The incidence of cystoid macular edema in patients with or without diabetes after uncomplicated phacoemulsification: A four-year study
Theodoros Mirachtsis, Konstantinos Markou, Eirini Georgiadou, Christos Sioulis, Simeon Lake, Vagia Moschou, Nick Georgiadis
The incidence of cystoid macular edema in patients with or without diabetes after uncomplicated phacoemulsification: A four-year study
Theodoros Mirachtsis1, Konstantinos Markou2, Eirini Georgiadou1, Christos Sioulis1, Simeon Lake3, Vagia Moschou3, Nick Georgiadis3
1Department of Ophthalmology, “424” General Military Hospital, Thessaloniki 54629, Greece
2Department of E.N.T., School of Medicine, Aristotle University, Thessaloniki 54631, Greece
3Department of Ophthalmology, School of Medicine, Aristotle University, Thessaloniki 54631, Greece
Correspondence to:Theodoros Mirachtsis. Department of Ophthalmology, “424” General Military Hospital, Thessaloniki 54629, Greece. theomirah@gmail.com
Received: 2016-05-09Accepted: 2016-06-17
目的:调查无糖尿病和糖尿病患者单纯超声乳化术后黄斑囊样水肿(post-operative cystoid macular edema,pCME)的发生率及其与术中累积释放能量(cumulative dissipated energy, CDE)、超声乳化时间的关系。
方法:研究纳入116例无糖尿病患者(A组)和101例糖尿病接受超声乳化手术患者(B组)。术前,两组经频域光学相干断层扫描(spectral-domain optical coherence tomography, SD-OCT)和眼底荧光造影(fundus fluorescein angiography, FFA)检测,均未发现黄斑病变或糖尿病黄斑水肿的表现。术后记录超声乳化相关指标。术后每2mo进行FFA检查。出现pCME迹象的患者再次进行SD-OCT评估。
结果:单纯超声乳化术后,两组之间的pCME发生率有显著差异(A组:6.9%,B组:15.8%,P=0.03)。24例患者中,出现19例亚临床pCME。pCME的发生和超声乳化参数(CDE、超声乳化时间和晶状体硬度)显著相关。发生pCME和未发生pCME患者之间糖化血红蛋白(glycosylated hemoglobin, HbA1c)有显著差异(P=0.005)。黄斑囊样水肿和眼轴长度无相关性。
结论:单纯超声乳化术后,无糖尿病和糖尿病患者pCME的发生率有显著差异。大多数患pCME的患者为亚临床表现。CDE和超声乳化时间是pCME的重要影响因素和预测因素。良好的血糖控制可以预防pCME发生。
引用:Mirachtsis Th, Markou K, Georgiadou E, Sioulis C, Lake S, Moschou V, Georgiadis N. 糖尿病患者单纯超声乳化术后黄斑囊样水肿的发生率.国际眼科杂志2016;16(8):1407-1411
Abstract
•AIM: To report the incidence of post-operative cystoid macular edema (pCME) in patients with or without diabetes and its correlation with cumulative dissipated energy (CDE) and phaco-time after uncomplicated phacoemulsification.
•METHODS: In the study 116 nondiabetic (Group A) and 101 diabetic patients (Group B) underwent phacoemulsification. Preoperatively none of the groups (A+B) had signs of maculopathy or diabetic macular edema documented by spectral-domain optical coherence tomography (SD-OCT) and fundus fluorescein angiography (FFA). Phaco metrics were documented after surgery. FFA was performed two months after each operation. Patients with indications of pCME were reassessed with SD-OCT.
•RESULTS: The incidence of pCME after uncomplicated phacoemulsification was statistically significant difference between the two groups (15.8% in Group B versus 6.9% in Group A,P=0.03<0.05). The subclinical pCME appeared in 19 out of 24 patients. There was a significant correlation between parametric values (CDE, phaco-time, hardness of the lens) and pCME occurrence. Glycosylated Hemoglobin (HbA1c) blood levels was statistically significant difference (P=0.005<0.05) between the patients who developed or not pCME. Cystoid macular edema did not correlate with the axial length of the eye.
