Recent studies reported that the C. fragile extract exhibits diverse pharmacological properties such as anti-edema, anti-viral, antibacterial, anti-allergic, anti-protozoal, and anti-carcinogenic activity[20,21]. Nevertheless, there are no reports on the anti-invasive properties of the C. fragile extract. Therefore, we investigated the anti-invasive activity of MECF in MDA-MB-231 cells. The present study showed that MECF suppressed TNF-α-induced MMP-9 expression and activity by inhibiting NF-κB activity, resulting in the anti-invasion of MDA-MB-231 cells.
Numerous reports have demonstrated that various MMPs are directly implicated in the cancer cell invasion process and therefore promote metastasis[1,3]. In particular, MMP-9 is implicated in carcinogenesis as a key enzyme that is overexpressed in different types of malignant cancers, and its expression and activity are associated with the aggressiveness of cancer cells[5]. Many studies also reported that elevated levels of MMP-9 are found in colorectal,prostate, brain, lung, breast, and ovarian cancers[22-24]. MMP-9 is directly involved in the degradation of the collagen IV component of the basement membrane, which plays a crucial role in cancer invasiveness[3]. According to our results, MECF suppressed TNF-α-induced MMP-9 expression and activity in MDA-MB-231 cells. Furthermore, the matrigel assay showed that MECF markedly downregulated TNF-α-induced invasion of the MDAMB-231 cells, which suggests that MECF is a promising strategy for regulating cancer invasion. Additionally, the activity of MMP-9 is traditionally postulated to be governed by tissue inhibitors of metalloproteinases (TIMPs), which are complex proteins composed of four proteins ranging in molecular size from 21-28 kDa[25]. Therefore, it is essential to understand whether TIMPs may be involved in MECF-induced MMP-9 regulation.
In past decades, numerous in vivo and in vitro studies indicated that the pleiotropic NF-κB plays a significant role in various types of biological activities including carcinogenesis, adhesion, invasion,and cell survival[26]. In particular, NF-κB regulates the expression of inflammatory and carcinogenic genes including MMP-9 in different types of cancers such as breast, lung, colon, hepatocellular,prostate and ovarian carcinomas[24]. Therefore, targeting NF-κ B activity is a possible method to attenuate cancer invasion by suppressing MMP-9 expression. Accordingly, recent studies have focused on macroalgae- and plants-derived natural compounds that suppress MMP-9 expression via the NF-κB signaling pathway[15,27]. The results from this present study demonstrated that MECF inhibited the invasiveness of MDA-MB-231 cells by downregulating MMP-9, which resulted from the suppression of NF-κB activity.
In conclusion, we investigated the anti-invasive property of MECF in MDA-MB-231 cells, through its suppression of NF-κB-dependent MMP-9 activity. Our results indicate that MECF might be a promising therapeutic candidate for the inhibition of cancer invasiveness through the suppression of NF-κB and the downstream target, MMP-9.
Conflict of interest statement
The authors declare to have no conflict of interest at all.
Acknowledgment
This study was supported by Basic Science Research Program(2015R1D1A1A01060538) through the National Research Foundation of Korea (NRF) funded from the Ministry of Education,Science and Technology of Korea.
References
[1] Herszényi L, Barabás L, Hritz I, István G, Tulassay Z. Impact of proteolytic enzymes in colorectal cancer development and progression. World J Gastroenterol 2014; 20: 13246-13257.
[2] Sekhon BS. Matrix metalloproteinases - an overview. Res Rep Biol 2010;1: 1-20.
[3] Löffek S, Schilling O, Franzke CW. Biological role of matrix metalloproteinases: a critical balance. Eur Respir J 2011; 38: 191-208.
[4] Aglund K, Rauvala M, Puistola U, Angström T, Turpeenniemi-Hujanen T, Zackrisson B, et al. Gelatinases A and B (MMP-2 and MMP-9) in endometrial cancer-MMP-9 correlates to the grade and the stage. Gynecol Oncol 2004; 94: 699-704.
[5] Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 2005; 115: 44-55.
[6] Harrison ML, Obermueller E, Maisey NR, Hoare S, Edmonds K, Li NF,et al. Tumor necrosis factor-α as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J Clin Oncol 2007; 25: 4542-4549.
[7] Brown ER, Charles KA, Hoare SA, Rye RL, Jodrell DI, Aird RE, et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-α inhibitor, in patients with advanced cancer. Ann Oncol 2008; 19: 1340-1346.
[8] Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 2010; 20: 87-103.
[9] Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest 2001; 107: 241-246.
[10] Chen YJ, Chang LS. NFκB- and AP-1-mediated DNA looping regulates matrix metalloproteinase-9 transcription in TNF-α-treated human leukemia U937 cells. Biochim Biophys Acta 2015; 1849: 1248-1259.
