Processed beetroot(Beta vulgaris L.)as a natural antioxidant in mayonnaise:Effects on physical stability,texture and sensory attributes
Vassilios Raikos,Angela McDonagh,Viren Ranawana,Garry Duthie
Metabolic Health,Rowett Institute of Nutrition and Health,University of Aberdeen,Aberdeen AB25 2ZD,Scotland,UK
Abstract The oxidative and physical stability of the reformulated mayonnaise with processed beetroot was investigated and compared with a control(mayonnaise without beetroot) and a commercially available product.Processing of beetroot had an impact on the structural integrity of the antioxidants present.Microwaving(960 W for 7 min)was advantageous for preserving the betalain and polyphenol content of beetroot compared to roasting(180°C for 90 min)and boiling(100°C for 30 min).The oxidative stability of mayonnaise samples was determined by Rancimat and the thiobarbituric (TBA) assay.The addition of microwaved beetroot significantly enhanced the oxidative stability of mayonnaise at the end of a storage period of 4 weeks (4°C).Although no significant differences (P>0.05) were detected between the mayonnaise samples containing beetroot and the commercial control,the latter was less susceptible to oxidation during storage.The turbiscan stability index(TSI)revealed that the commercial mayonnaise was less prone to destabilization phenomena.All the textural parameters increased with the incorporation of beetroot.The sensory evaluation revealed that,with the exception of graininess and uniformity,most of the sensory attributes are preserved if not improved with the addition of beetroot.
Keywords: Mayonnaise;Beetroot;Oxidative stability;Texture;Sensory analysis
1.Introduction
Mayonnaise is one of the most widely appreciated condiments added to various foods to improve the flavour and taste.Typically, the ingredients used for mayonnaise formulation include egg,either as a whole or egg yolk,vinegar,water,spices and vegetable oil [1].In the traditional recipe, the fat content varies from 65% to 80% and commonly used oils for mayonnaise production are soyabeen, rapeseed, sunflower and corn.These oils are produced at low cost and are preferred for their sensory properties and perceived texture of the final product.
Although the nutritional value of vegetable oils is highly appreciated mainly because of the high content of polyunsaturated fatty acids(PUFA),their utilisation as food ingredient may be problematic due to increased susceptibility to rapid oxidative deterioration [2,3].Lipid oxidation is known to impair product quality through the production of rancid odours,unpleasant flavours and even compromise the safety of foods because of the formation of harmful compounds [4].Furthermore, the oxidation process in multiphase food systems such as mayonnaise is not solely dependent on fatty acid composition.Numerous other factors include droplet and interfacial properties, the presence of antioxidants and/or pro-oxidants,ingredient partitioning and interactions and others are known to affect the oxidation process in food emulsions[5].
Numerous attempts have been made to develop healthier versions of mayonnaise and most of the efforts have focused on reducing the considerably high fat content[6].Lately there is an increased public concern for food products formulated with nonnatural,synthetic ingredients which are included in the recipe to perform different functionalities.In mayonnaise, the synthetic antioxidant ethylene diamine tetraacetic acid(EDTA)is used to control the oxidation process and prevent rapid deterioration.Despite the fact that synthetic antioxidants are cheap and effective, they are not widely accepted by consumers and therefore the food industry is currently looking for potential natural alternatives.Plant materials rich in phenolic compounds and other natural antioxidants have gained much attention because they exhibit a wide range of activities such as antioxidative,antimicrobial,antimutagenic,and anti-inflammatory activities[7].In particular, red beet is one of the most potent vegetables with respect to antioxidant activity mainly due to the presence of betalains [8–11].Betalains are water soluble, nitrogen containing pigments,currently approved for food applications(colourants)by the European Union and are labelled as E-162[12].Furthermore, the inclusion of vegetables in composite food products such as mayonnaise can be cost-effective.Approximately 30%of the vegetable production is not marketed simple because they do not meet the aesthetic criteria.However, poor organoleptic properties of the harvested vegetables are not an exclusion criterion for the formulation of processed products like mayonnaise.In this way, misshapen or blemished but nutritious vegetables can be re-introduced into the food market as ingredients of processed foods at a low cost and food waste can be reduced.
