Examining the Effects of Biochar Application on Soil Phosphorus Levels and Phosphatase Activities with Visible and Fluorescence Spectroscopy
ZHANG Yu-lan, CHEN Li-jun*, ZHANG Yu-ge, WU Zhi-jie,MA Xing-zhu, YANG Xiao-zhu
1. Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China
2. Key Laboratory of Regional Environment and Eco-remediation, College of Environment, Shenyang University, Shenyang 110044, China
3. Institute of Soil Fertilizer and Environment Resource, Heilongjiang Academy of Agricultural Sciences, Harbin 150086, China
Examining the Effects of Biochar Application on Soil Phosphorus Levels and Phosphatase Activities with Visible and Fluorescence Spectroscopy
ZHANG Yu-lan1, CHEN Li-jun1*, ZHANG Yu-ge2, WU Zhi-jie1,MA Xing-zhu3, YANG Xiao-zhu1
1. Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China
2. Key Laboratory of Regional Environment and Eco-remediation, College of Environment, Shenyang University, Shenyang 110044, China
3. Institute of Soil Fertilizer and Environment Resource, Heilongjiang Academy of Agricultural Sciences, Harbin 150086, China
Biochar could be got from crop straw which contain rich carbon under oxygen free or oxygen limited conditions at low temperature. The application of biochar into soil is beneficial to ease the pressure of handling straw, reduce pollution, reduce greenhouse gas emissions and improve soil quality. This study was carried out in a cornfield containing meadow brown soil at the lower reaches of Liao River which was treated with different amounts of biochar (0, 360, 1 800, 3 600 kg·ha-1) and fertilizer. We investigated the contents of soil available phosphorus (AP), organic P (OP) and total P (TP). We also investigated the enzyme activities of soil acid phosphatase (AcP), alkaline phosphatase (AlP) and phosphodiesterase (PD) via a fluorescence spectroscopy method by using a fluorescent conjugated polymer as the substrate. Soil AP contents increased drastically with the increasing application of biochar, whereas the OP and TP contents exhibited little change. The increase in AP contents was ascribed to the introduction of P into the soil via biochar. Soil AlP and PD activities increased with increasing biochar application. Soil AcP activity increased significantly after the application of the appropriate amount of biochar (1 800 kg·ha-1), whereas it was inhibited by the application of high levels of biochar (3 600 kg·ha-1), perhaps due to the intrinsic alkalinity of biochar. The effect of Biochar inputs on soil phosphorus element and phosphatase activity is the comprehensive embodiment of the soil physical properties, chemical properties, and microbial community structure and metabolic capacity. We should further study such item. The fluorescent microplate method used in this study has many advantages, such as accuracy, rapidness and simple to perform.
Biochar addition; Soil phosphorus; Soil phosphatase activity; Fluorescent microplate
Introduction
Phosphorus (P) is an essential macronutrient that is required for plant growth and development. The soil P supply for crops is insufficient in most areas of China although P fertilization has been increased. P for crops comes mainly in the form of soil-available P, which is insufficient, as applied P is unavailable after it is precipitated in the soil due to its active nature. On the other hand, excessive P application affects the environment because P in the soil is lost in runoff when the soil P concentration reaches a certain level, although it is difficult for P to move in the soil[1]. Biochar amendment to the soil is one of the most effective strategies for dealing with these problems, and it has been used to help alleviate the decline in soil quality, the food crisis, ecological pollution and other serious problems[2]. Biochar application improves the physical and chemical properties of soil[3]. The P in the biomass used to produce biochar is retained during the pyrolysis process, primarily in soluble form with high availability. P from biochar amendment directly enters into the soil, thereby significantly increasing the pool of available P in the soil[4-5]. In addition to directly releasing P into the soil, application of biochar influences the P status of the soil by altering soil pH and the adsorption/desorption of P[6-8]. The charge and functional groups at the biochar surface are beneficial for the conservation of soil nutrients, which reduces the possibility of P loss[4].
Soil phosphatase is directly involved in the hydrolysis of soil organic P and it plays an important role in the P cycle in the environment[9]. The P that biochar carries into the soil has some effect on soil phosphatase activity. Biochar adsorbs enzyme substrate and enhances enzymatic catalytic reactions, which leads to an increase in soil enzyme activity. Moreover, improving soil properties and structure through biochar application indirectly enhances soil enzyme activities[10].
