320排容积CT双血供灌注评估肺占位性病变的良恶性及与微血管密度的相关性
刘 慧 林 江△ 陆秀良 顾君英 姚家美
(1复旦大学附属中山医院放射科,2病理科 上海 200032)
320排容积CT双血供灌注评估肺占位性病变的良恶性及与微血管密度的相关性
刘慧1林江1△陆秀良1顾君英1姚家美2
(1复旦大学附属中山医院放射科,2病理科上海200032)
【摘要】目的评价肺占位性病变双重血供CT灌注(dual-input CT perfusion,DI-CTP)的可重复性、鉴别良恶性病变的能力及与病变微血管密度(microvessel density,MVD)的相关性。方法116例经病理证实的肺占位性病变患者接受320排容积CT的DI-CTP检查,由两名观察者单独进行DI-CTP参数测量,获得病变的肺动脉血流量(pulmonary flow,PF)、支气管动脉血流量(bronchial flow,BF)及血流灌注指数(perfusion index,PI),并计算灌注总量(total perfusion,TPF)。评价观察者内及观察者间的可重复性;分析良恶性病变DI-CTP参数的差异;并对其中94例外科手术切除病灶进行CD34免疫组化染色分析DI-CTP参数与MVD间的相关性。结果观察者间和观察者内的可重复性达到良好以上(ICC>0.90)。良恶性肺占位的BF、PF、PI差异有统计学意义(P<0.05),其中PI的ROC曲线下面积为0.936。良恶性肺占位间的MVD差异具有统计学意义(P<0.05);BF、PF及TPF与MVD呈正相关。结论320排容积DI-CIP可重复性良好,其参数可反映肺占位性病变的血管生成情况,并为鉴别肺占位的良恶性提供依据。
【关键词】肺部病变;CT灌注成像;双重血供;可重复性;微血管密度
CT 灌注成像(CT perfusion,CTP)作为一种无创性评价组织血流灌注的功能成像方式,已经成为评价肿瘤血管生成及抗血管生成治疗药物效果的主要检查方法[1]。以往肺癌CTP由于受扫描技术限制,一般只能采用主动脉作为输入动脉,即采用单血供灌注(single-input CT perfusion,SI-CTP)模型,该模型只能反映肺肿瘤的支气管动脉供血情况[2-3]。然而大量研究均证实肺癌的供血来自支气管动脉和肺动脉[4-6]。
320排容积CT成像由于探测器宽度达到16 cm,在无需移床的情况下,一次曝光扫描范围即可覆盖成人肺脏的大部,单次容积扫描一般可同时包含肺内病灶、肺动脉和主动脉,该扫描技术能够同时捕捉到肺动脉、主动脉以及病灶的动态强化特征,在此基础上建立的双血供灌注(dual-input CT perfusion,DI-CTP)模型可以获得反映肺部病变两套血供的参数,对于肺内病灶的诊治有重要意义。
目前使用320排容积CT进行肺占位DI-CTP的研究才刚刚起步,国内外文献报道不多[7-9],且主要用于良恶性病变的鉴别诊断,尚未见系统性地评估肺占位性病变DI-CTP观察者可重复性的研究,尚未见将DI-CTP参数与病变的微血管密度(micro-vessel density,MVD)这一评价病变血管生成“金标准”进行对照的报道。本研究目的是评价肺占位性病变DI-CTP观察者的可重复性和对肺占位性病变的鉴别诊断价值,并研究其与MVD之间的相关性。
资 料 和 方 法
一般资料本研究为前瞻性,经复旦大学附属中山医院医学伦理委员会批准。共116例肺占位性病变患者接受灌注成像检查,其中男93例,女23例,平均年龄60岁(42~80岁)。纳入标准:(1)病灶最大径≥1cm,为实性病灶;(2)检查前未接受任何治疗;(3) 肝、肾功能良好,无对比剂过敏史;(4)有能力配合检查。所有病例在CT检查后均经病理证实(外科手术切除94例,经支气管镜活检16例,CT引导下穿刺活检6例),其中恶性病变95例,包括腺癌39例、鳞癌34例、腺鳞癌3例、小细胞肺癌14例、类癌3例、肉瘤样癌l例、不能分型肺癌l例;良性病变21例,包括错构瘤4例、炎性病变14例、肺结核2例和肺硬化性血管瘤l例。
检查方法采用Toshiba Aquilion One 320排螺旋CT扫描仪,先进行常规平扫定位,确定灌注扫描范围,采用高压注射器从单侧上肢静脉注射60 mL碘普罗胺(370 mg/mL),注射流率8 mL/s,开始注药时即屏气,2 s后启动17个回合动态容积扫描(图1)。扫描参数:管电压80 kV,管电流80 mA,探测器宽度16 cm,转速0.5 s/r,层厚0.5 mm。一次灌注扫描的平均辐射量是5.07 mSv。
图像后处理及分析采用Toshiba双血供灌注软件,在肺门水平的肺动脉主干、降主动脉、左心房及肺内病灶内绘制感兴趣区(regions of interest,ROI)生成4条时间-密度曲线(time-density curve,TDC)(图2)。设置灌注窗宽范围为0~150 HU,以排除骨质及肺组织的干扰,确保病灶灌注测量图得到良好显示。运行灌注软件,自动生成512×512矩阵编码的伪彩图像,分别显示肺动脉血流量(pulmonary flow,PF)、支气管动脉血流量(bronchial flow,BF)及灌注指数[perfusion index,PI=PF/(PF+BF)](图3),并计算灌注总量(total perfusion flow,TPF)。为了解观察者间一致性(inter-observer agreement),患者的图像分析由两名高年资放射科医师采用盲法独立完成上述所有后处理程序,在病灶的3个代表层面各测量1次,最终采用3次测量值的平均值;为了解观察者内一致性(intra-observer agreement),并最大程度降低回忆偏倚,其中1位医师在1个月后再次对所有图像进行测量和分析。