Previous studies have indicated that beetroot, either raw or freeze-dried, may be successfully implemented in the mayonnaise recipe to replace synthetic antioxidants and prolong the shelf-life[13].The objective of this study was to determine the effect of different cooking methods on the antioxidant activity of beetroot in order to identify optimum processing conditions for mayonnaise reformulation.Furthermore,the impact of beetroot supplementation on the physical stability,textural and sensory properties of the end product was also investigated.A commercially available mayonnaise product was used a reference for comparative purposes.
2.Materials and metho ds
2.1.Materials
Pasteurised spray dried whole egg powder was purchased from International Egg Products(Essex,UK).Commercial mayonnaise(C2),fresh beetroot,organic rapeseed oil,vinegar and table salt were obtained the local supermarkets (Tesco, UK; Morrisons, UK).Folin-Denis’ reagent, gallic acid, 2-thiobarbituric acid and 1,1,3,3-tetramethoxypropane(TEP)were supplied by Sigma Aldrich (St.Louis, MO, USA).All reagents used were of analytical grade.
2.2.Beetroot preparation
The leaves and most of the stalk were removed from the beetroot.The beetroot was washed, dried and cooked using three different methods: microwave (M), oven-baking (R) and boiling(B).For microwaving,the beetroot was microwaved at 960 W for 7 min.Oven-baked samples were cooked at 180°C for 90 min.Boiled samples were placed in boiling water for 30 min and drained.The cooked beetroot was allowed to cool,vacuum packed and stored at −17°C until required for emulsion preparation.Aliquots of the three types of cooked beetroot were freeze-dried for 72 h using a HS−1freeze drier(Frozen in Time Ltd.,UK)and were ground into a fine powder with a Wahl James Martin spice grinder(Argos,UK)for determination of betalain,polyphenol and total antioxidant content.
2.3.Extraction of polyphenols and betalains from beetroot powders and characterisation
1 g of the freeze dried beetroot powders were added to 20 mL of a 50%(v/v)ethanol solution and mixed(Spiramin 5,Denley Instruments Ltd.,West Sussex,UK)for 30 min.Samples were sonicated in a water bath for 30 min to enhance dissolution and centrifuged at 3000×gfor 10 min.The residue was re-extracted with fresh solvent following the same procedure and the extracts were combined and stored at 4°C in a tube which was covered in aluminium foil.
2.3.1.Ferric reducing antioxidant power(FRAP)
The total amount of antioxidant present was assessed using the ferric reducing ability of plasma(FRAP)assay as described by Benzie and Strain[16].The FRAP reagent was prepared by mixing25 mol/Lof300 mol/Lacetatebuffer,2.5 mLof20 mol/L FeCl3and 2.5 mL of 10 mol/L TPTZ(2,4,6-tripyridy-s-triazine)and warmed to 37°C before use.30 μL aliquots of sample,Fe(II)standard or blank (dH2O) were added to 900 μL of the FRAP reagent and 90 μL of dH2O.The absorbance of the solution was measured at 593 nm following incubation at 37°C for 4 min.
2.3.2.Polyphenols
The amount of total soluble phenolics present in each sample was measured according to the Folin–Ciocalteu assay according to the method of Singleton and Rossi [17].2.0 mL of Folin–Ciocalteau solution was added to tubes containing 2 mL of dH2O and 0.2 mL of sample, standards and blank (dH2O).The solutions were allowed to stand for a minimum of 30 s and a maximum of 8 min before 2 mL of 7.5% sodium carbonate(Na2CO3)were added.The solutions were vortexed and incubated for 60 min at room temperature.The absorbance of the samples was measured at 765 nm against the standards and blank.
2.3.3.Betalains
The concentration of betalains in the samples was determined using the spectrophotometric methods of Stintzing et al.[18].The absorbances of the betalains were read at 538 nm for betacyanins and 480 nm for betaxanthins.The betalain content (BC) was calculated as BC(mg/L)=[(A×DF×MW×1000)/(e×l)], where A is the absorption,DF the dilution factor and l the pathlength(1 cm)of the cuvette.For quantification of betacyanins and betaxanthins,the molecular weights (MW) and molar extinction coefficients (e) (MW=550 g/mol; e=60,000 L/mol cm in H2O) and(MW=308 g/mol;e=48,000 L/mol cm in H2O)were applied.
