Pitfalls and optimal approaches to diagnose melioidosis
Paul Vijay Kingsley, Govindakarnavar Arunkumar, Meghan Tipre, Mark Leader, Nalini Sathiakumar
1Sultan Ismail Hospital, Johor Bharu, Malaysia2Manipal Center for Virus Research, Manipal University, Manipal, India3School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama, USA
Pitfalls and optimal approaches to diagnose melioidosis
Paul Vijay Kingsley1*, Govindakarnavar Arunkumar2, Meghan Tipre3, Mark Leader3, Nalini Sathiakumar3
1Sultan Ismail Hospital, Johor Bharu, Malaysia
2Manipal Center for Virus Research, Manipal University, Manipal, India
3School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama, USA
ARTICLE INFO ABSTRACT
Article history:
in revised form 16 March 2016 Accepted 15 April 2016
Available online 15 June 2016
Melioidosis pseudomallei;
Review
Diagnosis
Treatment
Pitfalls
Approaches route of infection Melioidosis is a severe and fatal infectious disease in the tropics and subtropics. It presents as a febrile illness with protean manifestation ranging from chronic localized infection to acute fulminant septicemia with dissemination of infection to multiple organs characterized by abscesses. Pneumonia is the most common clinical presentation. Because of the wide range of clinical presentations, physicians may often misdiagnose and mistreat the disease for tuberculosis, pneumonia or other pyogenic infections. The purpose of this paper is to present common pitfalls in diagnosis and provide optimal approaches to enable early diagnosis and prompt treatment of melidoidosis. Melioidosis may occur beyond the boundaries of endemic areas. There is no pathognomonic feature specific to a diagnosis of melioidosis. In endemic areas, physicians need to expand the diagnostic work-up to include melioidosis when confronted with clinical scenarios of pyrexia of unknown origin, progressive pneumonia or sepsis. Radiological imaging is an integral part of the diagnostic workup. Knowledge of the modes of transmission and risk factors will add support in clinically suspected cases to initiate therapy. In situations of clinically highly probable or possible cases where laboratory bacteriological confirmation is not possible, applying evidence-based criteria and empirical treatment with antimicrobials is recommended. It is of prime importance that patients undergo the full course of antimicrobial therapy to avoid relapse and recurrence. Early diagnosis and appropriate management is crucial in reducing serious complications leading to high mortality,and in preventing recurrences of the disease. Thus, there is a crucial need for promoting awareness among physicians at all levels and for improved diagnostic microbiology services. Further, the need for making the disease notifiable and/or initiating melioidosis registries in endemic countries appears to be compelling.
1. Introduction
Melioidosis is an infectious disease of humans and animals caused by a gram-negative bacillus, Burkholderia pseudomallei(B. pseudomallei) that live in moist soil and water. Although the distribution of melioidosis is concentrated in the tropics and subtropics, with increasing movement of people between countries,it may occur in any part of the world. Thus, it may be considered as a global health problem. Melioidosis has a wide range of clinical presentations and can be commonly misdiagnosed and mistreated for other diseases such as tuberculosis, common forms of pneumonia or other pyogenic infections. Hence, the disease is often called, ‘the Great Mimicker’[1]. Despite advances in treatment,the case fatality rates in acute severe melioidosis is about 30% to 47% and may range from 40%-75% in cases with sepsis[2];sepsis appears to be a major determinant of case fatality. A 20-year prospective study in Australia reports a 50% case fatality rate among cases with septic shock and only 4% in the absence of septic shock[3]. About 21%-48% of cases present with septic shock[3-5]. Thus, misdiagnosis of the disease has serious consequences.Although 20% of community-acquired pneumonia and 20% of community-acquired sepsis in endemic areas may be due to B. pseudomallei[1], the disease is not well known among physicians and microbiologists. Further, based on the ease of spread and severity of illness/death, the Centers for Disease Control and prevention has classified B. pseudomallei as a bioterrorism agent in Category B,the second highest priority group[6]. Thus, it is crucial for all health care providers to be aware of the disease. In this paper, we present the common pitfalls in diagnosis and provide optimal approaches to early diagnosis and prompt management.
2. Problems in diagnosis and optimal approaches
2.1. Under-diagnosis and under-reporting
Melioidosis occurs primarily in countries with tropical and subtropical climates lying between latitudes 20 °N and 20 °S. It is considered to be endemic in southeast Asia (Brunei, Cambodia,Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore,Thailand, Timor-Leste and Vietnam), northern Australia, most of the Indian subcontinent (India, Pakistan, Bangladesh, Nepal,Bhutan, Sri Lanka, Maldives), southern China, Hong Kong and Taiwan[7]. Although melioidosis is endemic in several countries,most published case reports of melioidosis are only from Thailand,Malaysia, Singapore and northern Australia (Table 1). Other endemic countries such as the Indian subcontinent report cases infrequently although the case volume is expected to be high[4,5,8]. Sporadic cases have been reported from parts of Africa, the Caribbean, Central and South America and Middle East. Hence, the known distribution of the disease is referred to as the ‘tip of the iceberg’[9].

Table 1Frequency of published case reports.
The low frequency of reporting in endemic countries reflects underdiagnosis and under-reporting[10]. Missed diagnosis by physicians coupled with lack of adequate diagnostic laboratories in rural settings where most of the cases are expected may contribute to these problems. The disease is not notifiable in most endemic countries[11]. Approach: Under-diagnosis. There is a crucial need to promote greater awareness and provide improved diagnostic facilities to enable early diagnosis and treatment. Starting with medical students,a differential diagnosis of melioidosis should be entertained,investigated and discussed in all pertinent clinical scenarios. Continuing medical education on melioidosis is a useful exercise in all hospitals to increase awareness among doctors. The topic should be given importance at local and regional conferences. Protocols for diagnosis and management need to be in place for urban and for rural hospital/clinic settings where diagnostic facilities may be unavailable.