Seventeen intermittent low-dose volume acquisitions were made 2 s after the bolus injection (1.5 s per acquisition for the first 8 acquisitions and 2s per acquisition for the subsequent 9 acquisitions).
图1动态容积扫描流程式图
Fig 1Dynamic volumetric scanning process diagram
The peak enhancement time point of the left atrium (indicated by the max line) was located between the two peaks of PA and aorta, distinguishing between pulmonary and bronchial circulation. The lesion had two ascending slopes representing pulmonary and bronchial circulation respectively. The latter was much steeper than the former, which suggests that bronchial circulation was dominant in this case.
图2输入动脉(肺动脉,主动脉)、左心房及病灶的时间密度曲线
Fig 2Time-density curves of input arteries
(PA and aorta),left atrium and lung lesion
BF was 40.9 mL·min-1·100 mL-1, PF was 27.4 mL·min-1·100 mL-1and PI was 42.2%. Microvessel density with CD34 immunostaining was 73 (Original magnification, ×200).
图363岁男性鳞癌病例
Fig 3Squamous cell carcinoma of a 63-year-old man
病理标本免疫组化处理及观察94例外科手术切除病灶进行CD34染色,其中恶性病变80例(腺癌34例、鳞癌31例、小细胞肺癌7例、腺鳞癌3例、类癌3例、肉瘤样癌l例、不能分型肺癌l例),良性病变14例(肺结核1例、炎性病变9例、错构瘤4例)。以PBS液代替一抗作为阴性空白对照,MVD计数采用Weidner方法,结果判定在盲法下进行。
统计学分析使用SPSS 21.0及MedCale 14.8.1软件完成。采用Bland-Altman法及组内相关系数(intraclass correlation coefficient,ICC)分析DI-CTP检查的观察者一致性;采用非参数秩和检验(Wilcoxon法)比较恶性与良性肺占位性病变DI-CTP参数,并绘制有鉴别诊断价值参数的受试者工作特性曲线(receiver operating characteristic curve,ROC曲线);运用Youden指数决定ROC曲线的最佳工作点(optimal operating point,OOP),并计算OOP、相应敏感性、特异性、似然比(1ikelihood ratio,LR)、阳性预测值(positive predictive value,+PR)和阴性预测值(negative predictive value,-PR);采用Spearman相关系数分析各DI-CTP参数与MVD的相关性。
结果
DI-CTP结果观察者间和观察者内的可重复性均达到良好以上(ICC>0.90),观察者间测定病灶的PF、BF、PI值的ICC分别为0.96、0.98和0.95;观察者内ICC则分别为0.97、0.99和0.97。病变最大径(40.96±17.74) mm,范围14~120 mm。良、恶性肺占位的支气管动脉血流量BF、肺动脉血流量PF、灌注指数PI均数间差异具有统计学意义(P<0.05);灌注总量TPF均数间的差异无统计学意义,具体统计结果见图4、表1。BF、PF、PI值鉴别良恶性肺占位的ROC曲线下面积(area under curve,AUC)分别为0.644、0.711、0.936,其中PI数值最大,具有最高的诊断准确性(图5)。PI=51.15%时,鉴别良、恶性肺占位的敏感度是0.95,特异度是0.84,+LR是17.54,-LR是0.17,+PR是17.54,-PR是0.17。
PF:Pulmonary flow;BF: Bronchial flow;PI:Perfusion index;TPF:Total pulmonary flow.
