微波法制备单晶金刚石的研究进展
黄 平,汪建华,刘 繁,翁 俊,王小安
等离子体化学与新材料湖北省重点实验室(武汉工程大学),湖北 武汉 430074
微波法制备单晶金刚石的研究进展
黄平,汪建华*,刘繁,翁俊,王小安
等离子体化学与新材料湖北省重点实验室(武汉工程大学),湖北 武汉 430074
人工合成金刚石的方法主要有高温高压法和化学气相沉积法两种.高温高压法制备的金刚石尺寸小,无法避免金属杂质使得制备的金刚石应用受到限制.在所有的化学气相沉积中,微波等离子体化学气相沉积法具有无放电污染,能量转换效率高,工艺参数易于调节等优点.用微波等离子体化学气相沉积法制备大尺寸、高速率、高质量的单晶金刚石受到广泛重视.介绍了微波等离子体化学气相沉积单晶金刚石的制备工艺,对提高金刚石生长速率,扩大金刚石单晶尺寸两个方面的研究进展进行了综述,并对单晶金刚石的前景进行了展望.
微波等离子体;化学气相沉积;单晶金刚石
1 引言
金刚石具有高硬度,高热导率,高的光学透过率,优异的电子迁移率等许多优异的物理化学特性,是一种用途十分广泛的功能性材料.金刚石可分为天然金刚石和人工合成两类,合成金刚石又分为单晶和多晶,多晶金刚石由于自身存在大量不规则晶界,无法继承单晶金刚石的许多优良特性.高质量的单晶金刚石可以用作金刚石高温半导体、电子器件、量子计算机信息处理、高能辐射离子探测器、高压设备顶砧等领域[1-5],宝石级单晶金刚石还能够作为首饰用[6].而天然金刚石在地球上的储存量十分稀少,且价格昂贵,因此人工合成在物理化学性质接近天然金刚石的高质量单晶金刚石显得十分必要.
目前,天然金刚石十分稀少,工业上应用的金刚石主要来自于人工合成的金刚石.制备单晶金刚石的方法主要分为高温高压法[7](high temperature and high pressure,HPHT)和化学气相沉积法(chemical vapor deposition,CVD).HPHT[8]生长技术一般只用来合成小颗粒金刚石(数个微米至毫米),高温高压使得成本昂贵,设备要求苛刻,而且HPHT金刚石由于使用了金属催化剂,使得金刚石中残留有金属颗粒杂质,从而限制了HPHT金刚石的大规模应用.与传统HPHT法相比,CVD制备单晶金刚石在成本、条件等方面更具优势,特别是对于合成尺寸,CVD法理论上不受限制.CVD合成金刚石单晶的方法主要有热丝CVD法[9-10]、直流等离子体喷射CVD法[11-12]、微波等离子CVD法[13].在这三种方法中,由于微波等离子体化学气相沉积(microwave plasma chemical vapor deposition diamond,MPCVD)具有无极放电无污染、能量转换效率高、微波等离子参数可控等优点,是国内外研究者们制备高质量单晶金刚石的首选方法[14].
2 CVD制备金刚石工艺
相对于高温高压沉积技术,微波等离子体化学气相沉积是一种低气压沉积技术.等离子体在进行化学气相沉积时,活性基团在衬底表面发生一系列复杂的化学或物理反应,最终得到需要的产物.A(气)+B(气)→C(固)+D(气).反应气体A、B被激发为等离子体状态,其活性基团发生反应生成所需要的固态物沉积在衬底上.
制备金刚石的常见混合气体是甲烷和氢气.在微波能量激励下,含碳混合气体电离生成含碳的活性粒子并在衬底上以sp2和sp3键重组形成石墨相和金刚石相.从甲烷中电离的CH3基团[15],是形成金刚石最主要的前驱体.氢气离解成的原子氢,能够刻蚀非金刚石相稳定金刚石相,在金刚石形成过程中起着至关重要的作用[16-17].含碳基团在基片表面进行结构重组,由于原子氢对sp2键碳原子的刻蚀作用远比对sp3键碳原子的刻蚀作用强,这样重组后的碳-碳键具有金刚石结构的sp3键保留下来,在合适的工艺下沉积得到金刚石.