Many methods are used to determine soil phosphatase activity, among which the spectrophotometry colorimetric method (using p-nitrophenol as the substrate) is widely used due to its low cost. However, this method has some drawbacks when detecting large numbers of samples, such as the high consumption of chemical regents, glassware and labor. We previously developed a method using 96-well plates in a microplate reader to determine soil enzyme activity, which is mainly based on measuring changes in fluorescence intensity after incubating a soil suspension, a fluorescently-labeled substrate and the corresponding buffer in a 96-well microplate[11].
In the present study, we examined the soil P contents in meadow brown soil after the addition of biochar from corn straw using a visible spectrophotometer combined with an autosampler. We also measured soil phosphatase activity in a cornfield after the application of biochar from corn straw after plant growth using a 96-well plate in a microplate reader with fluorescent material as substrate. The results of this study help elucidate the mechanism underlying the effects of biochar application on soil P and the effects of soil enzymes on the P cycle.
1 Materials and methods
1.1 Experiments
Preparation of biochar: Corn straw was dried in an 80 ℃ oven (48 hours) after harvesting and cleaning. The straw was then crushed and burned in a vacuum sintering furnace (OTF-1200X-5L, Shenyang Kejing company) at 350 ℃ for 4 hours (including a 2 hour period from room temperature to 350 ℃) to obtain the desired product.
1.1.1 Site description
The study was initiated using meadow brown soil in April, 2014. The experimental field was located at the Eco-experimental Station under the CAS Shenyang Branch in the Eastern Mausoleum district of Liaoning Province, Northeast China. The field was planted with maize (ZeamaysL.). The experiment was arranged in a random design with different amounts of biochar as treatments. There were four treatments and three replicates. The size of each plot was 1 m2(1 m×1 m). The four treatments included: (1) no biochar treatment as the control (CK); (2) 360 kg·ha-1; (3) 900 kg· ha-1and (4) 1800 kg·ha-1. Biochar was spread into soil uniformly and all fertilizers were applied based on local practices, i.e., 180 kg N·ha-1, 75 kg P2O5·ha-1and 75 kg K2O·ha-1, before crop planting.
1.1.2 Sample collection
Soil samples were collected after harvesting (October 25, 2014). A composite surface (0~20 cm) soil sample was collected within a plot by randomly sampling five locations and placing the soil into an isothermal bag after mixing well. After sieving (2.0 mm), each sample was divided into two parts. One part was air-dried and stored in a sealed container (for chemical property analysis), while the other part was stored at 4 ℃ (for enzyme activity analysis).
1.2 Instruments and reagents
The instruments utilized in this study include the following: an electric heating constant temperature incubator (EYELA, Japan); an 8-channel pipette (Eppendorf, Germany); a Tecan Genios (Infinite○R200 PRO, Switzerland); a Carry 50 UV-Vis Spectrophotometer with autosampler (SPS3, Varian, US) and standard laboratory glassware.
1.2.1 Analytical reagents
4-methylumbelliferyl phosphate (MUP) was used for soil phosphomonoesterase activity determination and bis-(4-methylumbelliferyl) phosphate (BIS-MUP) was used for soil phosphodiesterase (PD) activity determination (Table 1). Other reagents include sodium azide (NaN3), acetic acid (C2H4O2), maleic acid (C4H4O4), citric acid (C6H8O7), boric acid (H3BO4), methyl cellosolve (2-methoxyethanol) (CH3OCH2CH2OH), methylumbelliferone, sodium salt (C10H7O3Na), sodium acetate (C2H3O2Na), tris(hydroxymethyl) aminomethane (NH2C(CH2OH)3) and sodium hydroxide (NaOH).
1.3 Assay methods
1.3.1 The determination of soil P
Conventional methods were adapted to detect changes in soil P levels. Soil total P (TP) levels were determined after digestion with H2SO4-HClO4, and soil available P (AP) contents were determined by a colorimetric method using extractant in 0.5 mol·L-1NaHCO3(pH 8.5). Soil organic P (OP) content was determined based on the reduction of P extraction in H2SO4(1 mol·L-1) after burning soil and P extraction in H2SO4using a colorimetric method[12].