2.4.Mayonnaise preparation
A mayonnaise recipe was developed that contained the following ingredients in weight ratio (w/w): rapeseed oil (70%),egg powder(5%),water(20%),vinegar(3.5%)and salt(1.5%).The recipe containing cooked beetroot was adjusted as follows:rapeseed oil (70%), egg powder (5%), water (16%), beetroot(5%), vinegar (3.5%) and salt (0.5%).A coarse emulsion was initially formed by dissolving egg powder,sugar,salt and vinegar in water.Mayonnaise was prepared by adding the oil to the aqueous mixture at a steady rate and mixing the ingredients using a Morphy Richards hand blender at speed 1.5(Argos,UK).After preparation,the physical stability of the mayonnaise was determined.The remainder was stored at 4°C for 4 weeks and sampling was performed at timed intervals.
2.5.Analysis of lipid oxidation products in dispersed phase
2.5.1.Lipid extraction from mayonnaise
The oil phase was extracted using the method of Lagunes-Galvez et al.with modifications [14].Mayonnaise was gently mixed and approximately 50 g were poured into polypropylene centrifuge tubes.Samples were frozen at −70°C for 24 h and thawed at room temperature to break the emulsion.The oil phase was collected, centrifuged at 2400×gfor 5 min and stored at−70°C for further analysis.
2.5.2.Rancimat analysis
The oxidative stability of the emulsion samples was determined by using a 743 Rancimat device equipped with the 743 Rancimat control and evaluation program (Metrohm Ltd.,Herisau, Switzerland) as described by Läubli and Bruttel with minor modifications[15].Three grams of samples(extracted oil)were added to the reaction tubes.Oxidative stability was determined by calculating the induction time of the samples exposed to an air flow of 20 L/h at constant temperature (100°C).The default value 1 μS/cm was used for evaluation sensitivity of the induction time.
2.5.3.TBARS
Thiobarbituric acid reactive substances content in the protein digests were quantified using reverse-phase high performance liquid chromatography(RP-HPLC).Thiobarbituric acid(0.67%w/v) was added to the reaction mixture (150 extracted oil and 4 mL dH2O)before heating the samples for 30 min in a boiling water bath.Samples were allowed to cool and were centrifuged at 1800×gfor 15 min.HPLC analysis was performed using a Waters 2695 Separations Module(Waters Corporation,Milford,USA)equipped with a Waters 2475 fluorescence detector and a Luna®5 μm C18(2)100 Å,100×4.6 mm column.TBARS was determined with isocratic elution at a flow rate of 0.8 ml/min,sample run was 15 min,injection volume was 20 μL,and fluorescence detector wavelengths were set to 515 nm (excitation)and 546 nm(emission).
2.6.Turbiscan measurements
The physical stability of mayonnaise samples was monitored using a Turbiscan MA2000 (Formulaction, Ramonville St.Agne,France).The apparatus comprises of a detection head equipped with a near-infrared light source(880 nm)which scans the length of the sample,acquiring transmission and backscattering data every 40 μm.Samples were weighed into a cylindrical borosilicate glass tube(25 mm inner diameter and 60 mm high)so that the sample and the vial weighed 40 g to ensure consistency.Before being loaded into the Turbiscan the sample was centrifuged at 50×gfor 10 min.The light source scanned the sample at 5 min intervals from top to bottom and measured the percentage of light backscattered or transmitted during 3 h period at 30°C.The refractive indices used for particle size calculation were 1.47 for the dispersed phase and 1.33 for the continuous phase.The Turbiscan stability index(TSI)was calculated according to backscattering changes that indicate the particles aggregation and dynamic migration by Turbisoft 2.0.
2.7.Viscosity
The viscosity of the samples was assessed using a rotational viscometer (Cole-Parmer Instrument Co., Ltd., London, UK).100 g of the sample were weighed into a plastic beaker and refrigerated for 3.5 h.The probe(R7)rotated at a rate of 50 rpm.The viscosity was recorded 10 s after the measurement began to ensure the consistency of each measurement.
2.8.Texture profile analysis
The textural properties of the emulsions were assessed using a CT3 Texture Analyser(Brookfield Engineering Laboratories,Inc., USA) and a cylindrical mesh probe (TA-MP; Brookfield Engineering).Data was recorded using Texture Pro CT V1.3 Build 15 software.Following mayonnaise formation, 100 g of the sample were weighed into a 100 mL plastic beaker.Using a compression test setting,the probe was lowered at 1 mm/s to a trigger value of 10 g.The equipment recorded the hardness,total work done,adhesiveness and adhesive force of the sample.