金刚石成核所需要的表面能很高,很难在单晶硅表面实现异质外延.所以通常使用单晶金刚石作为单晶金刚石外延的衬底.同质外延单晶金刚石是在金刚石亚稳区利用CVD法在金刚石晶面上外延生长金刚石的方法.MPCVD法制备单晶金刚石的工作气压一般都很高,16 kPa~40 kPa,常见的微波功率600 W ~6 000 W[18-19].衬底温度在800℃~1 200℃[13-14,20].常见的微波发生器有2.45 GHz和915 MHz.2.45 GHz的微波输入功率通常低于10 kW,与2.45 GHz相比,915 MHz的MPCVD装置产生的等离子球的尺寸更大[21],能够同时放入许多片衬底(图1[20]),Asmussen等人在915 MHz的MPCVD装置中,放入了70片3.5 mm× 3.5 mm HPHT金刚石晶种同时外延单晶金刚石[21],生长条件如下:微波功率11.5 kW,气压16 kPa,5%~8%的CH4/H2体积比,放电直径>100 mm,基片温度1 125℃~1 200℃,该方法能够极大地提高金刚石的生长效率,节约成本.据报道[22],915 MHz的最大输出功率可以达到70 kW,最大等离子球直径达到了300 mm.

图1 使用915 MHz装置在70片金刚石晶种同时外延单晶金刚石Fig.1 Epitaxial deposition of single crystal diamond over 70 seed crystals with 915 MHz equipment
3 提高单晶金刚石生长速率
在能够生长出一定厚度单晶金刚石的情况下,提高金刚石的沉积速率能够缩短沉积时间,有效地控制成本.影响单晶金刚石生长速率的因素有很多,如衬底温度、微波功率密度、甲烷浓度、添加辅助性气体等.近年来,提高单晶金刚石生长速率的同时保证较好的金刚石质量对于研究者来说仍然是一项重大的挑战.
衬底温度对单晶金刚石生长十分重要.在适合单晶外延的温度范围内,适当升高衬底温度有利于提高金刚石的生长速率.这是由于衬底表面沉积温度升高后,衬底表面活性增强,促进含碳化合物的分解扩散[23-24],加速衬底表面的物理化学反应,进而提高金刚石的生长速率.单晶金刚石的生长温度一般在800℃~1 200℃.研究发现,其他生长条件相同的情况下,金刚石的沉积速率在1 000℃左右达到饱和[25].然而随着沉积温度的升高,金刚石表面形貌有可能会发生变化.Tallaire等人在单晶生长的研究中发现,当衬底温度超过900℃,单晶衬底表面形成了典型的金字塔缺陷[26],如图2(a).金字塔缺陷是单晶生长过程中常见的一种缺陷.这种金字塔的顶端含有非外延微晶金刚石.该微晶可能来自于衬底表面缺陷如二次形核或螺旋位错的攀移造成的[27-28].当衬底温度低于750℃时,生长速率极低,且观察到的薄膜粗糙,含有大量缺陷[26].当温度高于1 200℃时,易出现石墨化不利于单晶金刚石的生长.

图2 单晶生长过程中常见的两种缺陷(a)金字塔缺陷;(b)边缘多晶化Fig.2 Two kinds of common defects in the process of the growth of crystal diamond (a)Pyramid defect;(b)Polycrystallization of the edge
微波功率密度是输入功率与等离子体球尺寸的比率,对金刚石生长影响很大.高微波功率和高的气压都能够加速含碳气源的热分解,提高金刚石生长速率和晶体质量[29].气体的离解,特别是氢,在金刚石的生长过程中十分关键,能够为金刚石的生长提供前驱体.在反应腔内,气体的离解主要依靠来自等离子体球中心的热量来完成.要想提高原子氢的含量,就必须有更高的微波等离子体密度.早期的微波等离子体装置制备单晶金刚石的微波功率密度小于5 W/cm3,气压小于15 kPa,只有在低甲烷体积分数(<1%)下才能够得到质量较好的单晶金刚石,生长速率也小于1 μm/h.随着设备的不断改进,微波功率密度最大可提升到1 000 W/cm3[29].研究表明[30],在其他生长条件相同的情况下,随着微波功率密度的增加,金刚石的生长速率随之增加,如图3.由于反应腔体承受能力有限,微波功率密度不可能无限制的增加.太大的微波功率密度会使得腔体内壁过热,腔体内壁或者石英窗口可能会被刻蚀,同时也会影响晶体稳定的生长.据报道[31-32],使用脉冲微波能够有效的解决由于微波功率密度增加而导致腔体过热的这一问题.
