Estimating risk factors of urban malaria in Blantyre,Malawi:A spatial regression analysis
Lawrence N. Kazembe, Don P. MathangaStatistics Department,University of Namibia,Windhoek,NamibiaMalaria Alert Centre,College of Medicine,University of Malawi,Blantyre,MalawiDepartment of Community Health,College of Medicine,University of Malawi,Blantyre,Malawi
Estimating risk factors of urban malaria in Blantyre,Malawi:A spatial regression analysis
Lawrence N. Kazembe1*, Don P. Mathanga2,31Statistics Department,University of Namibia,Windhoek,Namibia
2Malaria Alert Centre,College of Medicine,University of Malawi,Blantyre,Malawi
3Department of Community Health,College of Medicine,University of Malawi,Blantyre,Malawi
ARTICLE INFO
Article history:
Received 26 Jan 2016
Receivedinrevisedform24 Feb2016
Accepted 5 Mar 2016
Available online 31 Mar 2016
Keywords:
Urban malaria
Travel history
Spatial regression
Geo-additive models
Blantyre
Malawi
ABSTRACT
Objective:To estimate risk factors of urban malaria in Blantyre, Malawi, with the goal of understanding the epidemiology and ecology of the disease, and informing malaria elimination policies for African urban cities that have markedly low prevalence of malaria.
Methods:We used a case-control study design, with cases being children under the age of five years diagnosed with malaria, and matched controls obtained at hospital and communities. The data were obtained from Ndirande health facility catchment area. We then fitted a multivariate spatial logistic model of malaria risk. Covariate and risk factors in the model included child-specific, household and environmental risk factor(nearness to garden, standing water, river and swamps). The spatial component was assumed to follow a Gaussian process and model fitted using Bayesian inference.
Results:Our findings showed that children who visited rural areas were 6 times more likely to have malaria than those who did not[odds ratio(OR)= 6.66, 95%confidence interval(CI): 4.79–9.61]. The risk of malaria increased with age of the child(OR = 1.01, 95%CI: 1.003–1.020), but reduced with high socio-economic status compared to lower status(OR = 0.39, 95%CI: 0.25–0.54 for the highest level and OR = 0.67, 95%CI: 0.47–0.94 for the medium level). Although nearness to a garden, river and standing water showed increased risk, these effects were not significant. Furthermore, significant spatial clusters of risk emerged, which does suggest other factors do explain malaria risk variability apart from those established above.
Conclusions:As malaria in urban areas is highly fuelled by rural-urban migration, emphasis should be to optimize information, education and communication prevention strategies, particularly targeting children from lower socio-economic position.
Original article http://dx.doi.org/10.1016/j.apjtb.2016.03.011
Tel: +264 61 206 4515
E-mail: lkazembe@unam.na
The study protocol was performed according to the Helsinki declaration and approved by the Malawi College of Medicine Research Ethics Committee. Informed written consent was obtained from the guardians/care-givers/mothers of the children who participated in the study.
Foundation Project: Supported by National Institutes of Health(Grant No. 5R01TW7599).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
1. Introduction
Over the past decade, an increasing number of resources and efforts have been made to reduce malaria burden in the sub-Saharan Africa[1–3]. This has lead to renewed interest to better understand the epidemiology and ecology of malaria for better informed intervention strategies[2]. Particularly, malaria has often been seen as a rural phenomenon, however, because of the rapid urbanization there has been considerable shift towards urban malaria[4–7], more especially towards understanding the epidemiology and transmission of the disease[4,5]. Although there are malaria cases in urban areas, lack of malaria transmitting mosquitoes have been reported[6], suggesting other factors contributing to the disease ecology, including a variety of vector breeding sites mostly artificially made through, for example, tyre tracts, urban farming[7–10], but also back and forth human movement between rural and urban areas have been hypothesized as the potential risk factor [11–13]. However, the complexity of the disease necessitatesone to explore other risk factors sustaining malaria transmission. We hypothesize that rural–urban migration and nearness to putative sources of malaria transmission, for instance, nearness to a dam, river, garden, swamp water or standing water should be associated with increased malaria risk.