2.9.Sensory analysis
The acceptability of the reformulated mayonnaise was assessed by 17 untrained volunteers (students and staff) at the Human Nutrition Unit of Rowett Institute of Nutrition& Health.A questionnaire was prepared to assess the sensory attributes of the mayonnaise.A 9-point Hedonic scale(1=‘dislike extremely’, 9=‘like extremely’) was used in part I of the questionnaire to evaluate taste, texture, odour, appearance and aftertaste.Part II of the questionnaire assessed the oiliness, graininess, saltiness, thickness, spreadability, stickiness,acidity and uniformity of the product using a 5-point scale(1=‘lowest’,5=‘highest’).Samples were tasted in no particular order and were blind coded with random two letter codes.Water and crackers were served to rinse and eat between tasting samples.
2.10.Statistical analysis
Data were averaged from at least n=3 measurements made on three different batches and results are presented as the mean of measurements±standard deviation.Statistical analysis was conducted using a one-way analysis of variance (ANOVA) by using Scheffe’s test at 5% significance level.An independent t-test was applied to detect significant differences between two samples(IBM SPSS statistics 22).
3.Results and discussion
3.1.Impact of processing on betalain content and antioxidant activity
The betanin, indicaxanthin and polyphenol content of processed beetroot in addition toin vitroantioxidant properties as characterised by FRAP are presented in Table 1.The betanin content was significantly affected (P<0.001) by the processing methods and followed the order M>R>B.The same trend was observed for indicaxanthin with difference levels being significant(P=0.017)between M and B.Total polyphenol content was similar for B and R samples but higher (P>0.05) for M.In agreement with the levels of antioxidants found to be present in processed beetroot in this study,thein vitroantioxidant potential of M beetroot was significantly higher(P<0.001)compared to R and B samples.Betalain stability is affected by a number of factors including pH, moisture content, light and oxygen with temperature being the most important amongst them [19].Recent studies demonstrated that the betalain content is retained and in some cases even increased when red beet is microwaved, whereas significant losses are observed when samples are roasted or boiled [20].Despite the fact that the treatment conditions differed in relation to this study, present and previous results suggest that mild thermal processing is beneficial for betalain preservation.This is attributed to betalain degradation by is omerisation,decarboxylation or cleavage during heat processing [21].Furthermore, mild heating conditions may also confer protection against betalain degradation by inactivating endogenous enzymes responsible for pigmentdegradation and color losses such as polyphenoloxidases and peroxidases[22,23].On the other hand,betalains are relatively stable over a pH range between 3 and 7,which makes them good candidates for applications to low acidity foods like mayonnaise[24].
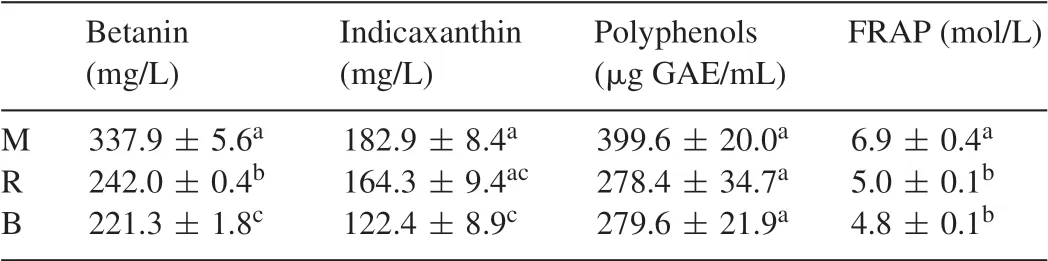
Table 1 Betalain/polyphenol content and antioxidant properties of ethanolic extract of processed beetroot(±SD).

Fig.1.Effect of storage time at 4°C on the oxidative stability of mayonnaise samples supplemented with processed beetroot as determined by the Rancimat accelerated oxidation test.Different letters denote significant differences between induction times(P<0.05)on separate days.