Table 1 Enzyme substrates
1.3.2 The determination of phosphomonoesterase and PD activities
The first row of plants was used to produce standard curves and the 11 remaining rows were used to produce soil suspensions. Eight holes were produced as replicates for one sample per row. Instrument parameters were set according to values in the literature[11]. Enzyme activities were expressed as nmol MU·g-1dry soil·min-1.
1.4 Data analysis
All determinations were performed with three replicates and all values were reported as means. The results were analyzed using Microsoft Excel 2007, and statistical analysis was performed using SPSS 21.0 (ANOVA). Significant differences were tested using a validation process among treatments (p<0.05). Figures were drawn using Origin 8.0 software.
2 Results and discussion
2.1 Effects of biochar application on soil AP, OP and TP levels
Soil AP contents increased with increasing biochar application. Only 1 800 kg·ha-1treatment significantly increased AP contents, although all three treatments increased soil AP. Soil OP and TP increased slightly after biochar treatment; however, the differences among plots were not significant (Figure 1). Soil AP had a significant positive correlation with the amount of biochar application, while soil OP and TP had no such correlation with biochar levels (Figure 2). These results demonstrate that application of biochar increases the levels of three types of P in the soil. P levels in the biochar were high enough to increase soil AP levels, but they did not increase soil OP or TP levels. At the same time, the adsorption of P by biochar helped retained the P from fertilization.
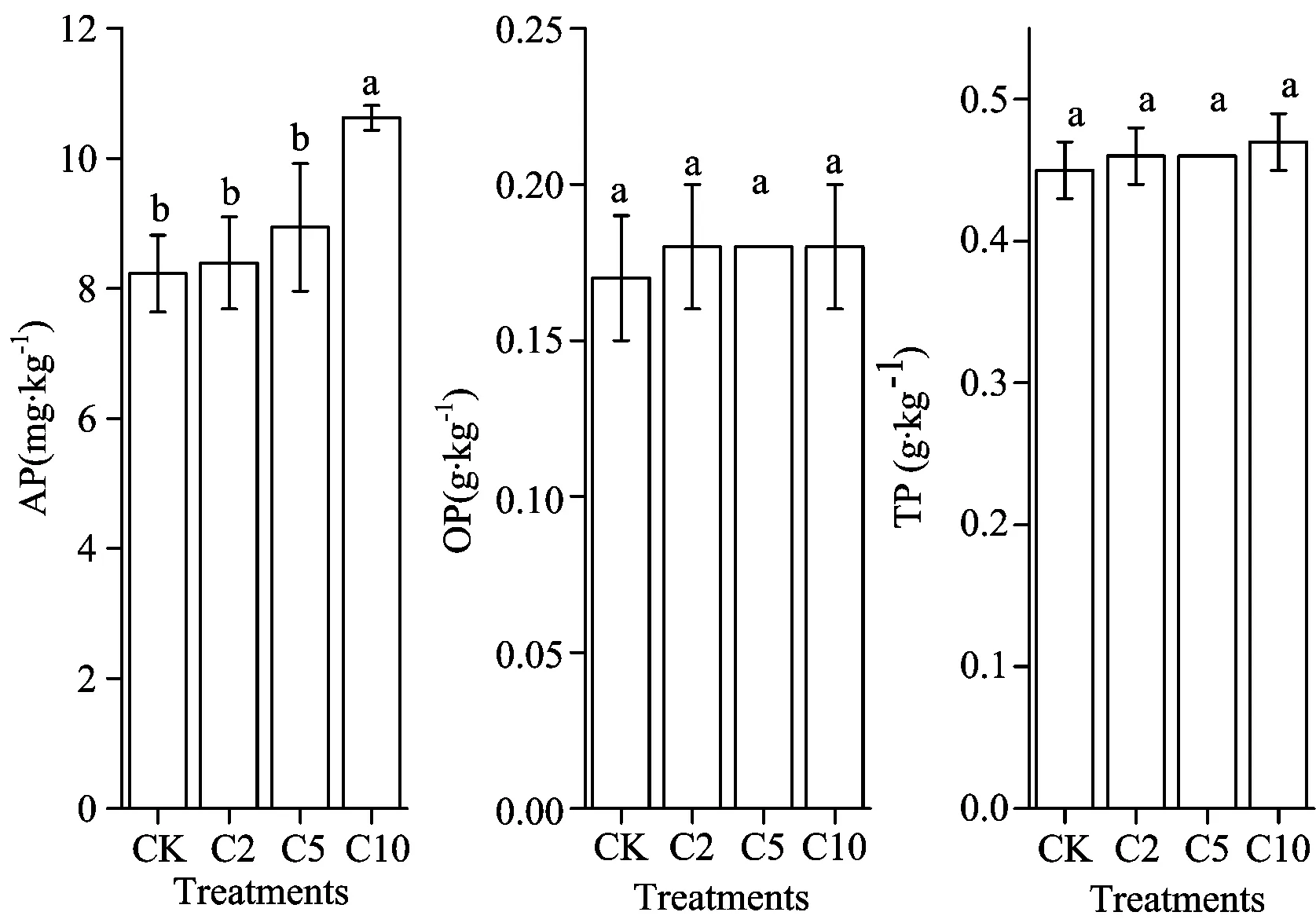
Fig.1 Effect of biochar amendment on available, organic and total P levels in meadow brown soil