Moreover, malaria risk has shown spatial heterogeneity, which in part may be explained by variability in environmental or socioeconomic factors[10,13]. This association has not been examined at a small-area level. It is evident that risk may depict different spatial patterns when assessed at small scale than at large scale. We approach this problem by developing a generalized linear mixed model for spatial data introduced by Diggle and Ribeiro[14], which is referred to as a spatial generalized linear mixed model. The uses of such models in malaria epidemiology are increasing[15–20].
In this study, our central objective is to fit a spatial model to estimating risk factors associated with malaria, and map malaria risk in Ndirande township, in Blantyre, Malawi.
2. Materials and methods
2.1. Setting
This study was conducted in 2010, in Ndirande(Figure 1), a densely populated township, in Central Blantyre consisting of three wards: Ndirande north, Ndirande south, and Ndirande west. The population of Ndirande is 109164 which is approximately 15%of total population in Blantyre City based on the 2008 Malawi Population and Housing Census. The population among paediatrics is structured into 3562(for those aged<1 year), 13917(for the 1–4 years old), 14597(for the 5–9 years old)and 12403 for those between 10 and 14 years old, respectively. Ndirande township is mainly serviced by Ndirande Health Centre and its catchment area extends beyond the three wards mentioned above. This governmentrun clinic is the only health unit offering free paediatric services and routine diagnostic procedures in the town. Malaria cases occur year round at Ndirande clinic, peaking during the rainy season, typically from November to May and approximately 30%of all children seen at the clinic are treated for malaria.
2.2. Study design and dataset
The study was a prospective case-control design. Cases were defined as children, aged below five years, who had an axillary temperature of 37.5°C or a history of fever within the last 48 h and a positive blood smear(any parasitaemia)for Plasmodium falciparum. Each case was matched to a control for age(±6 months)and sex. Controls consisted of two groups: clinic and community controls. Clinic controls were defined as children who came to the same clinic with an illness that was not malaria and had a negative blood smear. Community controls were randomly selected by escorting malaria cases to their homes, after which a random direction was chosen by spinning a marked ball. The fifth household in the chosen direction was selected. Subsequent households were approached if necessary, until a matching control was found. Community controls were defined as children with a negative blood smear and an axillary temperature<37.5°C who had not been ill within the last two weeks. Both clinic and community controls were recruited within 14 days of identifying the case.

Figure 1. Map of Ndirande Township in Blantyre City, Malawi.
For each case and control, we obtained geographical coordinates of homes from which they come from. We also captured geo-coordinates of any potential putative malaria vector breeding sites including dam, river, standing/swamp water, or a garden nearby selected homes. In addition, using a structured questionnaire, we collected information on household assets, availability and use of insecticide treated nets, whether the child visited a rural area, and for how long, and whether a net was used when away.
The study protocol was performed according to the Helsinki declaration and approved by the Malawi College of Medicine Research Ethics Committee. Informed written consent was obtained from the guardians/care-givers/mothers of the children who participated in the study.
2.3. Spatial modelling
A spatial generalized linear mixed model for binary outcomes (1 = if case, 0 = for control)was developed[14,21,22]. Specifically, let y=(y(s1),y(s2),K,y(sn))′be a set of pointreferenced data observed at location si=(latitude1,longitude1)in an area. The observed data are assumed independent conditional on a Gaussian process M(s)such that p(y|M)is a Bernoulli variable. That is to say,

where πiis the probability of observing a case, and ηiis a predictor. The predictor can be expanded to take into account all possible explanatory variables,

such that β is the vector of fixed effects corresponding to variables xTi=(xi1,…xip). These covariates include child and household-specific factors. The parameters φ(M(si)), which follow a Gaussian process, are random effects that capture the unobserved spatial heterogeneity at location si.
Model(3)can be extended to a semi-parametric geoadditive regression model to analyse the data. The spatial effects can be written as an interaction term f(lat,long)and modelled by twodimensional surface estimators, specified as two-dimensional first order random walks, to have a semi-parametric model,

Inference on the spatial model uses the Bayesian approach, by drawing samples from the posterior distribution, which combines the likelihood and the prior assumptions. Particularly, it is based on the empirical Bayesian(EB)approach, also called the mixed model methodology[21–23]. The EB approach is achieved by recasting the predictor model[21,22]as generalized linear mixed model after appropriate reparametrization. Details of the EB estimation approach can be found in Brezger et al. [21]and Fahrmeir et al.[22].