Under-reporting. Data are needed to assess the magnitude of the problem and disease trends across countries. Mandatory reporting allows for the collection of incidence and mortality statistics,assessing disease trends and in tracking disease outbreaks. Jeyaram(2005) states that there is a compelling need to make the disease notifiable in endemic countries[11]. The disease was made notifiable in Singapore in 1989; the data have been useful in monitoring the disease which indicate a decreasing trend of incidence and of case fatality[12]. Initiating a melioidosis registry will be another powerful tool to track the disease, assess treatment and outcomes and to follow-up patients. For example, Pahang state in Malaysia began a melioidosis case registry in 2005 which has allowed them to compile annual statistics and improve management[13]; further, it has helped to increase knowledge among physicians.
2.2. Highly variable incubation period
Although melioidosis is endemic in southeast Asia and northern Australia between latitudes 20 °N and 20 °S, large outbreaks have occurred outside this region as in southwestern Australia[14]. Cases reported from temperate climates are likely to be due to importing of the disease from the tropics by immigrants and travelers.
The incubation period (time from exposure to the bacteria to the onset of symptoms) for the disease may range from one to 21 d(mean, 9 d), but is highly variable[15]. Factors that influence the incubation period include the virulence of the strain, inoculating dose, mode of acquisition and comorbid conditions in the host. With a high inoculum, as may occur in cases of aspiration following neardrowning or severe weather events, the disease may manifest within 24 h[16]. The longest recorded incubation period was in a World WarⅡ veteran who had served as a prisoner of war in Southeast Asia and presented with the cutaneous form of melioidosis 62 years after initial exposure[17].
Approach: Physicians should be on high alert to a diagnosis of melioidosis both in endemic and nonendemic regions. In nonendemic regions, it is important to elicit even a remote history of travel to an endemic area including recreational travel, military service and other work-related travel. It is to be noted that symptoms may occur several years after initial exposure to the bacteria.
2.3. Inoculation and inhalation
B. pseudomallei have a natural environmental habitat in endemic areas and are found in muddy soil, surface water and plants. In Southeast Asia, the organism has been repeatedly isolated from irrigated, cleared agricultural land such as paddy fields and farms[18,19]. Most cases of melioidosis occur following exposure to contaminated soil or water though percutaneous inoculation via penetrating wounds and preexisting skin abrasions such as ulcers or burns[20]; inoculation at the time of a snake bite has been reported[21]. Skin contact tends to have a longer incubation period with less severe disease[7]. Inhalation via contaminated dust particles or water droplets (bacteria in aerosol form) is the next most common route of entry and is characterized by pneumonia and more severe infection[7]. Acquisition of bacteria through aspiration of contaminated water in near-drowning episodes has been recorded[16]. Ingestion has been suggested as a mode of acquiring infection[10]. Laboratory-acquired cases have been described[22]. Person-to-person transmission is very uncommon. Sexual transmission has been suggested but has not been established[23]. Few reports suggest perinatal transmission[24,25]. Transmission to infants through infected breast milk from mothers with mastitis has been reported[26]. Contaminated detergents have been implicated in reports of nosocomial infection[27,28].
Melioidosis is strongly associated with wet weather; about 75% to 81% of cases occur during the rainy season[29]. Cases and deaths from melioidosis have been found to increase linearly with increase in mean monthly rainfall[30]. Periods of heavy rainfall and extreme weather events such as floods and tsunamis have been found to be associated with inhalation transmission[31].
Approach: Inoculation and inhalation are the commonest modes of transmission. In endemic areas, a history of regular contact with soil,water or plants should be elicited; existing wounds or of recent injury should be examined for. However, absence of definite history of contact with soil or of the absence of the evidence of a portal of entry via the skin does not rule out the disease. Cases with pneumonic presentation during periods of extreme wet weather events should alert the physician to consider melioidosis as a diagnostic possibility.
2.4. Several risk factors predispose to melioidosis
(1) Age. The disease may occur at any age including newborns. The peak incidence is between 40 and 60 years of age, the age range during which most co-morbid conditions develop[32]. (2) Gender. A preponderance of the disease among males has been noted[33];the gender difference may be due to a higher potential for activities facilitating exposure. (3) Ethnicity. Aboriginality was found to be a risk factor in Australia, possibly to the potential for exposure to contaminated water and soil[20]. (4) Occupation/recreational exposure in endemic areas. The risk is highest for persons with regular contact with soil or water in endemic area. Workers in the agricultural sector, construction workers, military personnel,adventure travelers and ecotourists are groups at high risk because of their contact with contaminated soil or water[1]. A study in Thailand found that 81% of cases were rice farmers[34]. (5) Lifestyle factors. About 12% to 39% of patients report a history of heavy alcohol consumption[3]. Smoking has been identified as a risk factor[3,35].(6) Diet. In Australia, excessive use of Kava (Piper methysticum root) is documented as a risk factor[36]. (7) Co-morbid conditions. Several underlying medical conditions or drug therapy that may impair host defense predispose to meloidoisis[3,37]. Pre-existing or newly-diagnosed type 2 diabetes mellitus is the most common comorbid condition associated with melioidosis; about 23%-60% of melioidosis patients were found to have associated type 2 diabetes mellitus. In patients with cystic fibrosis, B. pseudomallei may cause chronic infection[38]. Other co-morbid conditions that predispose to melioidosis include chronic pulmonary disease (found in 12%-27% of melioidosis cases), chronic renal disease (10%-27%), thalassemia(7%), atypical mycobacterial disease (Mycobacterium tuberculosis or Mycobacterium lepra), steroid therapy (<5%) and cancer (<5%). Human immunodeficiency virus infection has not been identified as a risk factor for the disease, and it does not seem to alter the clinical features or the progression of the disease[39]; reasons for the lack of an interaction between human immunodeficiency virus and melioidosis is unclear.