AP: available p-mg·kg-1; OP: organic p-g·kg-1; TP: total p-g·kg-1). Values are means ±S.D. Values with different letters differ significantly (p<0.05), as determined by the LSD test

Fig.2 Relationship between BC levels and available P, organic P and total P contents in meadow brown soil
AP: available p-mg·kg-1; OP: organic p-g·kg-1; TP: total p-g·kg-1
2.2 Effects of biochar amendment on soil phosphatase activity
Soil phosphatases are enzymes (phosphomonoesterase and PD) that catalyze the hydrolysis of organic phosphate esters and anhydrides and the hydrolysis of soil OP into inorganic P (phosphate), which directly affect the decomposition and bioavailability of soil OP. Phosphatases include phosphomonoesterase (EC 3.1.3), PD (EC 3.1.4), and so on. Among phosphatases, soil phosphomonoesterase has been the most extensively studied. This enzyme, which catalyzes the hydrolysis of organic phosphate monoesters such as phytic acid, is a nucleotide phosphoprotein, which is mainly divided into acid (pH 6.5) and alkaline (pH 11) phosphatase (AlP) based on the optimum pH value of the reaction. PD hydrolyzes diesterases such as phospholipids and nucleic acids to produce phosphate monoester, which is then hydrolyzed into orthophosphate in the soil (catalyzed by phosphomonoesterase). Orthophosphate is used by both plants and microorganisms[9].
AlP and PD activity increased with increasing biochar treatment, and the soil AcP activity of treatment C5 was significantly higher than that of the other treatments (Figure 2). The alkaline property of biochar increases soil pH and improves acidic soil[13]. The higher pH value leads to an increase in soil AlP activity and a reduction in soil AcP activity[14-15].
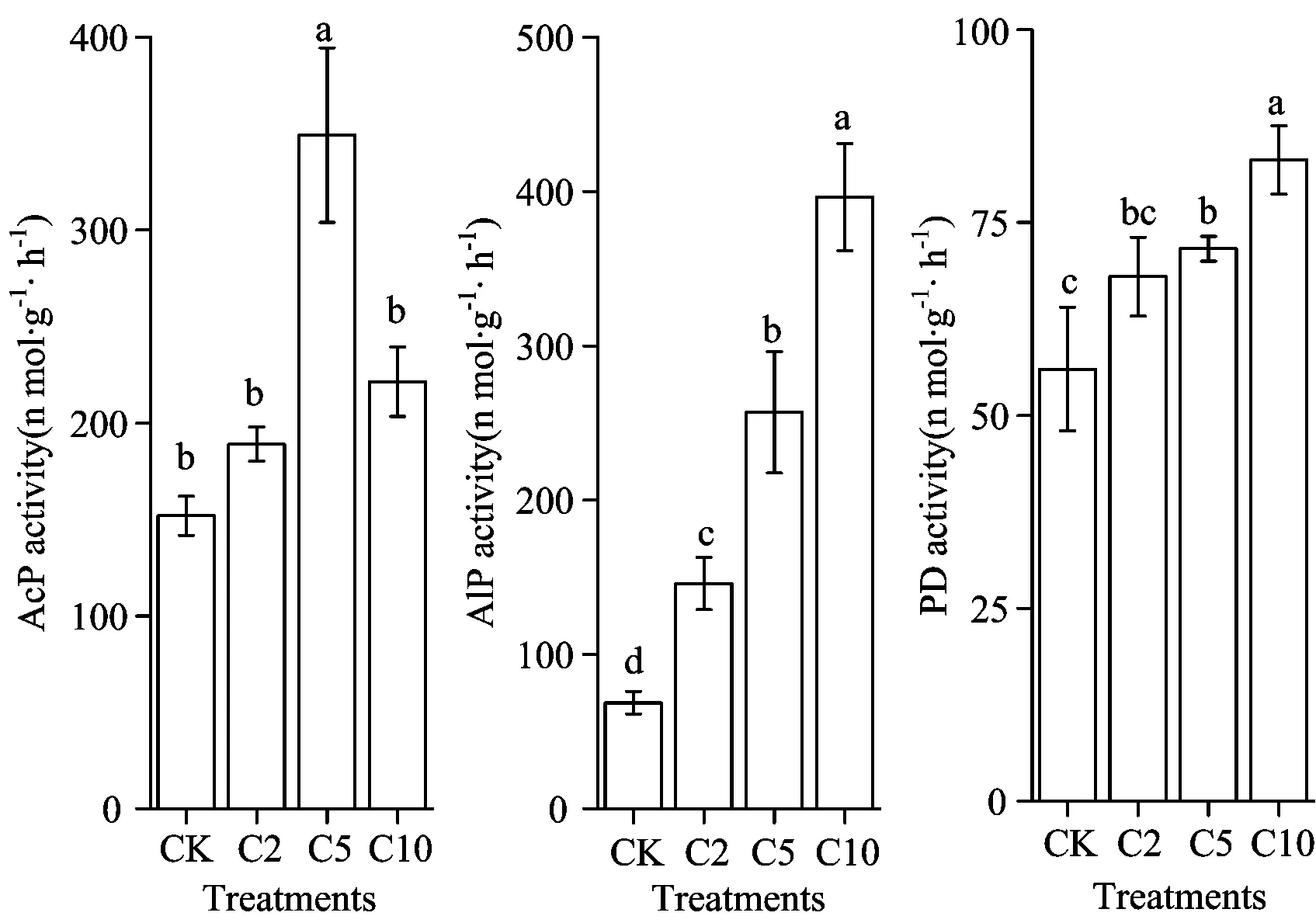
Fig.3 Response of phosphomonoesterase (acid and alkaline) and phosphodiesterase in meadow soil to biochar addition