[11] Abujamra AL, Spanjaard RA, Akinsheye I, Zhao X, Faller DV, Ghosh SK. Leukemia virus long terminal repeat activates NF-κB pathway by a TRL3-dependent mechanism. Virology 2006; 345: 390-403.
[12] Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/ NF-κB signaling transduction pathway. Oncogene 2006; 25: 7009-7018.
[13] Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol 2011; 12: 695-708.
[14] Bhadury P, Wright PC. Exploitation of marine algae: biogenic compounds for potential antifouling applications. Planta 2004; 219: 561-578.
[15] Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym 2011; 84: 14-21.
[16] Noda H, Amano H, Arashima K, Hashimoto S, Nishizawa K. Studies on the antitumour activity of marine algae. Bull Jap Soc Sci Fish 1989; 55: 1254-1264.
[17] Kim AD, Lee Y, Kang SH, Kim GY, Kim HS, Hyun JW. Cytotoxic effect of clerosterol isolated from Codium fragile on A2058 human melanoma cells. Mar Drugs 2013; 11: 418-430.
[18] Kim KN, Lee KW, Song CB, Jeon YJ. Cytotoxic activities of green and brown seaweeds collected from Jeju island against four tumor cell lines. J Food Sci Nut 2006; 11: 17-24.
[19] Ganesan P, Noda K, Manabe Y, Ohkubo T, Tanaka Y, Maoka T, et al. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim Biophys Acta 2011; 1810: 497-503.
[20] Kang CH, Choi YH, Park SY, Kim GY. Anti-inflammatory effects of methanol extract of Codium fragile in lipopolysaccharide-stimulated RAW 264.7 cells. J Med Food 2012; 15: 44-50.
[21] Lee C, Park GH, Ahn EM, Kim BA, Park CI, Jang JH. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013; 86: 54-63.
[22] Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, et al. Matrix metalloproteinase 9 is a mediator of epidermal growth factor dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res 2008; 68: 4606-4613.
[23] Yousef EM, Tahir MR, St-Pierre Y, Gaboury LA. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer 2014; 14: 609.
[24] Zhao M, Gao Y, Wang L, Liu S, Han B, Ma L, et al. Overexpression of integrin-linked kinase promotes lung cancer cell migration and invasion via NF-κB-mediated upregulation of matrix metalloproteinase-9. Int J Med Sci 2013; 10: 995-1002.
[25] Mannello F, Medda V. Nuclear localization of matrix metalloproteinases. Prog Histochem Cytochem 2012; 47: 27-58.
[26] Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009; 1: a000034.
[27] Nangia-Makker P, Raz T, Tait L, Shekhar MP, Li H, Balan V, et al. Ocimum gratissimum retards breast cancer growth and progression and is a natural inhibitor of matrix metalloproteases. Cancer Biol Ther 2013;14: 417-427.
Document heading 10.1016/j.apjtm.2016.04.010of 24 members, MMP-9 is known to be significantly upregulated in almost all tumor types[3]. Furthermore, MMP-9 expression positively correlates with cancer stage, grade, and prognosis[4]. Several studies have shown that cancer cells that were attributable to metastasis to distant organs, including the lungs, liver, lymph nodes, and adrenal medulla expressed high levels of MMP-9[3,5]. This suggests that MMP-9 is not only important in identifying invasion symptoms and its diagnosis, but also a promising strategy to prevent cancer invasion and metastasis. Therefore, MMP-9 expression may prove an important therapeutic target for preventing the invasion and metastasis of a broad-spectrum of cancers.
15 February 2016
*Corresponding author: Gi-Young Kim, Department of Marine Life Sciences, Jeju National University, Jeju 63243, Republic of Korea.
Tel.: +82 64 754 3427
Fax: +82 64 756 3420
E-mail: immunkim@jejunu.ac.kr
Foundation project: This study was supported by Basic Science Research Program(2015R1D1A1A01060538) through the National Research Foundation of Korea (NRF)funded from the Ministry of Education, Science and Technology of Korea.
 Asian Pacific Journal of Tropical Medicine2016年6期
Asian Pacific Journal of Tropical Medicine2016年6期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Taeniasis vs cysticercosis infection routes
- Are efforts up to the mark? A cirrhotic state and knowledge about HCV prevalence in general population of Pakistan
- ZIka virus infection in Asia: reappraisal on phylogenetic data of Asian lineage
- Experiment research of cisplatin implants inhibiting transplantation tumor growth and regulating the expression of KLK7 and E-cad of tumor-bearing mice with gastric cancer
- Comparative study of the chitooligosaccharides effect on the proliferation inhibition and radiosensitization of three types of human gastric cancer cell line
- Change of the peripheral blood immune pattern and its correlation with prognosis in patients with liver cancer treated by sorafenib