Approach: About 80% of patients have one or more risk factors;thus, knowledge of risk factors is essential. Type 2 diabetes mellitus has been found to be the commonest risk factor; thus, a high index of suspicion among type 2 diabetic patients is warranted. It is to be noted that otherwise healthy persons including children may get melioidosis.
2.5. Clinical symptoms mimic other diseases
Melioidosis presents as a febrile illness with protean manifestation ranging from chronic localized infection to acute fulminant septicemia with multiple organs affected. The disease is characterized by multiple abscess formation. Table 2 provides the salient features of clinical presentations, commonly misdiagnosed conditions along with certain clues to aid in diagnosis.
Acute pulmonary infection is the most common presentation occurring in about 50% of cases[7]. The infection may be primary acquired via inhalation or secondary occurring via hematogenous spread following inoculation. Patients present with extreme prostration and toxicity that is out of proportion to physical or radiographic findings. Clinical findings may vary from mild undifferentiated pneumonia to severe pneumonia or lung abscess.
Acute bacteremia may occur in about 50% of patients; the clinical picture may vary from a simple bacteremia with no evident focus of infection to sepsis with one/few organ involvement to fulminant septic shock and multi-organ involvement; about 21%-48% of cases present with septic shock[3-5]. In northern Australia and northeast Thailand, melioidosis has been reported to account up to 20% of community-acquired sepsis[1]. Patients present with a history of fever(median, 6 d; range, 3 d to several months) and often with no focus of infection. Sepsis of abrupt onset may progress to dissemination of the primary focus of infection evidenced by multiple abscesses most commonly found in spleen, liver, lung, skeletal muscle and prostate. Involvement of joints (septic arthritis), bone (osteomyelitis), lymph nodes (lymphadenopathy), skin (pustules) or central nervous system(cerebral abscess, brain stem encephalitis with flaccid paralysis) mayoccur. Other organs rarely involved include heart (pyopericardium,pericarditis), mycotic aneurysms, mediastinal infection, thyroid abscess, scrotal abscesses or epididymo-orchitis[40].

Table 2Characteristics, misdiagnosis and clues to diagnosis according to presentation type of melioidosis.
Acute localized infection, occurring in about 10% of cases, may present as skin ulcers, subcutaneous tissue abscesses, parotid abscess or ocular infection. Localized osteomyelitis has been described[41]. Localized infection may rapidly progress to more widespread infection[7].
Chronic infection may occur in about 8% of cases[42]. Clinical manifestations include chronic pneumonia, chronic skin ulcers/ abscesses and disseminated infection which may progress to sepsis. Melioidosis is less common in children than in adults even in endemic areas. In Thailand, about one-third of pediatric cases presented as acute suppurative parotitis[32]. In a 24-year prospective study in Northern Australia, 5% of cases were children[43]. In this study, most children presented in the wet season and had no known risk factor. In 42% of children, inoculation appeared to be the mode of transmission. Primary cutaneous manifestation was the commonest presentation (60% vs. 13% in adults), bacteremia was less common (16% vs. 59%), and brain stem encephalitis occurred in 3 out of 45 children. Bacteremic children presented with pneumonia or septic arthritis/osteomyelitis, some of whom progressed to septic shock. Mortality was about 7%. Among the subgroup of neonates,mortality was 72.5%. Acquisition of infection was attributed to infected breast milk, vertical transmission associated with placental micro abscesses or through contaminated detergent solutions.
Regional variations in disease presentation may occur[7,20,32,44]. Parotid abscess seen in 30%-40% of Thai children has been reported only in one case in Australia. On the other hand, prostatic abscess found in about 20% of Australian males has been rarely reported elsewhere. Similarly, brain stem encephalitis with flaccid paralysis noted in 4% of cases in northern Australia is reported in only 0.2% of Thai population.Despite advances in treatment, the case fatality is high. A 20-year prospective study in Australia reports a 50% case fatality rate among adult cases with septic shock and only 4% in the absence of septic shock[3].
Approach: There is no pathognomonic feature specific to melioidosis. Salient clinical features include patients presenting with fever, cough, lymphadenopathy, visceral/skeletal/subcutaneous abscesses and/or sepsis. Rapid progression to respiratory failure and profound weight loss are striking features. Diabetes is the most commonly associated co-morbid condition. Some clues may be helpful as noted in Table 2. In endemic areas, as a rule, physicians need to expand the diagnostic work-up to include melioidosis when confronted with clinical scenarios of: (1) any pyrexia of unknown origin; (2) progressive pneumonia; and (3) sepsis. Chest radiography and abdominal ultrasound are mandatory investigations. Where available, computed tomography scan of abdominopelvic area is useful to detect asymptomatic abscesses. On imaging, ‘honeycomb or Swiss cheese’ appearance of large abscesses (commonly in liver)or small dispersed abscesses and concurrent involvement of several organs may he highly supportive of a diagnosis of melioidosis.
Pediatric melidoidsis is uncommon. Diagnosis may be challenging as presenting clinical features may differ in children compared to adults; in Northern Australia, cutaneous manifestation was the commonest presentation, and in Thailand suppurative parotitis was most common. Neonates represent a high risk group. A high index of clinical suspicion is necessary to ensure prompt diagnosis and treatment.
2.5. Laboratory confirmation of the disease may be challenging
Figure 1 provides a schematic approach to the laboratory diagnosis of melioidosis. Despite its low sensitivity, the isolation of B. pseudomallei from blood, sputum, abscess aspirates, cerebrospinal fluid, pericardial fluid, skin lesions or other clinical specimens is considered as the ‘gold standard’ for a diagnosis of melioidosis. B. pseudomallei from sterile clinical specimens grow easily in common bacteriological culture media such as blood agar or MacConkey agar; however, it is often difficult to identify the organism in most laboratories even in endemic areas. Clinical specimens from nonsterile sites will require selective media such as Ashdown’s selective agar or the B. pseudomallei selective agar[45,46]. It should be noted that when B. pseudomallei is grown from blood culture it may take about 2 to 3 d for growth following subculture.
