Experiment research of cisplatin implants inhibiting transplantation tumor growth and regulating the expression of KLK7 and E-cad of tumor-bearing mice with gastric cancer
Gui-Feng Zhuang, Yan Tan, Yuan-Zheng Yang, Jie-Wei Zhang, Jing TangDepartment of Digestive System, Affiliated Hospital of Hainan Medical College, Haikou 570102, China
Experiment research of cisplatin implants inhibiting transplantation tumor growth and regulating the expression of KLK7 and E-cad of tumor-bearing mice with gastric cancer
Gui-Feng Zhuang, Yan Tan, Yuan-Zheng Yang*, Jie-Wei Zhang, Jing Tang
Department of Digestive System, Affiliated Hospital of Hainan Medical College, Haikou 570102, China
ARTICLE INFO ABSTRACT
Article history:
in revised form 16 March 2016 Accepted 15 April 2016
Available online 20 June 2016
Gastric cancer
Cisplatin implants
Tissue kallikrein - 7
E-cadherin
Objective: To study the influence of cisplatin implants on transplantation tumor growth and the expression of tissue kallikrein-7 (KLK7) and E-cadherin (E-cad) in tumor-bearing mice with gastric cancer. Methods: BALB/c nude mice were collected as experimental animal and were randomly divided into model control group (Group A), tail intravenous injection of cisplatin group (Group B), intratumor injection of cisplatin group (Group C) and cisplatin implants treatment group (Group D). After the drugs intervening, the weight and volume of transplantation tumors were measured on Day 20, Day 30 and Day 40 and serum and KLK7 and E-cad contents in transplanted tumor tissue were examined. Results: On Day 20, Day 30 and Day 40 after treatment, the weight and volume of transplantation tumors of tumorbearing mice in four groups were different (Group A > Group B > Group C > Group D). The contents of KLK-7 and E-cad in tumor tissue and serum of tumor-bearing mice in four groups were different (Group A > Group B > Group C > Group D in KLK-7) and (Group A < Group B < Group C < Group D in E-cad). The weight and volume, and KLK7 and E-cad contents of transplantation tumors in four groups were significant difference (P<0.05). Conclusion: Cisplatin implants can inhibit the growth of transplanted tumor tissue and down-regulated KLK7 expression and up-regulated E-cad expression of tumor-bearing mice with gastric cancer
Nowadays, new incidence of gastric cancer is more than 1 million each year all over the world and China accounts for more than 40%. The death toll caused by gastric cancer is about eight hundred thousand each year all over the world and China accounts for 35%. Its morbidity and mortality
1. Introduction
account for larger proportion around the world. At present, a quarter death causes of domestic people are gastric cancer and the causes of cancer death are about a quarter of gastric cancer. Surgical resection and intravenous chemotherapy are the mainly methods to treat gastric cancer in clinic. Cisplatin is a commonly used chemotherapy drug and intravenous administration is the most commonly used method by using cisplatin to conduct chemotherapy in clinic. Although cisplatin can exert a certain lethal effect on cancer cells, it has a lower concentration and relatively limited lethal effect as well as can cause many adverse reactions and whole poor tolerance and compliance of patients. In addition, intravenous administration cannot continuously exert the lethal effect on cancer cells in tumor tissues[1,2]. A new type of cisplatin implants is an agent with long-acting anti-tumor effect and can release cisplatin continuously and exert persistent antitumor effect [3]. Nowadays, the research on cisplatin implants used to treat gastric cancer is deficient and its specific effect on killing cancer cells and molecular mechanism are not entirely clear. In the following study, we built an animal model of tumorbearing mice with gastric cancer and analyzed the growth condition of transplantation tumor and the influence of KLK-7 expression and E-cad expression by administrating cisplatin implants.
2. Materials and methods
2.1. Experimental materials
A total of 60 male BALB/c nude mice were selected as experimental animals, which were purchased from Laboratory Animal Center,Hainan Medical College and this research was approved by Ethics Committee of Affiliated Hospital of Hainan Medical College. Cell strain SGC7901 was purchased from Shanghai Cell Bank of Chinese Academy Sciences. Cisplatin slow-release implants was purchased from Anhui BBCA Pharmaceutical Co., Ltd. and cisplatin injection was purchased from Shanghai Xudong Haipu pharmaceutical Co., Ltd. RPMI1640 medium and fetal calf serum were purchased from Hyclone Co., Ltd. Enzyme linked immunoabsorbent kit was purchased from Wuhan Boster Biological Engineering Co., Ltd.