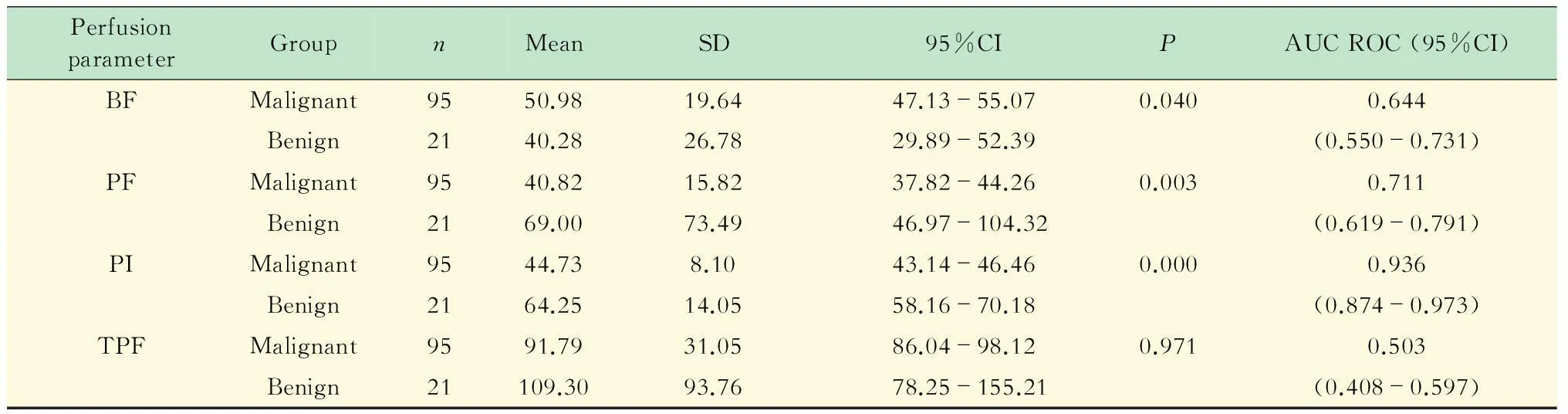
图4 良恶性病变CTP参数箱线图(n=116)
BF,PF,TPF:mL·min-1·100 mL-1;PI:100 %.
Parameters for identifying lung lesions.
图 5CT灌注成像四个参数诊断肺占位性病变的ROC曲线
Fig 5ROC curves of the four perfusion parameters
for identifying lung lesions
MVD计数结果良恶性肺占位的MVD分别为(139.49±107.85)条/视野和(62.04±35.43)条/视野,差异具有统计学意义(P=0.011)。
DI-CTP参数与MVD相关性分析 94例免疫组化染色的肺占位性病变BF、PF、TPF与MVD呈正相关(P值均<0.05),PI与MVD无相关性(P>0.05,表2)。

表2 CT灌注参数与MVD相关性分析
讨论
应用CTP对肺占位性病变进行鉴别诊断的病理生理基础是良恶性病变的血管生成和血管反应的性质不同造成其血液动力学差异。既往研究已证实肺部CTP检查可以用来鉴别诊断良恶性病变[10-12],并认为CTP参数与MVD具有较好的相关性[2,13]。
肺占位性病变320排容积CT双血供灌注成像研究的技术优势肺占位血流灌注不均匀[2],如CTP扫描只覆盖其中心区域,则可重复性有限,故需要扩大扫描覆盖范围,实现病灶容积CTP[13-14]。320排CT的覆盖范围达16 cm,可实现真正意义的容积灌注,另外,320排容积CT的球管转速为0.5 s/r,扫描时间间隔大大缩短,时间分辨率进一步提高,故能更真实准确地反映病变的血供情况,其可重复性明显提高。灌注研究一直受辐射剂量的限制,本研究采用低剂量扫描,平均辐射量是5.07 mSv,为欧共体标准的55.7%,极大地降低了受检者的辐射剂量。
目前,国内外灌注研究[2,11,15]多应用SI-CTP模型计算灌注参数,然而SI-CTP模型通常只能反映肺癌的优势供血来源,而供血相对少的那一部分血流灌注则被忽略。Yuan等[7]认为DI-CTP模型可以很好地应用于肺癌的灌注研究,Ohno等[16]对肺孤立结节的灌注研究发现DI-CTP模型比SI-CTP模型具有更好的定性诊断价值。本研究也发现,使用DI-CTP模型可以将肺占位性病变内肺动脉和支气管动脉供血分开观察,测量的灌注值能够更全面、准确地反映肺占位的血供特点,且使用DI-CTP模型进行灌注研究的可重复性很好,可能更有利于病灶血供的观察与比较。
CTP参数在肺占位病变鉴别诊断中的价值肺部病变具有肺动脉及支气管动脉双重血供,并且恶性病变多以支气管动脉供血为主,良性病变多以肺动脉供血为主[6,8]。本研究中BF反映了支气管动脉供血丰富程度,PF反映了肺气管动脉供血丰富程度,PI反映了肺动脉血流量在总灌注量中所占比例。本研究显示恶性病变组的BF值高于良性病变组,而PF及PI值低于良性病变组,该结果与不同性质肺占位的病理生理基础相一致,其中PI的ROC AUC最大,具有最高的诊断准确性,并且可用PI<51.15%作为良恶性病变的诊断阈值。本研究中恶性病变的PF及PI值均高于Yuan 等[8]的研究,LR和PR值也有所差异,这可能与对比剂注射方法和扫描技术不同有关,也可能与病变大小、位置和个体生理差异等因素有关。
肺占位性病变CTP参数与MVD相关性分析肿瘤内MVD可定量反映肿瘤血管生成状态,是目前评价肿瘤血管生成的“金标准”[14,17-18]。MVD可以通过一些特异性抗体进行标记,且多采用Weidner的计数标准[19]。CTP成像则是可以定量反映病变微循环特征的功能成像方法。既往CTP成像运用的是单血供模型,对肺占位病变CTP参数与MVD相关性分析显示,血流量和血容量与MVD有较好的相关性[12,20-21]。本文首次应用DI-CTP模型对其参数与MVD做相关性研究,结果发现肺占位病变BF、PF、TPF与MVD呈正相关;且良恶性肺占位的MVD差异具有统计学意义;另外TPF与MVD相关系数最高,故BF、PF及TPF可以无创性间接反映肺占位性病变的MVD这一病理特征,并具备帮助鉴别病变的良恶性、指导肿瘤治疗和评判疗效的潜能。本研究中良性病例多为炎性病变,可能导致良性病变的血流总量及MVD高于恶性病例。