Laboratory identification of B. pseudomallei from cultures can be difficult, especially in nonendemic countries where it is rarely seen. B. pseudomallei colonies are large and wrinkled, with a metallic appearance, and possesses an earthy, putrid odor (poses major biosafety concern). Colony morphology is also very variable and a single strain may display multiple colony types[28]. Colonies appear like environmental contaminants and are often discarded as being of no clinical significance[47].
Preliminary identification of B. pseudomallei from cultures include: Gram staining-organism appears as a Gram-negative rod with a characteristic ‘safety pin’ appearance (bipolar staining) and biochemical tests and the pattern of susceptibility to antimicrobials(organism is oxidase positive, Gentamicin resistant and Polymyxin resistant). The next step involves definitive phenotypic or genotypic identification of B. pseudomallei. Phenotypically, B.pseudomallei can be identified using commercial bacterial identification systems such as bioMérieux’s Analytical Profile Index 20NE and VITEK 1&VITEK 2; these tests may fail to distinguish B. pseudomallei from the closely related avirulent B. thailandensis in about 20% of cases(Lau et al, 2015). More precisely, B. pseudomallei can be identified by genotypic methods based on sequencing of 16s rRNA and groER genes[46].
Identification of B. pseudomallei at the molecular level by polymerase chain reaction (PCR) assays using specific B. pseudomallei primers continues to be a challenge due to the existing genetic similarity between closely related species of the Burkholderia genus[46]. Although some specific PCR assays are available for identification of B. pseudomallei from cultures, they lack validation in diagnostic settings. Matrix-assisted laser desorption ionization timeof-flight mass spectrometry is one of the state of the art pathogen identification tool used for the rapid and accurate identification of the B. pseudomallei from cultures in reference laboratories[46].
Culture-based diagnosis of melioidosis may cause significant biosafety concerns of laboratory exposures. Hence, direct detection of B. pseudomallei from clinical samples from sputum or blood, using specific primers is a preferred option for rapid diagnosis. However,there is no PCR assay with acceptable sensitivity and specificity thatis currently available. Detection of B. pseudomallei antigen in clinical specimens using point of care test such as lateral flow assay is an upcoming approach for the rapid diagnosis of melioidosis[48].

Figure 1. Schematic approach to laboratory diagnosis of melioidosis.
Antibody response and the importance of IgM/IgG detection in melioidosis remain unclear. Hence, serological assays for the detection of antibody response against B. pseudomallei such as indirect haemagglutination assay, enzyme linked immunosorbent assay-IgM and IgG or immunochromatographic tests are at best,only adjunct to culture-based diagnosis. Though a four-fold rise in titre in paired serum sample (acute and convalescent) could beconfirmatory of a diagnosis of melidoidosis, it is only useful in retrospective diagnosis. A result based on a single sample is not useful in a diagnosis of melioidosis[46].
Laboratory confirmation of melioidosis in most of the laboratories remains a major challenge because: culture-based methods pose significant biosafety risk; molecular diagnostic assays require sophisticated laboratories; and serological assays have poor sensitivity and specificity. Thus, a high degree of clinical suspicion should be the key for a diagnosis of melioidosis in endemic areas.
Approach: Routine baseline investigations do not help in the diagnosis of melioidosis. Culture results may take 2 to 3 d and confirmatory bacterial identification from cultures may require a well-equipped laboratory and trained personnel. In situations where the clinical picture is suggestive of melioidosis, and where Gram negative bacillus is grown in culture with characteristic colony morphology of B. pseudomallei without positive confirmatory tests should be considered supportive of a diagnosis of melioidosis.
2.6. Evidence-based criteria
In rural settings where the case volume is expected to be high,melioidosis may be suspected but laboratories may be unequipped to identify B. pseudomallei or there may be clinical situations when melioidosis is suspected but culture is negative.
In the above scenarios, using clinical criteria will be critical in making a diagnosis of melioidosis. Table 3 provides evidence-based criteria for a diagnosis of melioidosis[37,49]. As noted in the criteria,the characteristic features of abscesses should alert the physician to a diagnosis of melioidosis.

Table 3Evidence-based criteria for diagnosis of melioidosis.
†Large splenic or hepatic abscess with characteristic ‘Swiss cheese or honeycomb’ appearance or small dispersed abscesses or parotid abscess or prostate abscess in an endemic area where B. pseudomallei is the most probable cause.
Other factors that should be considered along with the clinical criteria include the evidence of exposure (residence or past travel to endemic area, known outbreak or laboratory accident) and the presence of major risk factors for melioidosis (type 2 diabetes mellitus, chronic renal disease, heavy alcohol use, chronic pulmonary disease, thalassemia, steroid therapy and malignancy). These factors may strengthen or weaken a diagnosis of melidoidosis in a clinically suspected case. For example, in a suspected case, a history of residence or travel to an endemic area or type 2 diabetes mellitus would strongly support a diagnosis of melioidosis.
Approach: Evidence-based criteria along with other factors(exposure and major risk factors) will aid in making a diagnosis of melioidosis when laboratory confirmation is either unavailable or negative. It is crucial to begin empirical therapy with antimicrobial therapy in all suspected melioidosis cases unless there are very definite alternate diagnoses or resolution of suspected symptoms with treatment with antimicrobial drugs that are not used in melioidosis.