2.2. Experimental methods
2.2.1. Dividing methods
Nude mice collected were randomly divided into model control group (Group A), tail intravenous injection of cisplatin group (Group B), intratumor injection of cisplatin group (Group C) and cisplatin implants treatment group (Group D) with 15 mice in each group. A tumor-burdened mouse model with gastric cancer was established in nude mice of four groups according to the following methods: culture cell strains SGC7901 were collected in logarithmic growth phase after amplification from generation to generation and adjusted the density of cells into 1.5 108/mL, and 0.2 mL cell suspension was injected into axilla of right anterior limb and the tumour was formed with diameter over 5 cm after 10 days. Mice in A-D Group were intervened according to the following methods: Group A was given PBS intratumor injection and caudal vein injection and the dose was referenced to Group B and Group C; Group B was given 1 mg/kg cisplatin injection by intratumor injection; Group C was given 1 mg/kg cisplatin injection by caudal vein injection and Group D was given 0.4 mg/kg cisplatin implants, which were placed in enterocoelia.
2.2.2. Animal specimen collection methods
On Day 20, Day 30, Day 40 after treatment, 5 mice were selected from each group and were sacrificed using cervical dislocation method. The blood was pumped through heart, and serum was obtained and preserved at -80 ℃ after centrifugation. Transplanted tumor tissue was dissected and tumor weight of each week was weighted. The longest diameter and the shortest diameter of tumor were measured by using micrometer. The volume of tumor tissue was calculated by the following formula: V=0.5 the longest diameter the shortest diameter2. After that, tumor tissue was quickly frozen in liquid nitrogen and preserved at -80 ℃ finally.
2.2.3. Detection methods of Animal specimen
Serum specimen was collected and the contents of KLK-7 and E-cad were measured using enzyme-linked immunosorbent determination kit. Tumor tissue was collected and homogenized after adding PBS. After homogenizing, the suspension was centrifuged and discarded sediment, and then the supernatant was reserved. The contents of KLK-7 and E-cad were measured using enzyme-linked immunosorbent determination kit
2.3. Statistical methods
Data were inputted using SPSS20.0 software and were statistically processed. ANOVA was used to compare the measurement data among four groups and pairwise comparison was conducted using LSD-t test. Differences were statistically significant at P<0.05.
3. Results
3.1. Weight and volume of tumor tissue
On Day 20, Day 30 and Day 40 after treatment, the weight and volume of transplantation tumors of tumor-bearing mice in four groups were different (Group A > Group B > Group C > Group D). The concrete analysis was as follows: transplanted tumor weight and volume of tumor-bearing mice in Group B, Group C and Group Dwere all lower than those in Group A. Transplanted tumor weight and volume of tumor-bearing mice in Group C and Group D were all lower than those in Group B and transplanted tumor weight and volume of tumor-bearing mice in Group D were all lower than those in Group C (Table 1).
3.2. KLK-7 content in tumor tissue and serum
On Day 20, Day 30 and Day 40 after treatment, KLK-7 content in tumor tissue and serum of tumor-bearing mice in four groups were different (Group A > Group B > Group C > Group D). The concrete analysis was as follows: KLK-7 content in tumor tissue and serum of tumor-bearing mice in Group B, Group C and Group D were all lower than those in Group A; KLK-7 content in tumor tissue and serum of tumor-bearing mice in Group C and Group D were all lower than those in Group B and KLK-7 content in tumor tissue and serum of tumor-bearing mice in Group D were all lower than those in Group C (Table 2).
3.3. E-cad content in tumor tissue and serum
On Day 20, Day 30 and Day 40 after treatment, E-cad content in tumor tissue and serum of tumor-bearing mice in four groups were different (Group A < Group B < Group C < Group D). The concrete analysis was as follows: E-cad content in tumor tissue and serum of tumor-bearing mice in Group B, Group C and Group D were all higher than those in Group A; E-cad content in tumor tissue and serum of tumor-bearing mice in Group C and Group D were all higher than those in Group B and E-cad content in tumor tissue and serum of tumor-bearing mice in Group D were all higher than those in Group C (Table 3).