•CONCLUSION: There was statistically significant difference in the incidence of pCME after uneventful phacoemulsification between nondiabetic subjects and diabetics. Most of these patients with pCME had subclinical appearance. CDE and phaco-time data were important factors and predictors to pCME. Good glycemic controls prevent the incidence of pCME.
KEYWORDS:•cataract; cystoid macular edema; cumulative dissipated energy; phacoemulsification; HbA1c
Citation:Mirachtsis Th, Markou K, Georgiadou E, Sioulis C, Lake S, Moschou V, Georgiadis N. The incidence of cystoid macular edema in patients with or without diabetes after uncomplicated phacoemulsification: A four-year study.GuojiYankeZazhi(IntEyeSci) 2016;16(8):1407-1411
INTRODUCTION
Postoperativecystoid macular edema (pCME) also known as Irvine-Gass syndrome is the most frequent complication leading to impairment of visual acuity and can occur even after a successful uncomplicated cataract surgery[1]. The pathogenesis of pCME is multifactorial and the incidence is controversial in clinical studies. Nowadays, spectral-domain optical coherence tomography (SD-OCT) evidenced that the incidence of subclinical CME varied between 4%-20% in healthy subjects after uncomplicated cataract surgery[2]and in well-controlled diabetic patients without retinopathy or diabetic macular edema, the incidence of pCME also reported to be as high as 56%[3]. The central subfoveal macular thickness (CSMT), in SD-OCT analysis, after uncomplicated cataract surgery, introduced a minimal, but statistically significant, increase postoperatively without impairment of best corrected visual acuity[4-6]. However the most patients with clinical pCME have spontaneous improvement by 3-12mo[7]. The anatomical recovery of the pCME does not sometimes accompany with functional rehabilitation[8]. Patients with BCVA 20/20 and subclinical pCME have abnormalities’ in contrast sensitivity[9]. According to recent studies the intraoperative use of anti-VEGF factor minimized the risk of pCME even in diabetic patients[10]. On the other hand, some studies showed that there was no statistically significance between the enhanced risk of pCME in healthy and in diabetics after mincroincision cataract surgery(MICS)[11-12]. In the bibliography there is a study regarding the Greek population which indicates that the incidence of pCME after cataract surgery in healthy patients was 4.0% and it reaches up to 28.6% in diabetics[13]. In our study the incidence of pCME following uncomplicated cataract surgery was evaluated, for a six-month period, in healthy and well-controlled diabetic patients with no macular changes in both groups. Parametric values were recorded after each cataract surgery. Cumulative dissipated energy (CDE) data measures in seconds and reflects how difficult was the surgery and how much energy has been expended[14]. We aimed to search a correllation between CDE data, phaco-time and the hardness of the cataractwith the occurrence of pCME. Adittionaly, we evaluated the blood levels of Hemoglobin A1c(HbA1c) of the patients and also the axial length between the eyes which appeared pCME or not. The SD-OCT analysis is a useful diagnostic tool regarding the volume, the severity and the extent of the macular edema[15]. The Diabetic Retinopathy Clinical Research Network defined diabetic macular edema using SD-OCT analysis as the macular thickness was higher than 310 μm[16]. Regardless that, Fundus fluorescein angiography (FFA) is still in general practice. Initial stages of macular edema showed fluorescein leakage although SD-OCT analysis was under the estimated limits[17].
Table 1Study protocol and demographics of studied groups

GroupsnGender(M/F)AgeParticipants217115(53%)/102(47%)72.9±8.3(39-90) GroupA11667/4972.2±9.2(39-88) GroupB10148/5373.7±7.0(52-90)
P=0.17.