2.7. High case fatality for failure to start appropriate antimicrobial therapy
Melioidosis may be successfully treated with appropriate antimicrobial therapy. Several randomized clinical trials have been conducted to evaluate effective regimens for melioidosis. Table 4 provides treatment regimens that have been found to be useful in adults[43,50,51]; treatment guidelines for children are currently based on adult studies. Treatment is accomplished in two phases:(1) Intensive phase with intravenous antimicrobial therapy for a minimum of 10 to 14 d with one of the first-line agents-ceftazidime,imipenem or meropenem; sulfamethoxazole-trimethoprim is added in cases of neurologic, cutaneous, bone, joint and prostate involvement and the treatment period extended to 4-8 weeks. Following initiation of therapy, patients’ blood cultures may become negative within a few days, but it may take 10 d or longer for patient to become afebrile[50,52]. Therapy is given until the patient is afebrile for more than 48 h. (2) Eradication phase on oral antimicrobial therapy for 3 to 6 months with sulfamethoxazole-trimethoprim alone(commonly used in Australia) or in combination with doxycycline(commonly used in Thailand)[32].
Variations in treatment regimens may be adopted depending on drug availability, experience in the local setting or patient factors. For example, amoxicillin-clavulanate may be used in intensive or the eradication phases for limited indications (Table 4), but its use is associated with high treatment failure/relapse rate[53].
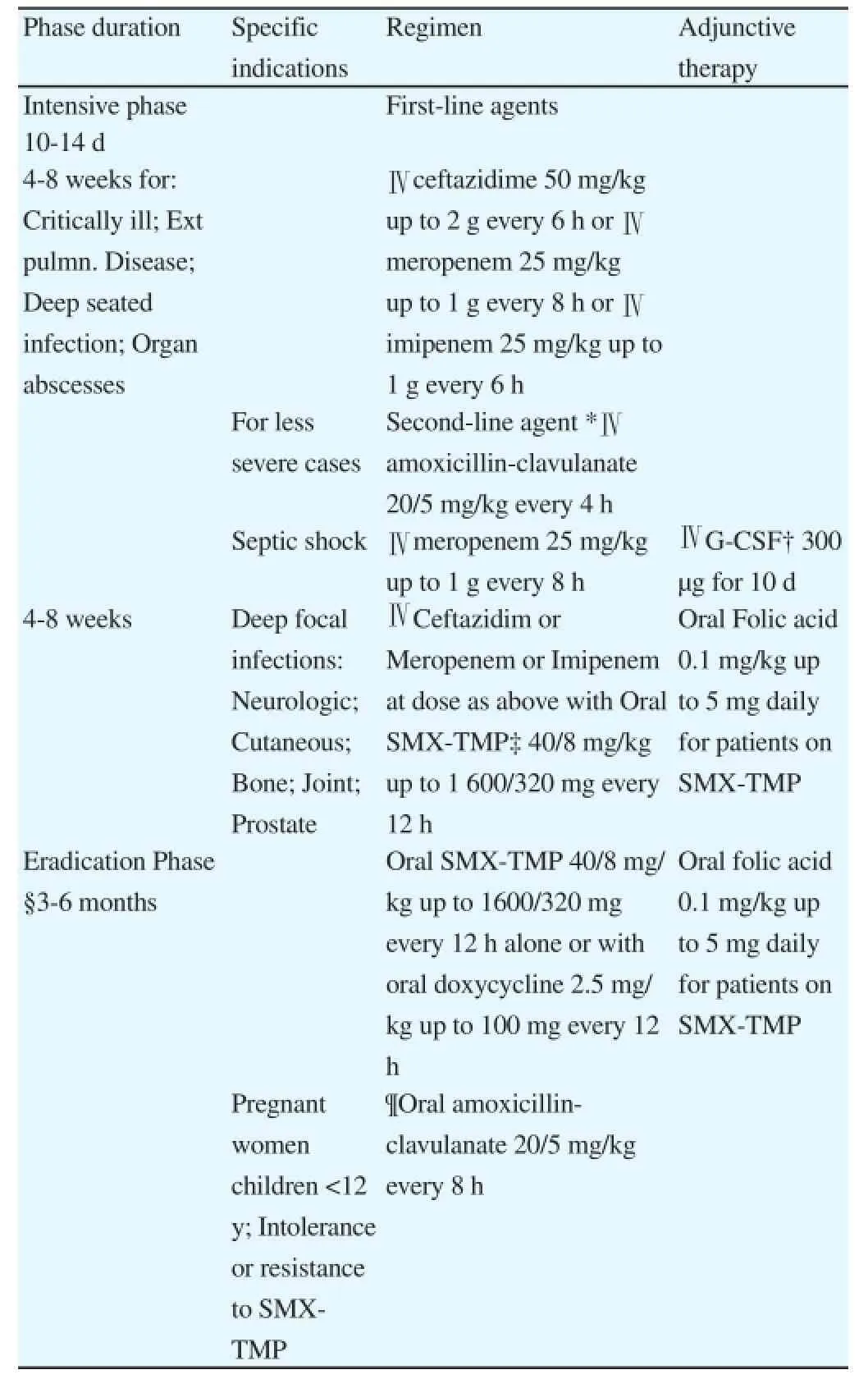
Table 4Antimicrobial regimens of melioidosis for adults and children.
*High rates of treatment failure, not recommended if first-line agents are available.
†Granulocyte-colony stimulating factor.
‡SMX-TMP, Sulphamethoxazole/Trimethoprim. In children, 30/6 mg/kg up to 1 200/240 mg every 12 h.
§Regimen may also be used as primary treatment in superficial infections(eg. localized cutaneous), provided there are no underlying risk factors and/or dissemination to other sites.
¶Associated with a higher relapse rate. For adult patients <60 kg, a dose of 1 000/250 mg every 8 h is suggested; for patients>60 kg, a maximum dose of 1 500/375 mg every 8 h is suggested. In countries where amoxicillinclavulanate is only available in fixed 2:1 combinations (i.e., 500 mg/250 mg),then 500/250 mg every 8 h plus amoxicillin 500 mg every 8 h to reach the required dosage is recommended.
Note: All doses should be adjusted in patients with renal impairment.
Source: McLeod et al, 2015[43]; Simpson et al, 1999[50].