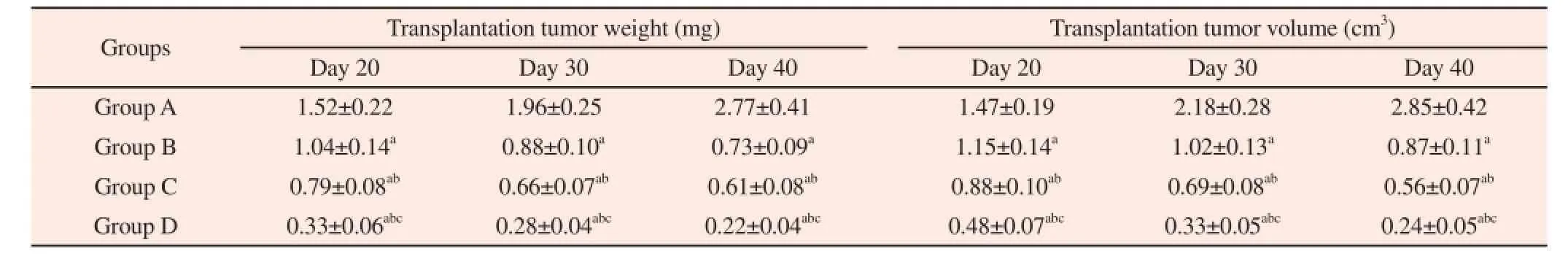
Table 1Comparison of transplanted tumor weight and volume of tumor-bearing mice in four groups on Day 20, Day 30 and Day 40.
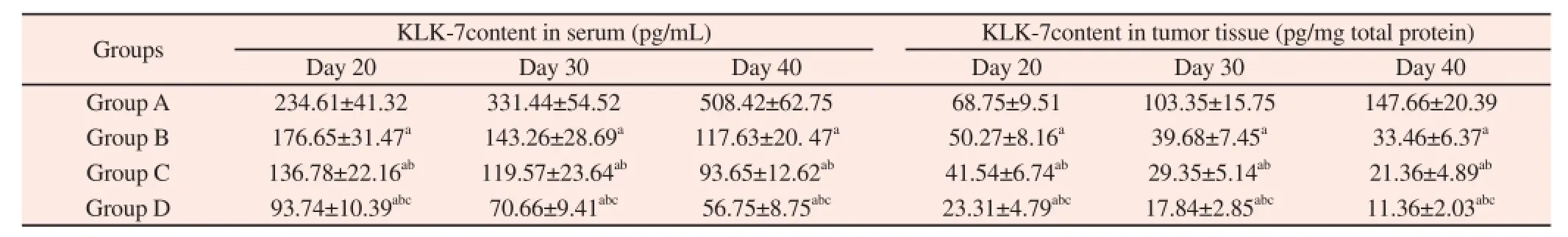
Table 2Comparison of KLK-7 content in tumor tissue and serum of tumor-bearing mice in four groups on Day 20, Day 30 and Day 40.

Table 3Comparison of E-cad content in tumor tissue and serum of tumor-bearing mice in four groups on Day 20, Day 30 and Day 40.
4. Discussion
Cisplatin is the commonly chemotherapy drug used in gastric cancer, which can effectively kill gastric cancer cells, induce tumor cell apoptosis and inhibit tumor growth. Intravenous administrationis the most commonly approach of chemotherapy administration,which though can effectively kill gastric cancer cells, the drug concentration within the tumor lesion is lower and lethal effect is effective. In addition, chemotherapy drug can induce viscera function damage effecting on each organ of the whole body through vein, and lead to a variety of adverse reactions and limit the dose of chemotherapy drugs and influence the effect of chemotherapy[4-6]. Chemotherapy drug injection within tumor tissue can form a higher drug concentration in local and has a stronger killing effect on cancer cells. However, the degree of difficulty of operation for this method of administration is bigger and is difficult to constant repeated administration of drugs, therefore it is not suitable for clinic treatment[7]. Cisplatin implants is a kind of cisplatin slow-release preparation, which is developed in recent years, and can release cisplatin slowly and continuously and exert a lethal effect on cancer cells[8-9].