SUBJECTS AND METHODS
Patients EnrollmentThis retrospective observational non-randomized study was performed at Ophthalmological Clinic of “424” General Military Hospital in Thessaloniki, Greece. The study was approved by the Department of Medical Law and Bioethics of the Aristotle University of Thessaloniki. All patients were treated according to the Declaration of Helsinki document and all of them underwent a process of informed consent. Patients with cataract and decreased visual acuity were enrolled in the study. All patients were above 18y. Patients were excluded if they had macular pathology or diabetic macular edema. They were also excluded if they had preexisting vein or artery occlusion, previous anterior or posterior uveitis, intraocular surgery, laser treatment, intravitreal injections or received topical pharmaceutical agents such as prostaglandins analogs or epinephrine. Patients systemically received drugs, recently approved that induced macular edema, such as antidiabetic agents as thiazolidinediones[18], anticancer agents such as tamoxifen or interferon[19]and modulators of the immune response such as fingolimod for the treatment of relapsing forms of multiple sclerosis (MS) were excluded from our study. Fingolimod associated macular edema seems to be dose-dependent[20]. Enrolled patients who had complications during cataract surgery (e.g., posterior capsule rapture with or without vitreous loss, dropped nuclear material or an eccentric intraocular lens, not placed in the capsular bag) were subsequently excluded.
Study ProtocolBetween Sep. 2011 and Jul. 2015, 217 patients (Group A: 116 healthy and Group B: 101 well-controlled diabetics) with cataract were pleaded for taking part in our 6-month study. The diabetic patients with HbA1c <7.5% took part in our study as well-controlled[21]. We completed an information protocol on demographics’ (birthday, sex, and age), medical and ocular history at the initial visit. Characteristics of patients included in the study are shown in Table 1. Preoperative assessment included best corrected visual acuity (BCVA), IOP, slit lamp examination of the cornea, the morphology and the hardness of the cataract, the axial length of the eye, the dilatation of the pupil and the existence of pseudoexfoliation or not was also recorded. Macular examination using OCT Cirrus HD Spectral Domain and fundus photographs were obtained and the values of HbA1c were also noted. The measure of HbA1c was taken place in the laboratory of our hospital which is certificated by the National Certification System of Quality (ISO 9001). Patients with HbA1c>7.5%, high level systemic systolic hypertension (>130 mm Hg) or renal failure were excluded from the study. The patients did not receive any anti-inflammatory non steroids drops preoperatively because of these drugs seems to reduce the incidence of post-operative CME[2,22].
Surgery was performed by the same surgeon (Mirachtsis Th). Micro-incision cataract surgery (MICS) was performed using Infinity Alcon unit (Alcon Inc. Fort Worth, Texas, USA) and an acrylic intraocular lens was inserted in the bag of all patients. Phaco energy ranged between 0-50 kHz according to the hardness and the severity of the cataract. The maturity and the hardness of the cataract were recorded using the slit-lamp examination and the ability to detect details of the fundus using a 66 D non-contact lens. Phaco metrics such as CDE and phaco-time were recorded after each procedure. Postoperative examination was performed at 1, 7, and 30d after surgery and included measurements of intraocular pressure, best corrected visual acuity, the intraocular inflammation, the “in the bag” lens centrality and fundus examination by the use of 78 D or 66 D non-contact lenses. All the patients received the same topical medication for 20d (mixed combination with antibiotic and dexamethasone installed every hour for 2d and tapering the next 3wk). Fundus fluorescein angiography (FFA) was performed 2mo after the phacoemulsification. Patients with indications of macula thickness changes were reassessed with OCT. FFA provides qualitative assessments for the diagnostic approach and in association with SD-OCT which provides quantitative measures of macular thickness and vitreous-macular changes leading us to the diagnosis[23].
Statistical AnalysisData were analyzed using SPSS software version 22.0, SPSS Inc. (IBM Co. Endicott, New York, USA).Pvalue less than 0.05 was considered as statistically significant.Pvalue less than 0.10 was also considered in borderline measurements. The one-way ANOVA was used to assess the risk of pCME occurrence of CDE data, phaco-time, HbA1c values and the hardness of the cataract separately. The Chi-square test was also used to compare the incidence of pCME between healthy and diabetic patients. The means of numerical variable between groups compared using the independent samples Student’st-test when the distributions were normal. In case of the means of those variable did not follow normal distributions we used the Mann-WhitneyUtest. The variability of the data was checking with Kolmogorov-Smirnov test.
RESULTS
Macular edema was recorded in 24 out of 217 patients (11.1%).