Approach: Treatment should be initiated early in the course of the disease since melioidosis may progress to a more severe form and become fatal. In endemic areas where laboratory diagnostic facilities are unavailable or delayed, empirical antibiotic regimens for rapidly progressing pneumonia and/or sepsis need to cover for melioidosis. It is crucial that patients be given the full course of antimicrobial therapy during the intensive phase, and is followed-up and monitored for adherence to therapy in the eradication phase. Incomplete treatment during the intensive phase or drug non-adherence during the eradication phase may result in relapse or recurrence.
2.8. Relapse and recurrence
Relapses and recurrence are not uncommon in melioidosis. Relapse is the reappearance of symptoms and signs after initial clinical response while still on antimicrobial therapy. A recurrent infection is a new episode of melioidosis occurring after full clinical recovery or convalescence. The rate of relapse or recurrence may vary from 15%-30%[21,41]. Most recurrences are due to the original infecting strain, but reinfection with another strain may occur[54]. Risk factors for relapse/recurrence include: severity of disease (positive blood culture, multifocal disease); incomplete or inadequate microbial treatment or choice of agent (amoxicillin-clavulanate) during the intensive phase of treatment; and improper eradication therapychoice of agents (amoxicillin-clavulanate, oral quinolones or doxycycline monotherapy), nonadherence or duration (less than twelve weeks)[41]. It is to be noted that relapses and recurrence may occur in immunocompromised patients despite the full course of microbial therapy.
Just as in tuberculosis, the infection may be dormant in melioidosis with prolonged latency (that is, infection is present without any clinical manifestations)[41]. Reactivation from a latent focus and recurrence into a fulminating form may occur when host defense is compromised as in diabetes mellitus[55].
Several episodes of infections may occur in a person over several years. Thus, patients need to be followed-up for at least five years or more after initial recovery.
Approach: Any patient with a prior history of melioidosis and presenting with a severe febrile illness or symptoms of sepsis should be suspected of having a relapse/recurrence and empirical antibiotic therapy covering B. pseudomallei should be begun without delay. Diabetes and other conditions that compromise host defense may predispose to relapse/recurrence. Patients need to be informed of a lifelong risk of recurrence and that they should provide their past history of melioidosis to their physician if they develop any severe febrile illness.
2.9. Prevention measures
No vaccine is currently available. Prevention of the infection in areas where the disease is endemic can be difficult since contact with contaminated soil is common. In endemic areas, persons with open skin wounds and those with diabetes or other comorbid conditions should avoid contact with soil and standing water in these areas as they are at increased risk for acquiring melioidosis. Wearing boots during agricultural work can prevent infection through thefeet and lower legs. In health care settings, using standard contact precautions (mask, gloves, gown and hand washing) is considered sufficient protection. For laboratories, B. pseudomallei is classified as a ‘Category 3’ pathogen because of the risk of infection to laboratory staff[1]. Therefore, microbiological and biomedical laboratories must have adequate facilities for safe work procedures and laboratory staff must engage in safe work practices such as safeguards for centrifugation, prohibiting the ‘sniff’ test (B. pseudomallei colonies have a characteristic putrid odor) and the use of a biological safety cabinet within a biosafety level-3 containment.
Approach: Educational interventions for prevention need to be part of the health care messages in endemic areas. In hospital and laboratories, adherence to standard control practices should be evaluated on an ongoing basis to ensure compliance; that is,infection control practices for hospitals and biosafety procedures for laboratories.
3. Conclusions
Cases of melioidosis are often missed and the diagnosis is delayed resulting in high case fatality in endemic areas. Because of the severity of symptoms, patients may not delay seeking care. Delays are most likely to be due to physicians not making a correct diagnosis and conducting appropriate investigations or in rural settings where laboratory facilities may be unavailable. Early diagnosis and appropriate management is crucial in reducing serious complications leading to high mortality, and in preventing recurrences of the disease. Radiological imaging is an integral part of the diagnostic workup. In situations of clinically highly probable or possible cases where laboratory bacteriological confirmation is not possible, using evidence-based criteria and empirical treatment with antimicrobial therapy is recommended. It is of prime importance that patients undergo the full course of antimicrobial therapy to avoid relapse and recurrence. Finally, there is a crucial need for promoting awareness among physicians at all levels and for improved diagnostic microbiology services. Further, the need for making the disease notifiable and/or initiating melioidosis registries in endemic countries appears to be compelling.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Chandni R. Melioidosis: The great mimicker. In: Medicine update 2013. Mumbai: The Association of Physicians of India; 2013, p. 14-18.
[2] Spickler AR, Roth JA, Gaylon J, Lofsted J. Emerging and exotic disease of animals textbook. 4th Edition. Iowa: Center for Food Security and Public Health Iowa State University; 2010.
[3] Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 2010; 4(11): e900. doi:10.1371/journal.pntd.0000900.
[4] Mukhopadhyay C, Chawla K, Krishna S, Nagalakshmi N, Rao SP, Bairy I. Emergence of Burkholderia pseudomallei and pandrug-resistant nonfermenters from southern Karnataka, India. Trans R Soc Trop Med Hyg 2008; 102(Suppl 1): S12-17. doi:10.1016/s0035-9203(08)70005-1.
[5] Saravu K, Mukhopadhyay C, Vishwanath S, Valsalan R, Docherla M,Vandana KE, et al. Melioidosis in southern India: epidemiological and clinical profile. Southeast Asian J Trop Med Public Heal 2010; 41(2): 401-409.
[6] Gilad J, Harary I, Dushnitsky T, Schwartz D, Amsalem Y. Burkholderia mallei and Burkholderia pseudomallei as bioterrorism agents: national aspects of emergency preparedness. Isr Med Assoc J 2007; 9(7): 499-503.
[7] Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 2005; 18(2): 383-416. doi:10.1128/ cmr.18.2.383-416.2005.