混合气体中甲烷体积浓度对金刚石的生长速率也有影响.较低的甲烷体积浓度下,金刚石的沉积速率相当低[33-34].低甲烷体积浓度下,特别是功率密度较低的情况下,表面易发生二次形核,不利于单晶金刚石的生长[28,34].Tallaire[35]等在功率3.2 kW,甲烷体积分数为2%~7%的参数下制备了单晶金刚石,发现当体积分数为2%时,制备的金刚石生长速率只有2 μm/h,而且表面粗糙,含有大量刻蚀坑;当甲烷体积分数增加至7%时生长速率达到15 μm/h.这是由于在较高的功率密度下,等离子体中会产生大量的原子氢,碳源浓度过低时,金刚石的刻蚀会相对加剧[26,36],从而降低了金刚石的生长速率.当碳源浓度升高时,原子氢会激发更多的含碳基团用于金刚石生长,进而提高金刚石的生长速率[26,37].

图3 生长速率与微波功率密度的关系曲线Fig.3 Relationship curves between the growth rate and the microwave power density
在传统的混合气体中添加少量的氮气能够快速提升金刚石的生长速率[14,38].据报道[14],卡内基实验室的研究人员发现,在反应气体中添加少量氮气的情况下,生长速率高达200 μm/h,是不添加氮气时2~3倍.在较高沉积温度的情况下,氮气的添加还能够阻止衬底表面孪晶的生成[39].毫米级厚度的单晶金刚石,添加少量氮气显得十分必要.虽然氮气的加入能够提高金刚石的沉积速率,对于单晶金刚石来说,N是杂质原子,不可避免地对金刚石的电学性能造成影响.添加杂质气体的方法适合制备热学力学应用方面的单晶金刚石[40].添加Ar能够提高金刚石的生长速率.由于Ar热传导率比氢气低,添加Ar后使得等离子球的热扩散变慢,从而等离子体中心的热量束缚更为集中,衬底温度上升得更快,从而提高生长速率[41-42].
4 大尺寸单晶的生长

图4 不同基片台上单晶金刚石的生长示意图(时间:t0<t1<t2)Fig.4 Schematic diagram of single crystal diamond growth on different substrates(time:t0<t1<t2)(a)Original open substrate;(b)Improved pocket substrate
CVD法制备单晶金刚石需要单晶金刚石作为衬底,理论上讲,金刚石衬底有多大面积,CVD单晶金刚石就有多大.据报道[43],目前HPHT单晶晶种最大尺寸达到了12 mm×12 mm.CVD金刚石单晶的生长与其他晶体的生长不一样,其他晶体生长基本上都是越长越大,而CVD金刚石单晶生长过程中存在边缘多晶化(PCD rim),单晶却是越长越小.这是因为外延单晶的同时,衬底边缘多晶金刚石也在同时生长,如图2(b)[44].由于边缘效应,晶种四周同时生长多晶层,而且随着单晶外延生长层厚度的增加,单晶表面积越来越小,而多晶表面积越来越大,甚至有可能完全演变成多晶生长. 在CVD单晶金刚石生长研究中,边缘多晶化是另一个需要解决的难题.Nad[45]等通过特殊的基片台设计,如图4[45],成功地在3.5 mm×3.5 mm×1.4 mm单晶衬底上制备了5.3 mm×5.2 mm×4.5 mm(激光切割后)的单晶金刚石,研究结果表明改进后的口袋型基片台不仅能够有效的遏制边缘多晶化的现象,外延单晶表面积还有所扩大.
马赛克拼接技术可以生产大尺寸的单晶.马赛克拼接法[46](图5)是先选用一块单晶衬底模板通过离子注入与剥离的方法克隆出许多个相同的CVD单晶片,然后将这些相同的CVD单晶片拼接在一起作为衬底,再在上面外延单晶.2012年,Yamada等使用马赛克法利用4~8片10 mm× 10 mm×1.0 mm的克隆单晶片成功外延了1 inch的大单晶片[46].2014年,他们使用相同的方法采用24片10 mm×10 mm×1.0 mm的克隆片成功制备了40 mm×60 mm(约2 inch)的大单晶片[47].可以设想,如果等离子球尺寸足够大,采用马赛克法将能够更有效的扩大单晶尺寸,已见报道[22]的等离子球直径尺寸能达到直径300 mm.