Table 2The appearance of postoperative cystoid macular edema (pCME) in diabetic patients and healthy
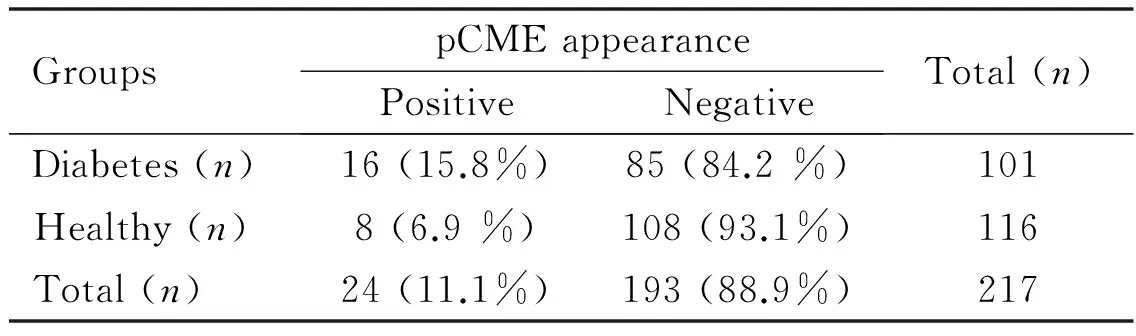
GroupspCMEappearancePositiveNegativeTotal(n)Diabetes(n)16(15.8%)85(84.2%)101Healthy(n)8(6.9%)108(93.1%)116Total(n)24(11.1%)193(88.9%)217
P=0.03.
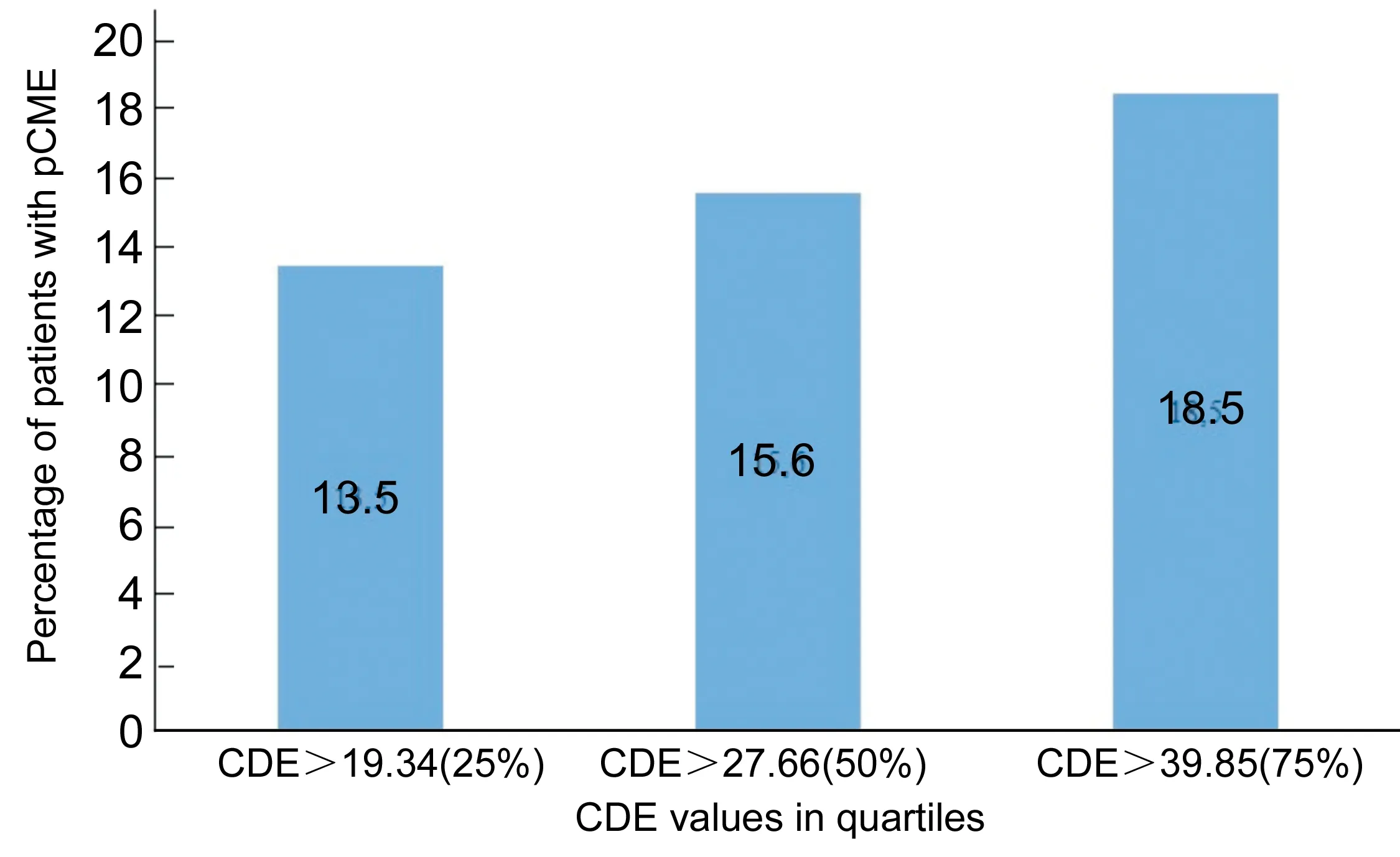
Figure 1The risk of pCME according to CDE values.
The results of CME appearance in healthy and diabetic patients are shown in Table 2. Seven out of these 8 healthy patients (87.5%) and 12 out of 16 diabetic patients (75%) appeared with subclinical pCME with BCVA over 20/40 using Snellen optotype which was verified with FFA and SD-OCT examination. The central subfoveal macular thickness (CSMT) in the patients with pCME was 324.41μm. Details which concerned these patients with pCME (clinical, subclinical or partial) are shown in Table 3. None of the enrolled patients had clinical evidence of worsening of the retinal disease. The mean CDE value concerning the 24 patients who occurred pCME was 43.74s (SD±29.41). The minimum value was 13.26s and the maximum 143.90s. On the other hand, the mean CDE value concerning the 193 patients with no pCME was 31.46s (SD±18.79). The minimum value was 4.74s and the maximum 139.90s. There was a significant difference using one-way ANOVA in CDE values between the patients who appeared pCME or not (P=0.005).
Processing statistical data, we divided the CDE values in quartiles and we ensured the progressive risk of pCME increasing with this parameter. We confirmed that in the first quartile (25% of the patients) when the CDE data was over 19.34s, the risk of pCME occurrence was 13.5%. In the second quartile (50% of the patients) when the CDE data was over 27.66s, the risk increased to 15.6% and at last in the third quartile when the CDE data was over 39.85s, the risk of pCME occurrence reached to 18.5% (Figure 1). Additionally, the mean hardness of the cataract in patients with pCME was 3.9 (SD±1.17) and in patients who did not occur pCME was 3.0 (SD±1.0) according to Lens Opacities Classification System version Ⅲ (LOCS Ⅲ). This is statistically significant (Chi-square,P<0.05) and it seems to be a factor in correlation with the CDE data which leads to pCME.