[8] Vidyalakshmi K, Lipika S, Vishal S, Damodar S, Chakrapani M. Emerging clinico-epidemiological trends in melioidosis: analysis of 95 cases from western coastal India. Int J Infect Dis 2012; 16(7): e491-e497. doi:10.1016/j.ijid.2012.02.012.
[9] Dance DA. Melioidosis: the tip of the iceberg? Clin Microbiol Rev 1991;4(1): 52-60.
[10] John TJ. Emerging&re-emerging bacterial pathogens in India. Indian J Med Res 1996; 103: 4-18.
[11] Jayaram M. Melioidosis-should it be a notifiable disease in Malaysia?Med J Malaysia 2005; 60(5): 531-534.
[12] Lo TJ, Ang LW, James L, Goh KT. Melioidosis in a tropical city state,Singapore. Emerg Infect Dis 2009; 15(10): 1645-1647. doi:10.3201/ eid1510.090246.
[13] How SH, Ng TH, Jamalludin AR, Tee HP, Kuan YC, Alex F, et al. Pahang melioidosis registry. Med J Malaysia 2009; 64(1): 27-30.
[14] Currie B, Smith-Vaughan H, Golledge C, Buller N, Sriprakash KS, Kemp DJ. Pseudomonas pseudomallei isolates collected over 25 years from a non-tropical endemic focus show clonality on the basis of ribotyping. Epidemiol Infect 1994; 113: 307-312.
[15] Currie BJ, Fisher DA, Howard DM, Burrow JN, Selvanayagam S,Snelling PL, et al. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop 2000; 74(2-3): 121-127.
[16] Pruekprasert P, Jitsurong S. Case report: septicemic melioidosis following near drowning. Southeast Asian J Trop Med Public Heal 1991; 22(2): 276-278.
[17] Ngauy V, Lemeshev Y, Sadkowski L, Crawford G. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World WarⅡ. J Clin Microbiol 2005; 43(2): 970-972. doi:10.1128/ jcm.43.2.970-972.2005.
[18] Wuthiekanun V, Smith MD, Dance DA, White NJ. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. Trans R Soc Trop Med Hyg 1995; 89(1): 41-43.
[19] Kaestli M, Mayo M, Harrington G, Watt F, Hill J, Gal D, et al. Sensitive and specific molecular detection of Burkholderia pseudomallei, the causative agent of melioidosis, in the soil of tropical northern Australia. Appl Env Microbiol 2007; 73(21): 6891-6897. doi:10.1128/aem.01038-07.
[20] Currie BJ, Fisher DA, Howard DM, Burrow JN, Lo D, Selva-Nayagam S, et al. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis 2000; 31(4): 981-986. doi:10.1086/318116.
[21] Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, DavisTM, et al. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis 1989; 159(5): 890-899.
[22] Schlech Ⅲ WF, Turchik JB, Westlake Jr. RE, Klein GC, Band JD, Weaver RE. Laboratory-acquired infection with Pseudomonas pseudomallei (melioidosis). N Engl J Med 1981; 305(5): 1133-1135. doi:10.1056/nejm198111053051907.
[23] Webling DD. Genito-urinary infections with Pseudomonas pseudomallei in Australian aboriginals. Trans R Soc Trop Med Hyg 1980; 74(1): 138-139.
[24] Halder D, Zainal N, Wah CM, Haq JA. Neonatal meningitis and septicaemia caused by Burkholderia pseudomallei. Ann Trop Paediatr 1998; 18(2): 161-164.
[25] Lumbiganon P, Pengsaa K, Puapermpoonsiri S, Puapairoj A. Neonatal melioidosis: a report of 5 cases. Pediatr Infect Dis J 1988; 7: 634-636.
[26] Ralph A, McBride J, Currie BJ. Transmission of Burkholderia pseudomallei via breast milk in northern Australia. Pediatr Infect Dis J 2004; 23(12): 1169-1171.
[27] Gal D, Mayo M, Smith-Vaughan H, Dasari P, McKinnon M, Jacups SP,et al. Contamination of hand wash detergent linked to occupationally acquired melioidosis. Am J Trop Med Hyg 2004; 71(3): 360-362.
[28] Pumpuang A, Chantratita N, Wikraiphat C, Saiprom N, Day NP, Peacock SJ, et al. Survival of Burkholderia pseudomallei in distilled water for 16 years. Trans R Soc Trop Med Hyg 2011; 105(10-2): 598-600. doi:10.1016/ j.trstmh.2011.06.004.
[29] Meumann EM, Cheng AC, Ward L, Currie BJ. Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature. Clin Infect Dis 2012; 54(3): 362-369. doi:10.1093/cid/cir808.
[30] Hassan MR, Pani SP, Peng NP, Voralu K, Vijayalakshmi N, Mehanderkar R, et al. Incidence, risk factors and clinical epidemiology of melioidosis: a complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infect Dis 2010; 10: 302. doi:10.1186/1471-2334-10-302.
[31] Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis,Australia. Emerg Infect Dis 2003; 9(12): 1538-1542. doi:10.3201/ eid0912.020750.
[32] White NJ. Melioidosis. Lancet 2003; 361: 1715-1722.
[33] Currie BJ, Jacups SP, Cheng AC, Fisher DA, Anstey NM, Huffam SE, et al. Melioidosis epidemiology and risk factors from a prospective wholepopulation study in northern Australia. Trop Med Int Heal 2004; 9(11): 1167-1174. doi:10.1111/j.1365-3156.2004.01328.x.
[34] Suputtamongkol Y, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Ruchutrakool T, et al. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis 1999; 29(2): 408-413. doi:10.1086/520223.
[35] Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000; 342(10): 681-689. doi:10.1056/nejm200003093421002.
[36] Morse LP, Moller CC, Harvey E, Ward L, Cheng AC, Carson PJ, et al. Prostatic abscess due to Burkholderia pseudomallei: 81 cases from a 19-year prospective melioidosis study. J Urol 2009; 182(2): 542-547. doi:10.1016/j.juro.2009.04.010.