In summary, for the fixed regression effects,β, we assumed diffuse priors, i.e., p(β)∝const. For the spatial random effects we assume a stationary Gaussian random field prior and twodimensional first order random walks are specified. We adopt the most commonly used prior specification based on the four nearest neighbours that is defined by

where B11,…B1K1are basis functions in latitude(lat)direction and B21,…B2K2in the longitude(long)direction. The terms βksare defined as first difference random walk coefficients.
2.4. Analysis of case-control malaria data
The proposed models were applied to analyse malaria casecontrol data. Our response variable is a binary(yi= 1 if case or 0 if control). Exploratory data analyses using Chi-square test of association were conducted to assess the association of the response variable with a set of explanatory variables and covariates. These included child-specific variables, maternal and household covariates, and environmental risk factors. Variables significant at P<0.2 were included in a multiple logistic spatial model. Implementation of these models were carried out in R2BayesX[23]. In R2BayesX, regression coefficients were estimated iteratively, and convergence was achieved when the change in regression parameters was 0.001 and terminated at 400 iterations if convergence was not achieved. However, our fitted model converged at less than 25 iterations.
3. Results
The study was conducted for a period of 1 year, and a total of 258 cases(33.6%)and 509 controls(66.6%)were identified. Figure 2 shows the locations of households of cases and controls in the area.
Table 1 presents Chi-square summaries. Travel to rural areas and socio-economic status(SES)were significantly associated with risk of malaria, but none of the other variables showed any significant association at P<0.05. However those with P-value less than 0.20 were included in the subsequent analysis. These included travel, SES, household nearness to garden, standing water and nearness to river, marital status, and woman employment status.

Figure 2. Locations for the malaria cases and controls in Ndirande, Blantyre.
Table 2 gives summaries of multivariate model. Malaria risk was associated with travel, with those who travelled out to rural areas six times more likely to have malaria than those who did not travel[odds ratio(OR)= 6.66, 95%confidence interval(CI): 4.79–9.61]. Age of the child also had a positive relationship of malaria risk, although the CI is very narrow(OR = 1.01, 95%CI: 1.00–1.02). The effect of SES showed a decreasing trend in risk with increasing SES class. Those at the highest and high classes showed OR = 0.39(95%CI: 0.25–0.54)and OR = 0.67(95%CI: 0.47–0.94)respectively. Malaria risk in children also displayed a positive association with unemployed women compared to employed women(Table 1). Nearness to either a swamp, river, standing water or garden did not show any significant relationship with malaria risk, although a house within 500 m of a swamp, river or garden displayed a positive association.
The spatial heterogeneity in malaria risk is captured in Figure 3. The risk considerably varied over this small area, with hotspots and cold spots of risk identified in the township. The cold spots at the corner can be explained by presence of Ndirande mountain, which do not provide suitable conditions for mosquito breeding. The hotspots located in the centre and bottom-right corner might be explained by presence of Nasolo river or maybe nearness to Ndirande health facility.

Table 1 Chi-square test of association between malaria and covariates.