AcP: acid phosphatase; AlP: alkaline phosphatase; PD: phosphodiesterase). Values are means ±S.D. Values with different letters differ significantly (p<0.05), as determined by the LSD test
Soil PD activity increased with increasing biochar treatment. P carried by biochar in the soil increases soil PD activity, and more adsorption of substrate is beneficial for enzyme-catalyzed reactions. Meanwhile, when the soil is slightly alkaline, porous and able to trap air and moisture, conditions are suitable for the assembly of microorganisms, which leads to an increase in microbial biomass[4-8]. Soil phosphatase is mainly derived from soil microbes and plant roots. The increase in soil microbial biomass also contributes to the sec-retion of PD. The increase in soil phosphatase activity also increases soil AP to a certain extent[9, 14-15]. Galvez et al. found that biochar input increased the soil AlP activity in two farmlands. Masto et al.[15]also showed that biochar input enhances soil AcP and AlP activity, which increases with increasing biochar application. In the current study, the amount of applied soil biochar had a significant positive correlation with soil AlP and PD activity (Figure 4).

Fig.4 Relationship between BC level and phosphomonoesterase (acid and alkaline) and phosphodiesterase activity in meadow soil
AP: available p-mg·kg-1; OP: organic p-g·kg-1; TP: total p-g·kg-1
3 Conclusions
Biochar application significantly increased the soil AP. Results obtained with fluorescence spectroscopy method demonstrated that biochar application significantly enhances AlP and PD activity, which increased with the increasing application of biochar. The application of suitable amounts of biochar also increased AcP activity to a certain degree.
[1] Beck MA,Sanchez PA. Plant and Soil, 1996, 184(1): 23.
[2] Chen W F, Zhang W M, Meng J, et al. Engineering Sciences, 2011, 9(2): 83.