Table 3The appearance of pCME after phacoemulsification according to study groups, CDE values and imaging findings (FFA, SD-OCT)
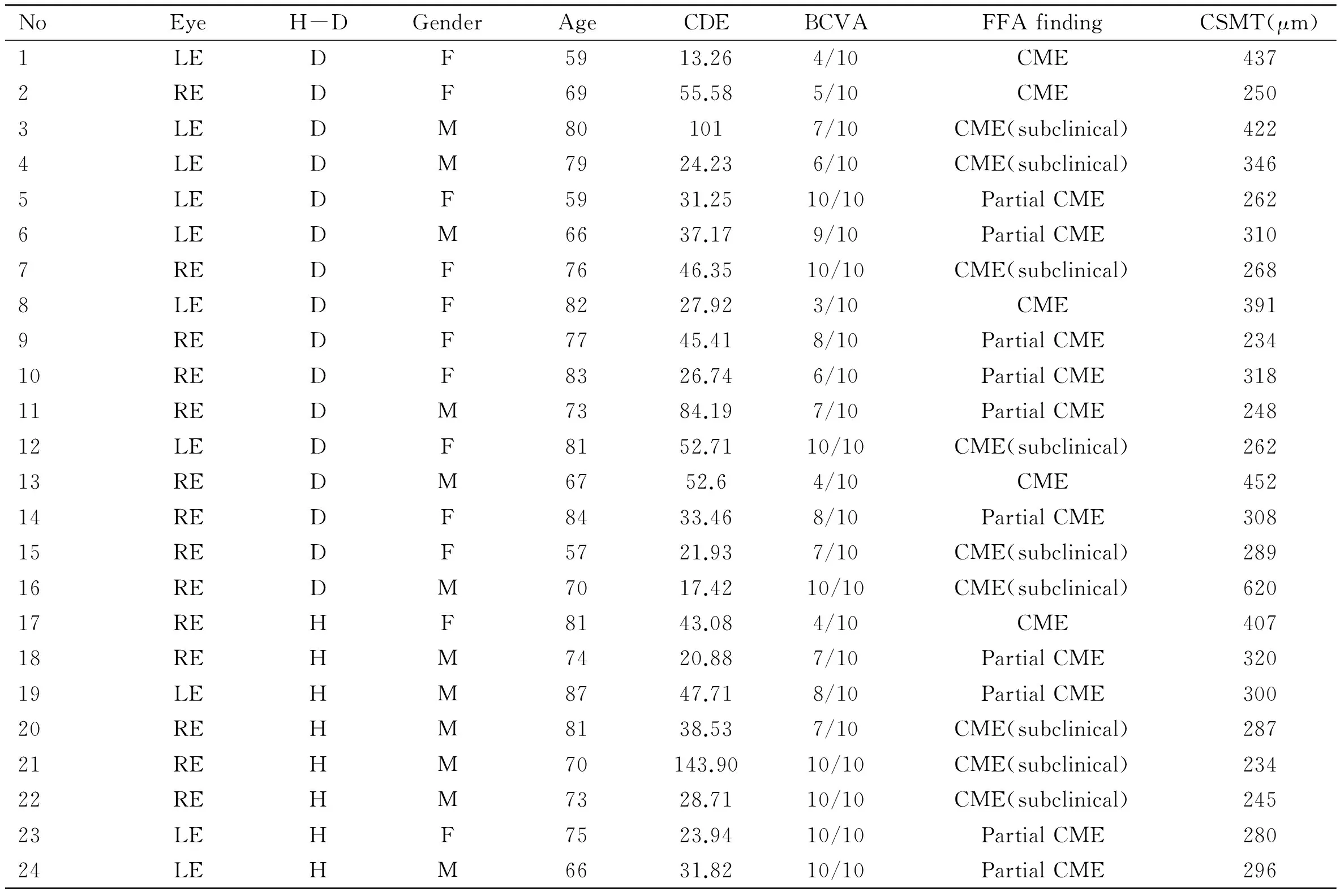
NoEyeH-DGenderAgeCDEBCVAFFAfindingCSMT(μm)1LEDF5913.264/10CME4372REDF6955.585/10CME2503LEDM801017/10CME(subclinical)4224LEDM7924.236/10CME(subclinical)3465LEDF5931.2510/10PartialCME2626LEDM6637.179/10PartialCME3107REDF7646.3510/10CME(subclinical)2688LEDF8227.923/10CME3919REDF7745.418/10PartialCME23410REDF8326.746/10PartialCME31811REDM7384.197/10PartialCME24812LEDF8152.7110/10CME(subclinical)26213REDM6752.64/10CME45214REDF8433.468/10PartialCME30815REDF5721.937/10CME(subclinical)28916REDM7017.4210/10CME(subclinical)62017REHF8143.084/10CME40718REHM7420.887/10PartialCME32019LEHM8747.718/10PartialCME30020REHM8138.537/10CME(subclinical)28721REHM70143.9010/10CME(subclinical)23422REHM7328.7110/10CME(subclinical)24523LEHF7523.9410/10PartialCME28024LEHM6631.8210/10PartialCME296
RE: Right eye; LE: Left eye; H: Healthy patients; D: Diabetic patients; BCVA: Best corrected visual acuity; CSMT: Central subfoveal macular thickness (μm); CDE: Cumulative dissipated energy (s); mean CSMT: 324.41μm; FFA:Fundus fluorescein angiography.