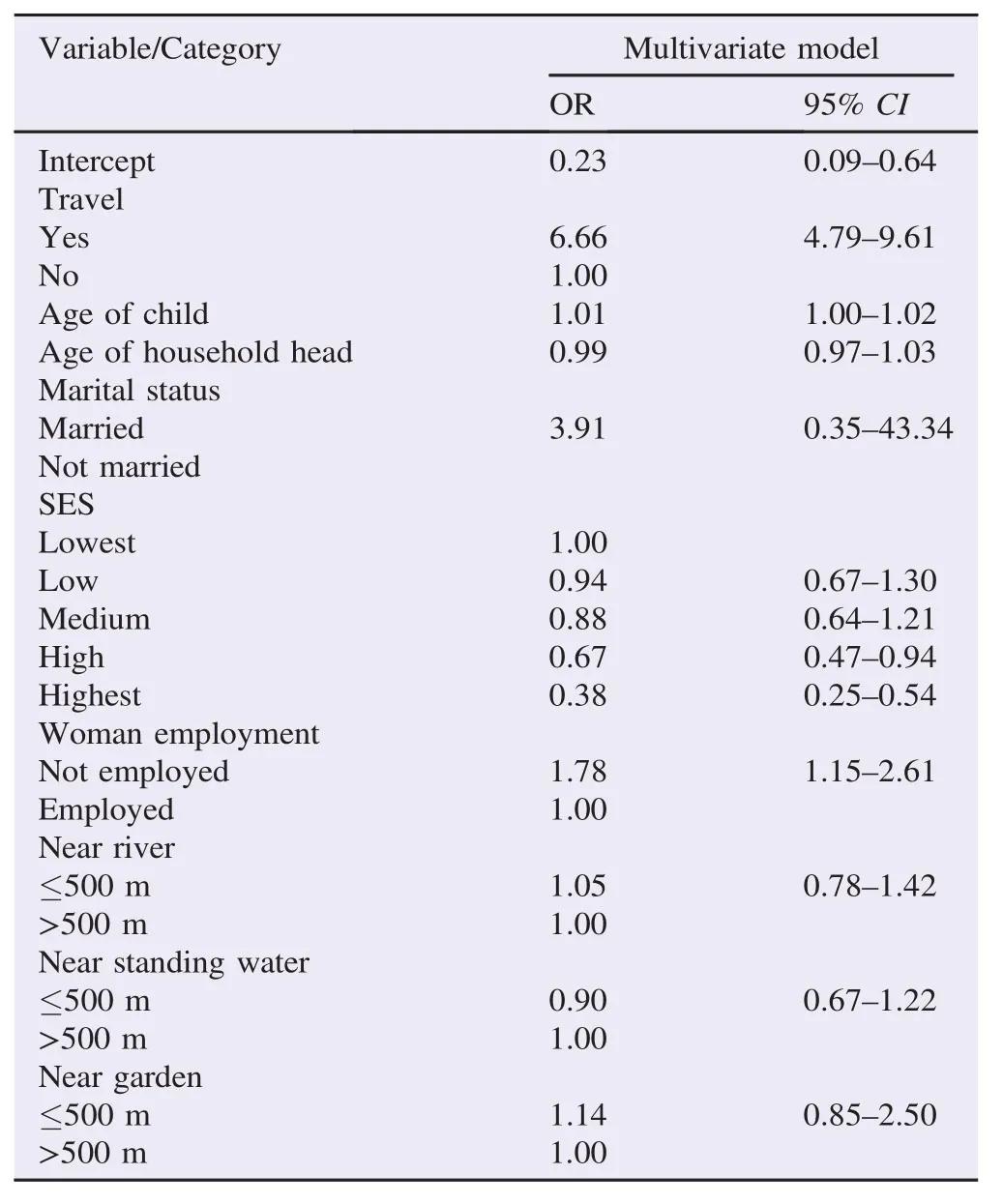
Table 2 Estimates from the multivariate spatial logistic regression of risk factors of urban malaria, Ndirande, Blantyre.
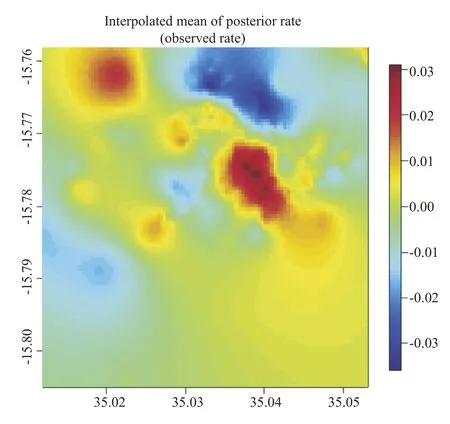
Figure 3. Residual spatial effects of urban malaria, presented on log odds scale.
4. Discussion
It is well established that urban areas have a markedly low prevalence[4–8], however, coupled with varied urban ecology, little is known about the risk factors associated with urban malaria in Blantyre, Malawi. Knowledge of these risk factors will help in the creation of malaria free urban areas and eventual elimination of malaria in African cities[4,5].