[37] Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N,Wongsuvan G, Chaisuksant S, Chetchotisakd P, et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg 2010; 82(6): 1113-1117. doi:10.4269/ajtmh.2010.10-0038.
[38] Holland DJ, Wesley A, Drinkovic D, Currie BJ. Cystic fibrosis and Burkholderia pseudomallei infection: An emerging problem? Clin Infect Dis 2002; 35(12): e138-e140. doi:10.1086/344447.
[39] Chierakul W, Wuthiekanun V, Chaowagul W, Amornchai P, Cheng AC,White NJ, et al. Short report: disease severity and outcome of melioidosis in HIV coinfected individuals. Am J Trop Med Hyg 2005; 73(6): 1165-1166.
[40] Leelarasamee A. Epidemiology of melioidosis. J Infect Dis Antimicrob Agents 1986; 3(2): 84-93.
[41] Puthucheary SD. Melioidosis in Malaysia. Med J Malaysia 2009; 64(4): 266-274.
[42] Sanford JP, Moore Jr. WL. Recrudescent melioidosis: a southeast asian legacy. Am Rev Respir Dis 1971; 104(3): 452-453.
[43] McLeod C, Morris PS, Bauert PA, Kilburn CJ, Ward LM, Baird RW,et al. Clinical presentation and medical management of melioidosis in children: a 24-year prospective study in the Northern Territory of Australia and review of the literature. Clin Infect Dis 2015; 60: 21-26. doi:10.1093/cid/ciu733.
[44] WoodsⅡ ML, Currie BJ, Howard DM, Tierney A, Watson A, Anstey NM, et al. Neurological melioidosis: seven cases from the Northern Territory of Australia. Clin Infect Dis 1992; 15(1): 163-169.
[45] Wuthiekanun V, Dance DA, Wattanagoon Y, Supputtamongkol Y,Chaowagul W, White NJ. The use of selective media for the isolation of Pseudomonas pseudomallei in clinical practice. J Med Microbiol 1990;33(2): 121-126.
[46] Lau SK, Sridhar S, Ho CC, Chow WN, Lee KC, Lam CW, et al. Laboratory diagnosis of melioidosis: Past, present and future. Exp Biol Med 2015; 240(6): 742-751. doi:10.1177/1535370215583801.
[47] Chantratita N, Wuthiekanun V, Boonbumrung K, Tiyawisutsri R,Vesaratchavest M, Limmathurotsakul D, et al. Biological relevance of colony morphology and phenotypic switching by Burkholderia pseudomallei. J Bacteriol 2007; 189(3): 807-817. doi:10.1128/jb.01258-06.
[48] Houghton RL, Reed DE, Hubbard MA, Dillon MJ, Chen H, Currie BJ,et al. Development of a prototype lateral flow immunoassay (LFI) for the rapid diagnosis of melioidosis. PLoS Negl Trop Dis 2014; 8(3): e2727. doi:10.1371/journal.pntd.0002727.
[49] Cheng AC, Currie BJ, Dance DA, Funnell SG, Limmathurotsakul D,Simpson AJ, et al. Clinical definitions of melioidosis. Am J Trop Med Hyg 2013; 88(3): 411-413. doi:10.4269/ajtmh.12-0555.
[50] Simpson AJ, Suputtamongkol Y, Smith MD, Angus BJ, Rajanuwong A, Wuthiekanun V, et al. Comparison of imipenem and ceftazidime as therapy for severe melioidosis. Clin Infect Dis 1999; 29(2): 381-387. doi:10.1086/520219.
[51] Inglis TJJ. The treatment of melioidosis. Pharmaceuticals 2010; 3: 1296-1303. doi:10.3390/ph3051296.
[52] Chetchotisakd P, Porramatikul S, Mootsikapun P, Anunnatsiri S,Thinkhamrop B. Randomized, double-blind, controlled study of cefoperazone-sulbactam plus cotrimoxazole versus ceftazidime plus cotrimoxazole for the treatment of severe melioidosis. Clin Infect Dis 2001; 33(1): 29-34. doi:10.1086/320878.
[53] Cheng AC, Chierakul W, Chaowagul W, Chetchotisakd P,Limmathurotsakul D, Dance DA, et al. Consensus guidelines for dosing of amoxicillin-clavulanate in melioidosis. Am J Trop Med Hyg 2008;78(2): 208-209.
[54] Maharjan B, Chantratita N, Vesaratchavest M, Cheng A, Wuthiekanun V,Chierakul W, et al. Recurrent melioidosis in patients in northeast Thailand is frequently due to reinfection rather than relapse. J Clin Microbiol 2005;43(12): 6032-6034. doi:10.1128/jcm.43.12.6032-6034.2005.
[55] Lim KS, Chong VH. Radiological manifestations of melioidosis. Clin Radiol 2010; 65(1): 66-72. doi:10.1016/j.crad.2009.08.008.
Document heading 10.1016/j.apjtm.2016.04.003
15 February 2016
*
Paul Vijay Kingsley, MBBS, Sultan Ismail Hospital, Johor Bharu, Malaysia.
E-mail: paulkvijay@gmail.com
 Asian Pacific Journal of Tropical Medicine2016年6期
Asian Pacific Journal of Tropical Medicine2016年6期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Taeniasis vs cysticercosis infection routes
- Are efforts up to the mark? A cirrhotic state and knowledge about HCV prevalence in general population of Pakistan
- ZIka virus infection in Asia: reappraisal on phylogenetic data of Asian lineage
- Experiment research of cisplatin implants inhibiting transplantation tumor growth and regulating the expression of KLK7 and E-cad of tumor-bearing mice with gastric cancer
- Comparative study of the chitooligosaccharides effect on the proliferation inhibition and radiosensitization of three types of human gastric cancer cell line
- Change of the peripheral blood immune pattern and its correlation with prognosis in patients with liver cancer treated by sorafenib