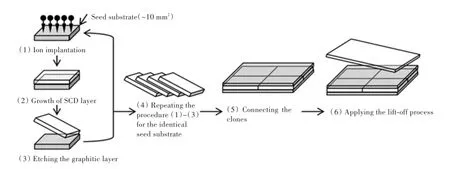
图5 马赛克法生产大尺寸单晶的示意图Fig.5 Schematic diagram of large size crystals produced by mosaic growth
5 展 望
近10年来,研究者们关于MPCVD单晶金刚石的研究方面已经取得了很大的成就.然而,单晶金刚石的制备仍然存在不少问题,比如说,光学透明度不够,单晶边缘多晶化,衬底缺陷延伸到外延层等.获得大尺寸、高速率、高质量的金刚石一直是研究者们不断追求的目标.人工合成金刚石技术的不断成熟,制备单晶金刚石的成本的逐步下降,将会更有利于金刚石的应用范围进一步的扩大.特别是单晶金刚石的掺杂技术一旦突破,半导体领域将会迎来全新的时代.总之,人造单晶金刚石将会在未来的社会和科学技术发展中产生巨大的影响.
[1] UEDA K,SOUMIYA T,ASANO H.Ferromagnetic schottky junctions using diamond semiconductors[J]. Diamond and related materials,2012,25:159-162.
[2] AZADEGAN B,WAGNER W.Simulation of planar channeling-radiation spectra of relativistic electrons and positrons channeled in a diamond-structure or tungsten single crystal(classical approach)[J].Nuclear instruments and methods in physics research B,2015,342:144-149.
[3]BAYN I,MEYLER M,LAHAV A,et al.Processing of photonic crystal nanocavity for quantum information in diamond[J].Diamond and related materials,2011,20:937-943.
[4] FREGEAUA MO,OBERSTEDT S,BRYS T,et al.First use of single-crystal diamonds as fission-fragment detector[J].Nuclear instruments and methods in physics research A,2015,791:58-64.
[5]OHTANI E,MIBESOUND K,SAKAMAKI T,et al. Velocity measurement by inelastic X-ray scattering at high pressure and temperature by resistive heating diamond anvil cell[J].Russian geology and geophysics,2015,56:190-195.
[6] 王东胜,王志勇,董耀华.人造大单晶金刚石合成技术及应用研究现状[J].广东建材,2010(4):36-40.
WANG D S,WANG Z Y,DONG Y H.Research situation on artificial big mono-crystalline diamond synthesis technology and application[J].Guangdong building materials,2010(4):36-40.
[7] 田宇.高温高压生长宝石级金刚石单晶的实验与理论研究[D].长春:吉林大学,2009.
[8]SU Q,ZHANG J,LI M,et al.Defects of diamond single crystal grown under high temperature and high pressure [J].Thin solid films,2013,546:457-460.
[9]HIEMKE J,SCHWARZ S,ROTTMAIR C,et al.Diamond single crystal growth in hot filament cvd[J].Diamond and related materials,2005,15:536-541.
[10]YAMAZAKI K,FURUICHI K,TSUMURA I,et al. The large-sized diamond single-crystal synthesis by hot filament cvd[J].Journal of crystal growth,2008,310:1019-1022.
[11]HEI LF,LIU J,LI C,et al.Fabrication and characterization of large homoepitaxial single crystal diamond grown by dc arc plasma jet cvd[J].Diamond and related materials,2012,30:77-84.
[12]LIU J,HEI L F,SONGJH,et al.High-rate homoepitaxial growth of cvd single crystal diamond by dc arc plasma jet at blow-down(open cycle)mode[J].Diamond and related materials,2014,46:42-51.
[13] YAMADA H,CHAYAHARA A,MOKUNO Y,et al. Numerical microwave plasma discharge study for the growth of large single-crystal diamond[J].Diamond and related materials,2015,54:9-14.
[14]CHAYAHARA A,MOKUNO Y,HORINO Y,et al. The effect of nitrogen addition during high-rate homoepitaxial growth of diamond by microwave cvd[J].Diamondandrelatedmaterials,2004,13(11) :1954-1958.
[15]HARRIS S J.Mechanism for diamond growth from methy radicals[J].Applied physics letters,1990,56:2298-2300.