In this research, we built an animal model of tumor-bearing mice with gastric cancer and analyzed the effect of tail intravenous injection of cisplatin, intratumor injection of cisplatin and cisplatin implants treatment. Firstly, we evaluate the growth condition of transplantation tumor by measuring the weight and volume of tumor tissue and the results showed that the weight and volume of transplantation tumors of tumor-bearing mice in four groups were different (Group A > Group B > Group C > Group D). Thus, our analysis is as follows: (1) transplanted tumor weight and volume of tumor-bearing mice in Group B, Group C and Group D were all lower than those in Group A and intravenous injection of cisplatin,intratumor injection and cisplatin implants can all inhibit the gastric cancer transplanted tumor growth; (2) transplanted tumor weight and volume of tumor-bearing mice in Group C and Group D were all lower than those in Group B and transplanted tumor weight and volume of tumor-bearing mice in Group D were all lower than those in Group C, which reveal that among the three methods of administration, cisplatin implants have a statistically significant tumor suppression effect, followed by intratumor injection of cisplatin and intravenous injection of cisplatin has the worst tumor suppression effect.
KLK family is a molecule family to regulate the cells growth,adhesion and proliferation, including KLK1-KLK15 (a total of 15 members), among which abnormal expression of KLK-7 is associated with the occurrence and development of digestive tract malignancies. Some clinical researches have verified that the level of KLK-7 expression in colon cancer tissue has increased significantly and is closely related with proliferation of cancer cells[10-11]. The research on cells level has showed that silencing of targeting KLK-7 by siRNA in gastric cancer cells after the reaction of KLK-7, the cell cycle and cell proliferation were significantly suppressed[12]. We analyzed the KLK-7 content in transplanted tumor tissue and serum sample after cisplatin treatment and confirmed that different cisplatin preparation and route of administration are all can inhibit the KLK-7 expression, and reduce KLK-7 content in transplanted tumor tissue and serum. Moreover, cisplatin implants have a statistically significant effect on inhibiting KLK-7 expression, followed by intratumor injection of cisplatin and intravenous injection of cisplatin has the weakest effect on inhibiting KLK-7 expression.
E-cad is a labeled molecule of epithelial cell, which has the function to regulate the adhesion between cells and cells, and cells and extracellular matrix. E-cad can maintain the integrality of morphological structure of epithelial tissue and its deficient expression will cause diastasis and reattachment of cancer cells and further induce local infiltration and distant transfer[13-14]. Besides,In the process of occurrence and development of gastric cancer,epithelial-mesenchymal transition is a significant pathological feature and epithelial cell will lose polarity and acquire interstitial cell phenotype. The expression level of epithelial cell markers(E-cad) is reduced significantly and further promote invasion of cancer cells[15]. Several studies have reported that the expression level of E-cad in gastric cancer tissue is reduced significantly and the process of EMT is enhanced[16-17]. We analyzed the E-cad content in transplanted tumor tissue and serum sample after cisplatin treatment and confirmed that different cisplatin preparation and route of administration are all can promote the E-cad expression,and increase E-cad content in transplanted tumor tissue and serum. Moreover, cisplatin implants have a statistically significant effect on promoting E-cad expression, followed by intratumor injection of cisplatin and intravenous injection of cisplatin has the weakest effect on promoting KLK-7 expression.
In conclusion, cisplatin implants can inhibit the growth of transplanted tumor tissue and down-regulated KLK7 expression and up-regulated E-cad expression of tumor-bearing mice with gastric cancer.
Declare of interest statement
We declare that we have no conflict of interest.
Reference
[1] Izuishi K, Mori H. Recent strategies for treating stage iv gastric cancer:roles of palliative gastrectomy, chemotherapy, and radiotherapy. J Gastrointestin Liver Dis 2016;25(1):87-94.
[2] Shim HJ, Kim KR, Hwang JE, Bae WK, Ryu SY, Park YK, et al. A phase II study of adjuvant S-1/cisplatin chemotherapy followed by S-1-based chemoradiotherapy for D2-resected gastric cancer. Cancer Chemother Pharmacol 2016;77(3):605-612.
[3] Cai TT, Yan LP, Li H, Shi XP, Liang Y, Shen MR. Antitumor effects of cisplatin implants against human gastric cancer in nude mice. World Chin J Dig 2014;22(20):2817-2825.
[4] Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3,randomised controlled trial. Lancet Oncol 2016; 17(3):309-318.
[5] Wang J. Curative effect of HDLF combined with abdominal perfusion chemotherapy for advanced gastric cancer. J Hainan Med Univ 2012;18(6):794-796.