综上所述,320排容积DI-CTP可重复性好,其参数可反映肺占位性病变的血管生成情况,并对肺占位的良恶性鉴别提供帮助。
参考文献
[1]MILES KA,LEE TY,GOH V,etal.Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography[J].EurRadiol,2012,22(7):1430-1441.
[2]MILES KA,GRIFFITHS MR.Perfusion CT:a worthwhile enhancement?[J].BrJRadiol,2003,76(904):220-231.
[3]MA SH,LE HB,JIA BH,etal.Peripheral pulmonary nodules:relationship between multi-slice spiral CT perfusion imaging and tumor angiogenesis and VEGF expression[J].BMCCancer,2008,8:186.
[4]MILNE EN,ZERHOUNI AE.Blood supply of pulmonary metastases[J].ThoracicImaging,1987,2(4):15-23.
[5]TACELLI N,REMY-JARDIN M,COPIN M C,etal.Assessment of non-small cell lung cancer perfusion:pathologic-CT correlation in 15 patients[J].Radiology,2010,257(3):863-871.
[6]KIESSLING F,BOESE J,CORVINUS C,etal.Perfusion CT in patients with advanced bronchial carcinomas:a novel chance for characterization and treatment monitoring?[J].EurRadiol,2004,14(7):1226-1233.
[7]YUAN X,ZHANG J,AO G,etal.Lung cancer perfusion:can we measure pulmonary and bronchial circulation simultaneously?[J].EurRadiol,2012,22(8):1665-1671.
[8]YUAN X,ZHANG J,QUAN C,etal.Differentiation of malignant and benign pulmonary nodules with first-pass dual-input perfusion CT[J].EurRadiol,2013,23(9):2469-2474.
[9]柳维义,谭理连,李志铭,等.320排螺旋 CT 低剂量容积灌注在孤立性肺结节鉴别诊断中的价值[J].实用放射学杂志,2014,30(5):755-758,803.
[10]SITARTCHOUK I,ROBERTS HC,PEREIRA AM,etal.Computed tomography perfusion using first pass methods for lung nodule characterization[J].InvestRadiol,2008,43(6):349-358.