[16]BATTAILE C,SROLOVITZ D,OLEINIKI,et al. Etching effects during the chemical vapor deposition of (100)diamond[J].The journal of chemical physics,1999,111(9):4291-4299.
[17]GORDON MH,DUTEN X,GICQUE A,et al.Energy coupling efficiency of a hydrogen microwave plasma reactor[J].Journal of applied physics,2001,89(3):1544-1549.
[18] 严垒,马志斌,曹为,等.高温高压金刚石衬底上的同质外延生长研究[J].人工晶体学报,2014,43(6):1420-1424. YAN L,MA Z B,CAOW,et al.Homoepitaxial growth of diamond single crystal on hpht substrates[J]. Journal of synthetic crystals,2014,43(6):1420-1424.
[19]AHARONOVICH I,LEE J C,MAGYAR A P,et al. Homoepitaxial growth of single crystal diamond membranesforquantuminformationprocessing[J]. Advanced materials,2012,24(10):54-59.
[20] SCHRECK M,ASMUSSEN J,SHIKATA S,et al. Large-area high-quality single crystal diamond[J].Materials research society,2014,39:504-510.
[21] ASMUSSEN J,GROTJOHN TA,SCHUELKE T,et al. Multiple substrate microwave plasma-assisted chemical vapor deposition single crystal diamond synthesis [J].Applied physics letters,2008,93:031502-1-031502-3.
[22]LIANG Q,YAN C,LAI J,et al.Large area single-crystal diamond synthesis by 915 MHz microwave plasma-assisted chemical vapor deposition[J].Crystal growth design,2014,14(7):3234-3238.
[23] MARINELLIA M,MILANIA E,PAOLETTI A,et al. Analysis of traps in high quality cvd diamond films through the temperature dependence of carrier dynamics[J].Diamond and related materials,2003,12:1733-1737.
[24] DEMLOWA SN,RECHENBERG R,GROTJOHN T,et al.The effect of substrate temperature and growth rate on the doping efficiency of single crystal boron doped diamond[J].Diamond and related materials,2014,49:19-24.
[25]MAEDA H,OHTSUBO K,GROTJOHN M,et al. Growth behavior of boron-doped diamond in microwave plasma-assisted chemical vapor deposition using trimethy boron as the dopant source[J].Diamond and related materials,1998,7:88-95.
[26] TALLAIRE A,ACHARD J,SILVA F,et al.Homoepitaxial deposition of high-quality thick diamond films:effect of growth parameters[J].Diamond and related materials,2005,14:249-254
[27] ENCKEVORT WJ,JANSSEN G,SCHERMER J,et al. Step-related growth phenomena on exact and misoriented{001}surfaces of cvd-grown single-crystal diamonds [J].Diamond and related materials,1995,4:250-255.
[28] OKUSHI H,WATANABE H,RI S,et al.Device-grade homoepitaxial diamond film growth[J].Journal of crystal growth,2002,237:1269-1276.
[29] GU Y,LU L,GROTJOHNT,et al.Microwave plasma reactor design for high pressure and high power density diamond synthesis[J].Diamond and related materials,2012,24:210-214.
[30]ACHARD J,SILVA F,TALLAIRE A,et al.High quality microwave plasma chemical vapor deposition diamond single crystal growth:high microwave power density regime[J].Journal of physics D:applied physics,2007,40:6175-6188.
[31] DUTEN X,ROUSSEAU A,GICQUEL A,et al.Timeresolved measurements of the gas temperature in a hydrogen/methanemediumpressuremicrowave915 MHz pulsed plasma[J].Journal of physics D:applied physics,2002,35:1939-1945.
[32]TALLAIRE A,ACHARD J,SILVA F,et al.Effect of increasing the microwave density in both continuous and pulsed wave mode on the growth of monocrystalline diamond films[J].Physica status solidi(a),2005,202:2059-2065.
[33] RI SG,YOSHIDA H,YAMANAKA S,et al.Misorientation angle dependence of surface morphology in homoepitaxial diamond film growth at a low hydrogen/methane ratio[J].Journal of crystal growth,2002,235:300-306.
[34]TERAJI T,ITO T.Homoepitaxial diamond growth by high-power microwave-plasma chemical vapor deposition[J].Journal of crystal growth,2004,271:409-419
[35]TERAJI T,MITANI S.High rate growth and luminescence properties of high-quality homoepitaxial diamond(100)films[J].Physica status solidi(a),2003,198(2):395-406.