3.2.Oxidative stability of mayonnaise supplemented with processed beetroot
The oxidative stability of the extracted lipid phase of mayonnaise samples during storage at 4°C was determined by Rancimat analysis and is presented in Fig.1.Rancimat analysis is an accelerated oxidation test which measures the time required by edible oils to form by-products of thermally-induced peroxidation.At day 1,the M sample had the longer induction period(15.88±0.25 h)compared to all other samples including the commercial control(C2).After 28 days of storage,the induction period of C1 was significantly reduced by 39.4%(P<0.001)and was significantly lower than the samples containing beetroot(M,R and B)and C2.The induction period of the M sample was also significantly reduced by 33.8%(P<0.001)but was not significantly lower than R,B or C2 at the end of the storage period.C2 was proven to be the most stable among the samples with a reduction of 6.19% throughout the storage period followed by B (6.60%).The lipid peroxidation process of the dispersed phase was followed by TBARS determination(Fig.2).At day 1, C2 had higher TBARS values than C1, M and B and significantly higher compared to R(P=0.023).In agreement with the results obtained from Rancimat,TBARS formation was significantly high for C1 (93.9% increase,P<0.001) during the storage period.On the other hand,C2 was less susceptible to the lipid peroxidation process as indicated by the TBARS increase(33.7%).M (P=0.028) and C2 (P=0.005) had significantly lower TBARS compared to C1 at the end of the storage period.
Overall, the addition of beetroot confers protection against lipid peroxidation during storage.Both Rancimat and TBARS results indicate that mayonnaise samples supplemented with beetroot demonstrate similar resistance to the oxidation process with C2.The interactions between hydroperoxides located at the droplet surface and transition metals from the continuous phaseis considered the most common cause of oxidative instability in aqueous colloidal systems[25,26].Hence,metal chelators such as EDTA are effective antioxidants in mayonnaise by preventing the metal-catalysed breakdown of newly-formed peroxides.In addition, EDTA’s antioxidant effect is further enhanced in low acidity foods due to the pH-triggered ionization of carboxylate groups which form complexes with metal ions[27].In the present study,the antioxidant effect in reformulated mayonnaise with beetroot may be attributed to the water-soluble fractions of betalains and phenolic substances.The efficacy of an antioxidant in an oil-in-water emulsion is determined to a large extent by its polarity,which in turn affects its partition into the different phases [28].In oil-in-water emulsions, non-polar antioxidants are thought to be more effective because they are present in higher concentrations in the oil phase[29].However,high proportions of polar antioxidants may also be localised at the droplet interfacial layer of mayonnaise where they interact with egg yolk constituents and perform their role as antioxidants[30].A few different studies demonstrated the ability of polar antioxidants to protect oil-in-water emulsions from oxidation[31–33].At the interface polar antioxidants may act either as free radical scavengers due to their hydrogen-donating ability or they can bind to pro-oxidants thanks to their metal chelating activity[34].
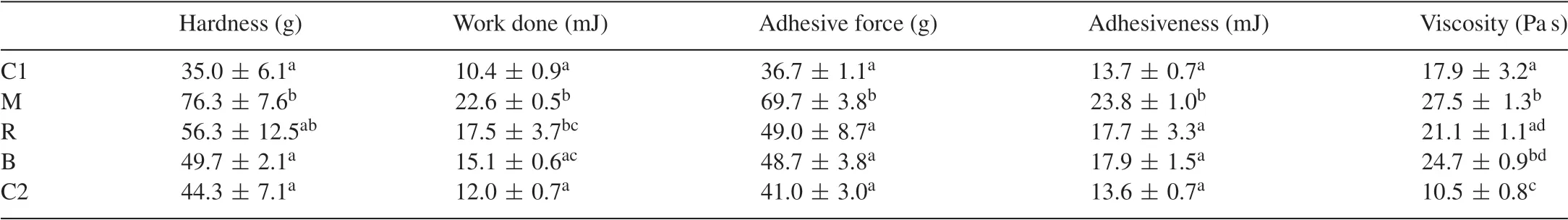
Table 2 Texture profile analysis and viscosity of mayonnaise samples(±SD).