[6] Lee JY, Son T, Cheong JH, Hyung WJ, Noh SH, Kim CB, et al. Association between chemotherapy-response assays and subsets of tumorinfiltrating lymphocytes in gastric cancer: a pilot study. J Gastric Cancer 2015;15(4):223-230.
[7] Okabe Y, Yajima K, Ishikawa T, Kosugi S, Sakamoto K, Sato Y, et al. Urgent gastrectomy in a patient who developed perforated gastric cancer during preoperative chemotherapy with S-1 plus cisplatin. Gan To Kagaku Ryoho 2014;41(1):95-98.
[8] Emoto S, Ishigami H, Hidemura A, Yamaguchi H, Yamashita H, Kitayama J, et al. Complications and management of an implanted intraperitoneal access port system for intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Jpn J Clin Oncol 2012; 42(11):1013-1019.
[9] Li H, Cai TT, Yan LP, Shi XP, Tian CH, Shen MR, Zhang M. Effect of cisplatin implantation on tumor expression change of SYK, HER2,CD44V6 and PTEN in human gastric carcinoma xenograft of nude mice. Chin J Modern Med 2015;25(5):8-13.
[10] Walker F, Nicole P, Jallane A, Soosaipillai A, Mosbach V,Oikonomopoulou K, et al. Kallikrein-related peptidase 7 (KLK7) is a proliferative factor that is aberrantly expressed in human colon cancer. Biol Chem 2014;395(9):1075-1086.
[11] Devetzi M, Trangas T, Scorilas A, Xynopoulos D, Talieri M. Parallel overexpression and clinical significance of kallikrein-related peptidases 7 and 14 (KLK7KLK14) in colon cancer. Thromb Haemost 2013;109(4):716-725.
[12] Su XF Wang WL, Cai HP. Inhibitory effect of KLK7siRNA on gastric cancer AGS cell lines. Chin J Cancer Biother 2013;20(2):207-211.
[13] Caldeira J, Figueiredo J, Brás-Pereira C, Carneiro P, Moreira AM, Pinto MT, et al. E-cadherin-defective gastric cancer cells depend on Laminin to survive and invade. Hum Mol Genet 2015; 24(20):5891-5900.
[14] Su YJ, Chang YW, Lin WH, Liang CL, Lee JL. An aberrant nuclear localization of E-cadherin is a potent inhibitor of Wnt/β-cateninelicited promotion of the cancer stem cell phenotype. Oncogenesis 2015;15(4):e157.
[15] Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res 2015;7(11):2141-2158.
[16] Han Y, Ye J, Dong Y, Xu Z, DU Q. Expression and significance of annexin A2 in patients with gastric adenocarcinoma and the association with E-cadherin. Exp Ther Med 2015;10(2):549-554.
[17] Ferraz MA, Zabaglia LM, Pereira WN, Orcini WA, de Labio RW, Neto AC, et al. Downregulated Expression of E-cadherin and TP53 in Patients with Gastric Diseases: the Involvement of H. pylori Infection and Its Virulence Markers. J Gastrointest Cancer 2016;47(1):20-26.
Document heading 10.1016/j.apjtm.2016.04.022
15 February 2016
*Corresponding author: Corresponding
author: Yuan-Zheng Yang, Affiliated Hospital of Hainan Medical College, Haikou 570102, China. Tel: 15103048576 E-mail: hhyangyuanzheng@163.com Foundation Project: This study was supported by Health Department of Hainan Province (Qiong 2013- self-financing 08).
 Asian Pacific Journal of Tropical Medicine2016年6期
Asian Pacific Journal of Tropical Medicine2016年6期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Comparative study of the chitooligosaccharides effect on the proliferation inhibition and radiosensitization of three types of human gastric cancer cell line
- Study on prevention effect of Zishen Yutai pill combined with progesterone for threatened abortion in rats
- Correlation study of biological characteristics of non-small cell lung cancer A549 cells after transfecting plasmid by microbubble ultrasound contrast agent
- Expression and significance of angiostatin, vascular endothelial growth factor and matrix metalloproteinase-9 in brain tissue of diabetic rats with ischemia reperfusion
- Change of the peripheral blood immune pattern and its correlation with prognosis in patients with liver cancer treated by sorafenib
- Are efforts up to the mark? A cirrhotic state and knowledge about HCV prevalence in general population of Pakistan