The phaco-time in the patients who had pCME was 14.27s (SD±11.62). In the contrary, the phaco-time in the patients who did not have pCME was 10.17s (SD±10.69). We concluded using the Kolmogorov-Smirnov test that this distribution of the means was not normal. As a result, we used Mann-Whitney test and we found statistically significant correlation between phaco-time and pCME occurrence (P=0.036). Consequently, we also divided the phaco-time, we had already recorded after the cataract surgery, in quartiles. The median data was 8.1s and there were 7 patients who had pCME with phaco-time under this cut-off point (6.4%) and 17 patients (15.7%) with pCME who had phaco-time over 8.1s. This difference was statistically significant (P=0.023).
The HbA1c value was also statistically high in patients with pCME (6.85±1.33vs6.16±1.09,P=0.005). The axial length of the 24 patients with pCME was 23.10 mm (SD±0.92) and the axial length of the patients without pCME was 23.18 mm (SD±1.02). Axial length seems not to provoke pCME (P=0.702).
DISCUSSION
The occurrence of pCME is a complication which provokes a feel of stress and worry about the accomplishment of the cataract surgery and the visual acuity outcome. According to Erikssonetal[3], diabetic patients have an increased risk in developing CME even after uneventful cataract surgery which is up to 56% in patients without diabetic retinopathy and diabetic macular edema. In our study the incidence of pCME after uncomplicated micro-incision cataract surgery was 15.8% in the diabetic patients and 6.9% in the healthy patients. The use of OCT confirmed the statistically significant appearance of subclinical pCME (75% in diabetics and 87.5% in healthy). Biro and Balla[4]and many other researchers, as our study confirmed, that SD-OCT is a helpful tool to provide accurate information about partial or subclinical pCME with minimal or no impairment of visual acuity[5-6]. Additionally, improved glycemic control minimized the risk of pCME and this is also our conclusion. Increased CDE data during phaco-procedure increase the incidence of pCME. We have to keep it in mind, although these results might have its cause in the individual settings of the used phaco-machine or in the individual technique of the surgeon. Phaco-time over 8.1s is also an important factor and predictor to pCME.
In conclusion cystoid macular edema is more often recorded in diabetic patients than healthy after uncomplicated cataract surgery. The large number of patients with pCME was subclinical or partial (75% in diabetics and 87.5% in healthy) with minimal macular changes in SD-OCT and they had good visual outcome. CDE and phaco-time are reliable data for each cataract surgeon to monitor and check the surgical outcomes. Smaller CDE values and phaco-time under 8s show less phacoemulsification energy, less damage to corneal endothelium and faster recovery time. Good glycemic control is necessary to minimize the risk of pCME. These instructions may also help to reduce the financial costs of the healthcare system among the patients with pCME[24].
REFERENCES
1 Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema.CurrOpinOphthalmol2012;23(1):26-32
2 Wielders H.P. Laura, Schouten S.A.G. Jan, Van Den Biggelaar J.H.M. Frank, Winkens Bjorn, Nuijts M.M.A. Rudy. Prevention of CME after Cataract Surgery.Cataract&RefractiveSurgeryTodayEurope2013;8(7):53-55
3 Eriksson U, Alm A, Bjärnhall G, Granstam E, Matsson AW. Macular edema and visual outcome following cataract surgery in patients with diabetic retinopathy and controls.GraefesArchClinExpOphthalmol2011;249(3):349-359
4 Biro Z, Balla Z. Foveal and perifoveal retinal thickness measured by OCT in diabetic patients after phacoemulsification cataract surgery.BrJOphthalmol2009;53(2):54-60
6 Von Jagow B, Ohrloff C, Kohnen T. Macular thickness after uneventful cataract surgery determined by optical coherence tomography.GraefesArchClinExpOphthalmol2007;245(12):1765-1771
7 Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update.Retina2011;31(1):4-12
8 Hunter AA, Modjtahedi SP, Long K, Zawadzki R, Chin EK, Caspar JJ, Morse LS, Telander DG. Improving visual outcomes by preserving outer retina morphology in eyes with resolved pseudophakic cystoid macular edema.JCataractRefractSurg2014;40(4):626-631
9 Bobrow JC.AmericanAcademyofOphthalmology:BasicandClinicalScienceCourse, section 11: Lens and Cataract, Singapore, 2011.