[36]LOMBARDI G,HASSOUNI K,STANCU G,et al. Modeling of microwave discharges of hydrogenadmixed with methane for diamond deposition[J].Ap-plied physics letters,2005,98:053303-1-053303-12.
[37] DERKAOUI N,ROND C,HASSOUNI K,et al.Spectroscopic analysis of hydrogen/methane microwave plasma and fast growth rate of diamond single crystal [J].Applied physics letters,2014,115:233301-1-233301-8.
[38]TALLAIRE A,COLLINS A T.CHARLES D,et al. Characterisation of high-quality thick single-crystal diamond grown by chemical vapor deposition with a low nitrogen addition[J].Diamond and related materials,2006,15:1700-1707
[39]ACHARD J,SILVA F,BRINZA O,et al.Coupled effect of nitrogen addition and surface temperature on the morphology and the kinetics of thick chemical vapor deposition diamond single crystals[J].Diamond and related materials,2007,16:685-689.
[40]TALLAIRE A,ACHARD J,SILVA F,et al.Growth of large size diamond single crystals by plasma assisted chemical vapour deposition:recent achievements and remaining challenges[J].Comptes rendus physique,2013,14:169-184.
[41] BOLSHAKOVAP,RALCHENKOVG.High-rate growth of single crystal diamond in microwave plasma in hydrogen/methane and argon/hydrogen/methane gas mixtures in presence of intensive soot formation[J]. Diamond and related materials,2016,62:49-57.
[42]CICAL G,MONEGER D,CORNACCHIA D,et al. Toward smooth microwave plasma chemical vapor deposition diamond films:exploring the limits of the hydrogen percentage in argon/hydrogen/methane gas mixture[J].Surface and coatings technology,2012,211:152-157.
[43] SUMIYAA H,HARANOA K.High temperature and high pressure synthesis and crystalline quality of large high-quality(001)and(111)diamond crystals[J]. Diamond and related materials,2015,58:221-225.
[44]吕反修,黑立富,刘杰,等.CVD金刚石大单晶外延生长及其高技术应用前景[J].热处理,2013,28 (5):1-11. LYU F X,HEI L F,LIU J,et al.Epitaxial growth of large size single crystal diamonds prepared by chemical vapor deposition technique and the prospect in high technology applications[J].Heat treatment,2013,28(5):1-11
[45]NAD S,GU Y,ASMUSSEN J,et al.Growth strategies for large and high quality single crystal diamond substrates[J].Diamond and related materials,2015,60:26-34.
[46] YAMADA H,CHAYAHARA A,UMEZAWA H,et al. Fabrication and fundamental characterizations of tiled clones of single-crystal diamond with 1-inch size[J]. Diamond and related materials,2012,24:29-33.
[47] YAMADA H,CHAYAHARA A,MOKUNO Y,et al. A 2-in.mosaic wafer made of a single-crystal diamond [J].Applied physics letter,2014,104:102110-1-1021110-4.
本文编辑:龚晓宁
Research Progress in Preparation of Mono-Crystal Diamond by Microwave Method
HUANG Ping,WANG Jianhua*,LIU Fan,WENG Jun,WANG Xiao'an
Hubei Key Laboratory of Plasma Chemical and Advanced Materials(Wuhan Institute of Technology),Wuhan 430074,China
The high temperature and high pressure and the chemical vapor deposition are the main methods for preparing synthetic diamond.The diamond prepared by the high temperature and high pressure has too small sizes with metal impurities,which restricts its application.Microwave plasma chemical vapor deposition has advantages of no discharge pollution,high energy conversion efficiency,and adjustable process parameters,compared with other chemical vapor deposition,and more attention was paid on it to obtain the single diamond with large size,high speed and high quality.The technologies for preparaing single crystal diamond by microwave plasma chemical vapor deposition diamond were introduced.The research progresses in improving the diamond growth rate and scaling the size of single crystal diamond were summarized.Finally,the application of single diamond was prospected.
microwave plasma;chemical vapor deposition;single-crystal diamond
汪建华,博士,教授,博士研究生导师.E-mail:wjhwz@126.com
TB43
A
10.3969/j.issn.1674-2869.2016.04.009
2016-02-28
湖北省教育厅科学技术研究计划优秀中青年人才项目(Q20151517);武汉工程大学科学研究基金项目(K201506)
黄平,硕士研究生.E-mail:fletly@126.com.