[3] Streubel J D, Collins H P, Garcia-Perez M, et al. Soil Science Society of America Journal, 2011, 75(4): 1402.
[4] Angst T E, Sohi S P. GCB Bioenergy, 2013, 5(2):221.
[5] Laird D A, Fleming P, Davis D D, et al. Geoderma, 2010, 158(3): 443.
[6] Chintala R, Schumacher T E, McDonald L M, et al. Clean-Soil, Air, Water, 2014, 42(5): 626.
[7] Yao Y, Gao B, Zhang M, et al. Chemosphere, 2012, 89(11):1467.
[8] Hale S E, Alling V, Martinsen V, et al. Chemosphere, 2013, 9: 1612.
[9] Wei K, Chen Z H, Zhang X P, et al. Geoderma,2014, 217-218: 37.
[10] Muhittin O A, Ayten N. European Journal of Soil Science,in press, 2015.
[11] Zhang Y L, Chen L J, Duan Z H, et al. Spectrosc. Spectr. Anal.,2014, 34(2): 455.
[12] Kuo S. In: Methods of Soil Analysis, Part 3, Chemical Methods (Sparks, D. L., et al., eds.), SSSA Book series No 5. Soil Science of America, Madison, WI, 1996. 869.
[13] DeLuca T H, MacKenzie M D, Gundale M J. In: Biochar for Environmental Management: Science and Technology. London, UK: Earthscan,2009. 251.
[14] Galvez A, Sinicco T, Cayuela M I, et al. Agriculture, Ecosystems and Enviroment. 2012, 160: 3.
[15] Masto RE, Kumar S, Rout T K, et al. Catena,2013, 111: 64.
*通讯联系人
S153.6
A
生物炭施用对土壤磷及磷酸酶活性影响的可见和荧光光谱法研究
张玉兰1,陈利军1*,张玉革2,武志杰1,马星竹3,杨晓竹1
1. 中国科学院沈阳应用生态研究所,辽宁 沈阳 110016
2. 沈阳大学生物与环境工程学院,辽宁 沈阳 110044
3. 黑龙江省农业科学院土壤肥料与环境资源研究所,黑龙江 哈尔滨 150086
含碳丰富的秸秆在无氧或限氧的条件下低温热解后得到生物炭可施入土壤,有利于缓解秸秆处理压力、减少污染、减少温室气体排放,并改良土壤。在重要的农粮基地辽宁潮棕壤上布置了生物炭还田试验。玉米田施用不同量的生物炭(0,360,1 800和3 600 kg·ha-1)一个生长季后,研究土壤有效磷(P)、有机P和全P含量的变化,并采用荧光共轭物质作为测定底物,通过酶解产生荧光物质研究土壤磷酸酶活性的响应。结果表明,生物炭添加到土壤后,土壤有效P含量随生物炭施用量增加而显著升高、有机P和全P的含量没有显著的变化。其原因是生物炭携带有效P而引起的。碱性磷酸单酯酶和磷酸二酯酶活性随生物炭添加量的增加而增大,适量生物炭处理(1 800 kg·ha-1)可显著增加酸性磷酸酶,而高量生物炭处理(3 600 kg·ha-1)对酸性磷酸酶略有抑制,可能是生物炭自身的偏碱性使土壤pH值增大所致。生物炭的添加对土壤磷素和磷酸酶活性的影响是土壤物理性质、化学性质及土壤微生物群落结构和代谢能力的综合体现,需要进一步深入研究。
生物炭添加; 土壤磷; 土壤酶活性; 荧光方法
2015-02-10,
2015-06-08)
Foundation item: The National Natural Science Foundation of China (41301325, 41171241), The Special Fund for Agro-scientific Research in the Public Interest (201303095-9), The National Key Basic Research Development Program of China (973 Program) (2011CB100506) and The National Key Technologies R&D Program (2012BAD14B04,2012BAD14B02,2013BAD07B01)
10.3964/j.issn.1000-0593(2016)07-2325-05
Received: 2015-02-10; accepted: 2015-06-08
Biography: ZHANG Yu-lan, (1974—),female, PhD working at the Institute of Applied Ecology, Chinese Academy of Sciences e-mail: ylzhang@iae.ac.cn *Corresponding author e-mail: ljchenchina@hotmail.com; ljchen@iae.ac.cn