[11]SHAN F,ZHANG Z,XING W,etal.Differentiation between malignant and benign solitary pulmonary nodules:use of volume first-pass perfusion and combined with routine computed tomography[J].EurJRadiol,2012,81(11):3598-3605.
[12]LI Y,YANG ZG,CHEN TW,etal.First-pass perfusion imaging of solitary pulmonary nodules with 64-detector row CT:comparison of perfusion parameters of malignant and benign lesions[J].BrJRadiol,2010,83(993):785-790.
[13]YI CA,LEE KS,KIM EA,etal.Solitary pulmonary nodules:dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density[J].Radiology,2004,233(1):191-199.
[14]NG QS,GOH V,KLOTZ E,etal.Quantitative assessment of lung cancer perfusion using MDCT:does measurement reproducibility improve with greater tumor volume coverage?[J].AJRAmJRoentgenol,2006,187(4):1079-1084.
[15]KAN Z,PHONGKITKARUN S,KOBAYASHI S,etal.Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model[J].Radiology,2005,237(1):151-158.
[16]OHNO Y,NISHIO M,KOYAMA H,etal.Comparison of quantitatively analyzed dynamic area-detector CT using various mathematic methods with FDG PET/CT in management of solitary pulmonary nodules[J].AJRAmJRoentgenol,2013,200(6):W593-W602.
[17]JAIN RK.Normalization of tumor vasculature:an emerging concept in antiangiogenic therapy[J].Science,2005,307(5706):58-62.
[18]WEIDNER N.Intratumor microvessel density as a prognostic factor in cancer[J].AmJPathol,1995,147(1):9-19.
[19]WEIDNER N,SEMPLE JP,WELCH WR,etal.Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma[J].NEnglJMed,1991,324(1):1-8.
[20]SPIRA D,NEUMEISTER H,SPIRA SM,etal.Assessment of tumor vascularity in lung cancer using volume perfusion CT (VPCT) with histopathologic comparison:a further step toward an individualized tumor characterization[J].JComputAssistTomogr,2013,37(1):15-21.
[21]BAI RJ,CHENG XG,QU H,etal.Solitary pulmonary nodules:comparison of multi-slice computed tomography perfusion study with vascular endothelial growth factor and microvessel density[J].ChinMedJ(Engl),2009,122(5):541-547.
Dual-input perfusion of lung lesions with 320-detector-row CT:its value in differentiating malignant from benign lesions and correlation with lesion micro-vessel density
LIU Hui1, LIN Jiang2△, LU Xiu-liang3, GU Jun-ying4, YAO Jia-mei5
(1DepartmentofRadiology,2DepartmentofPathology,ZhongshanHospital,FudanUniversity,Shanghai200032,China)
【Abstract】ObjectiveTo investigate the reproducibility of dual-input CT perfusion (DI-CTP) of lung lesions with 320-detector-row CT,its value in differentiation of malignant and benign lesions and the correlation between CTP parameters and microvessel density (MVD).MethodsOne hundred and sixteen patients with various lung lesions confirmed later by pathology underwent DI-CTP with 320-detector-row CT.The pulmonary trunk and the descending aorta were selected as input arteries for measuring contributions from pulmonary and bronchial circulation to the lesions.Pulmonary flow (PF),bronchial flow (BF),and perfusion index (PI) were recorded by two independent radiologists,and then total perfusion (TPF) was calculated.Intraclass correlation coefficient (ICC) and Bland-Altman statistics were used to evaluate intra-and inter-observer agreement.DI-CTP parameters were compared between malignant and benign lesions.The correlation between DI-CTP and MVD was analysed in 94 cases of lesions with immunohistochemical staining of CD34.ResultsBoth intra- and inter-observer agreements were good to excellent (ICC>0.90).PF and PI of benign lesions were higher than those of malignant lesions.BF of malignant lesions was higher than that of benign lesions.Statistically significant differences of BF,PF and PI were found between malignant and benign lesions (P<0.05) with the area under the ROC curve of PI being 0.936,the largest of the three perfusion parameters.There was statistically significant difference in MVD between benign and malignant lesions (P<0.05).BF,PF and TPF values were positively correlated with MVD .ConclusionsDI-CTP is reproducible and reflects the angiogenesis of lung lesions.It can provide additional information for differential diagnosis of malignant from benign lung lesions.
【Key words】lung lesions;CT perfusion;dual-input;reproducibility;micro-vessel density
【中图分类号】R812
【文献标识码】A
doi:10.3969/j.issn.1672-8467.2016.03.012
(收稿日期:2015-11-03;编辑:王蔚)
△Corresponding authorE-mail:lin.jiang@zs-hospital.sh.cn