This article explored the epidemiological factors that explain malaria risk in Ndirande township, Blantyre, Malawi. Of interest in Blantyre City is that previous entomological studies have failed to establish presence of Anopheles vectors[6]. Our analysis, in principal explored the effect of travel to rural areas as a risk factor. There was overwhelming evidence that a visit to rural areas is the major source of malaria infection in urban areas, confirming recent reviews[4,8,9].
The significance of SES seems to suggest that malaria risk is highest among low status households, which in part can be explained by lack of adequate resources to prevent from malaria. It is therefore evident that children from low social status who have travelled are at a greater risk than those from high social status[7,10–13].
In our analysis, we explicitly controlled for spatial heterogeneity to capture any unobserved or unmeasured covariates that influence disease risk. The ecology of diseases particularly that of malaria, which is driven by both abiotic and biotic factors, may be best described by such a model[11,24]. This model tries to capture both observed and unobserved because as in any model misspecification of possible risk factors may arise[13–15,18,20]. It is evident that the pattern that emerged in Figure 3, demonstrate that malaria risk is driven by other factors beyond what we measured. What actually these are is a matter of conjecture, most probably defined towards knowledge, attitude, behaviour and practices on malaria[25–29]. We outline a few here. First, these disparities may be due to use of preventative measures such as insecticide treated nets[26,27]. Second, they may be to differential utilization of health care, in such a way that those that have easy accessed to health facility may report more often for treatment than those afar[28,29]. Be as it may, it should be observed that this study was carried out in an urban area with easy access and most households within 3 km of Ndirande health facility. Third, it can be due to gaps in knowledge, leading to delayed uptake of care [28]. Nevertheless, the spatial effects may also assist in generating hypothesis for further research, for instance of knowledge, attitude, behaviour and practices, and other ecological studies on malaria transmission[24].
In conclusion, malaria in urban areas is highly fuelled by travel, and the need for information, education and communication prevention strategies should be emphasized, particularly targeting children from lower socio-economic position. Message campaigns on malaria prevention that are targeted at travellers would help in raising awareness of what is required to prevent malaria transmission[29].
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We acknowledge support from the National Institutes of Health(Grant No. 5R01TW7599).
References
[1]Snow RW, Guerra CA, Mutheu JJ, Hay SI. International funding for malaria control in relation to populations at risk of stable Plasmodium falciparum transmission. PLoS Med 2008;5: e142.
[2]Snow RW, Okiro EA, Gething PW, Atun R, Hay SI. Equity and adequacy of international donor assistance for global malaria control: an analysis of populations at risk and external funding commitments. Lancet 2010;376: 1409-16.
[3]Omumbo JA, Noor AM, Fall IS, Snow RW. How well are malaria maps used to design and finance malaria control in Africa?PLoS One 2013;8(1): e53198.
[4]Wilson ML, Krogstad DJ, Arinaitwe E, Arevalo-Herrera M, Chery L, Ferreira MU, et al. Urban malaria: understanding its epidemiology, ecology, and transmission across seven diverse ICEMR network sites. Am J Trop Med Hyg 2015;93(Suppl 3): 110-23.
[5]Kigozi SP, Pindolia DK, Smith DL, Arinaitwe E, Katureebe A, Kilama M, et al. Associations between urbanicity and malaria at local scales in Uganda. Malar J 2015;14: 374.
[6]Tambala P, Macheso A, Ziba C. Malaria vector assessment. Lilongwe: Ministry of Health Government of Malawi;1992.
[7]Fobil JN, Levers C, Lakes T, Loag W, Kraemer A, May J. Mapping urban malaria and diarrhoea mortality in Accra, Ghana: evidence of vulnerabilities and implications for urban health policy. J Urban Health 2012;89: 977-91.
[8]Tatem AJ, Gething PW, Smith DL, Hay SI. Urbanization and the global malaria recession. Malar J 2013;12: 133.
[9]Pond BS. Malaria indicator surveys demonstrate a markedly lower prevalence of malaria in large cities of sub-Saharan Africa. Malar J 2013;12(1): 313.
[10]Matthys B, Vounatsou P, Raso G, Tschannen AB, Becket EG, Gosoniu L, et al. Urban farming and malaria risk factors in a medium-sized town in Cˆote d′Ivoire. Am J Trop Med Hyg 2006;75(6): 1223-31.