10 Kanellopoulos A. John. Prophylactic Use of Anti-VEGF Therapy in Cataract surgery.Cataract&RefractiveSurgeryTodayEurope2013;8(7):56-57
11 Rashid S, Young LH. Progression of diabetic retinopathy and maculopathy after phacoemulsification surgery.IntOphthalmolClin2010;50(1):155-166
12 Shah AS, Chen SH. Cataract surgery and diabetes.CurrOpinOphthalmol2010;21(1):4-9
13 Katsimpris JM, Petropoulos IK, Zoukas G, Patokos T, Brinkmann CK, Theoulakis PE. Central foveal thickness before and after cataract surgery in normal and in diabetic patients without retinopathy.KlinMonblAugenheilkd2012;229(4):331-337
14 Chen M, Chen M. Comparison of CDE data in phacoemulsification between an open hospital-based ambulatory surgical center and a free-standing ambulatory surgical center.ClinOphthalmol2010;4:1287-1289
15 Ghanchi F; Diabetic Retinopathy Guidelines Working Group. The Royal College of Ophthalmologists Clinical Guidelines for Diabetic Retinopathy: a summary.Eye(Lond) 2013;27(2):285-287
16 Ossewaarde-van Norel A, Rothova A. Imaging Methods for Inflammatory Macular Edema.IntOphthalmolClin2012;52(4):55-66
17 Barteselli G, Chhablani J, Lee SN, Wang H, El Emam S, Kozak I, Cheng L, Bartsch DU, Azen S, Freeman WR. Safety and Efficacy of Oral Fluorescein Angiography in Detecting Macular Edema in Comparison with Spectral-Domain Optical Coherence Tomography.Retina2013;33(8):1574-1583
18 Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes.ArchInternMed2012;172(13):1005-1011
19 Abd El-Badie Mohamed M, Abd-El Azeem Eed K. Retinopathy associated with interferon therapy in patients with hepatitis C virus.ClinOphthalmol2012;6:1341-1345
20 Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management.Neurology2012;78(9):672-680
21 International Council of Ophthalmology. ICO Guidelines for Diabetic Eye Care 2014
22 Miyake K, Ota I, Miyake G, Numaga J. Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery.JCataractRefractSurg2011;37(9):1581-1588
23 Goatman KA. A reference standard for the measurement of macular oedema.BrJOphthalmol2006;90(9):1197-1202
24 Schmier JK, Halpern MT, Covert DW, Matthews GP. Evaluation of costs for cystoid macular edema among patients after cataract surgery.Retina2007;27(5):621-628
(作者单位:154629希腊塞萨洛尼基424军事总医院眼科;254631希腊塞萨洛尼基亚里士多德大学医学院耳鼻喉科;354631希腊塞萨洛尼基亚里士多德大学医学院眼科)
通讯作者:Theodoros Mirachtsis. theomirah@gmail.com
DOI:10.3980/j.issn.1672-5123.2016.8.03
关键词:白内障;黄斑囊样水肿;术中累积释放能量;超声乳化术;糖化血红蛋白
糖尿病患者单纯超声乳化术后黄斑囊样水肿的发生率
Theodoros Mirachtsis1, Konstantinos Markou2, Eirini Georgiadou1, Christos Sioulis1, Simeon Lake3, Vagia Moschou3, Nick Georgiadis3
摘要