[11]Martens P, Hall L. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis 2000;6(2): 103-9.
[12]De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-Saharan Africa: a systematic review. J Trop Med 2012;2012: 819563.
[13]Ngom R, Siegmund A. The key role of socio-demographic and socio-environmental factors in urban malaria occurrence and control–an illustration using the city of Yound´e. Soc Sci Med 2015;133: 269-79.
[14]Diggle P, Ribeiro P. Model-based geostatistics. New York: Springer-Verlag;2007, p. 232.
[15]Chirombo J, Lowe R, Kazembe L. Using structured additive regression models to estimate risk factors of malaria: analysis of 2010 Malawi malaria indicator survey data. PLoS One 2014;9(7): e101116.
[16]Nkurunziza H, Gebhardt A, Pilz J. Geo-additive modelling of malaria in Burundi. Malar J 2011;10: 234.
[17]Sedda L, Qi Q, Tatem AJ. A geostatistical analysis of association between armed conflicts and Plasmodium falciparum malaria in Africa, 1997–2010. Malar J 2015;14(1): 500.
[18]Giardina F, Kasasa S, Si´e A, Utzinger J, Tanner M, Vounatsou P. Effects of vector-control interventions on changes in risk of malaria parasitaemia in sub-Saharan Africa: a spatial and temporal analysis. Lancet Glob Health 2014;2(10): e601-15.
[19]Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, et al. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000–10: a spatial and temporal analysis of transmission intensity. Lancet 2014;383(9930): 1739-47.
[20]Gething PW, Patil AP, Hay SI. Quantifying aggregated uncertainty in Plasmodium falciparum malaria prevalence and populations at risk via efficient space-time geostatistical joint simulation. PLoS Comput Biol 2010;6(4): e1000724.
[21]Brezger A, Kneib T, Lang S, Bayes X. Analysing Bayesian structured additive regression models. J Stat Softw 2005;14(11): 1-11.
[22]Fahrmeir L,Kneib T,Lang S.Penalizedstructuredadditiveregression forspace-timedata:a Bayesianperspective.Stat Sin2004;14:731-61.
[23]Umlauf N, Adler D, Kneib T, Lang S, Zeileis A. Structured additive regression models: an R interface to BayesX. J Stat Softw 2015;63(21): 1-42.
[24]Ferrari G, Ntuku HM, Schmidlin S, Diboulo E, Tshefu AK, Lengeler C. A malaria risk map of Kinshasa, Democratic Republic of Congo. Malar J 2016;15(1): 27.
[25]Heggenhougen HK, Hackethal V, Vivek P. The behavioural and social aspects of malaria and its control:an introduction and annotated bibliography. Geneva: World Health Organization;2003.
[26]Mwenesi HA. Social science research in malaria prevention, management and control in the last two decades: an overview. Acta Trop 2005;95(3): 292-7.
[27]Brenyah RC, Osakunor DN, Ephraim RK. Factors influencing urban malaria: a comparative study of two communities in the Accra Metropolis. Afr Health Sci 2013;13(4): 992-8.
[28]Ahmed SM, Haque R, Haque U, Hossain A. Knowledge on the transmission, prevention and treatment of malaria among two endemic populations of Bangladesh and their health-seeking behaviour. Malar J 2009;8: 173.
[29]Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, et al. Operational strategies to achieve and maintain malaria elimination. Lancet 2010;376(9752): 1592-603.
*Corresponding author:Lawrence N. Kazembe, Statistics Department, University of Namibia, Windhoek, Namibia.
 Asian Pacific Journal of Tropical Biomedicine2016年5期
Asian Pacific Journal of Tropical Biomedicine2016年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimalarial qinghaosu/artemisinin:The therapy worthy of a Nobel Prize
- Insecticide susceptibility in larval populations of the West Nile vector Culex pipiens L. (Diptera:Culicidae)in Saudi Arabia
- Agave sisalana extract induces cell death in Aedes aegypti hemocytes increasing nitric oxide production
- Phenolics-saponins rich fraction of defatted kenaf seed meal exhibits cytotoxicity towards cancer cell lines
- Comparative investigation of the free radical scavenging potential and anticancer property of Diospyros blancoi(Ebenaceae)
- Breastfeeding counsel against cancers