3.3.Effect of beetroot addition on physical and textural properties
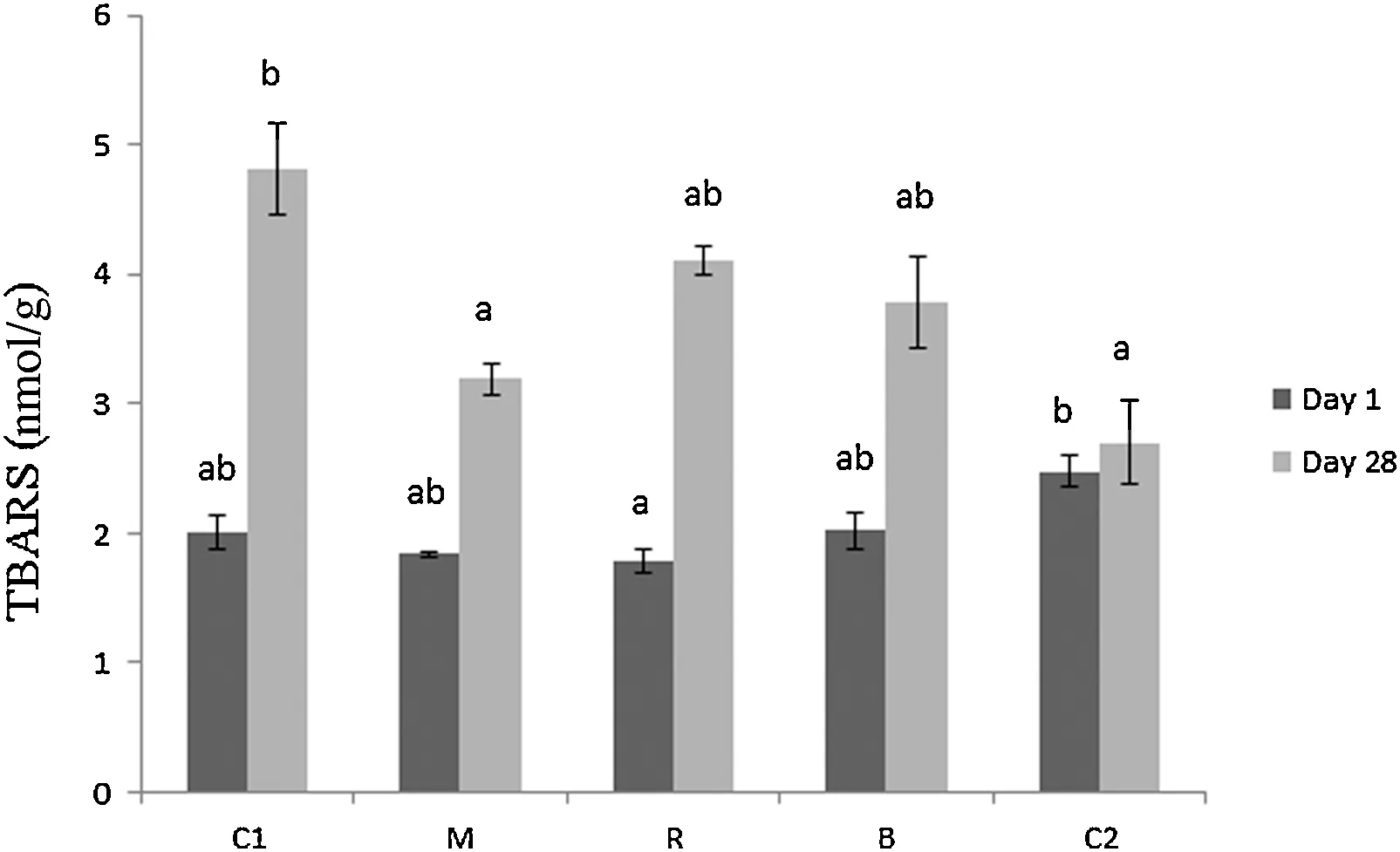
Fig.2.TBARS of mayonnaise samples supplemented with fresh vegetables as affected by storage time at 4°C.Different letters denote significant differences between samples(P<0.05)on separate days.
Turbiscan analysis enables continuous monitoring of the optical properties of emulsions and hence provides real-time information on destabilization phenomena.Fig.3 shows theΔBS% profiles in the reference mode (ΔBS=BSt−BS0) as a function of the sample height (total height=48 mm) for the control mayonnaise (C1).BS% is a parameter that is directly dependent on the droplet’s mean diameter (D) and the droplet volume fraction(Φ),i.e.BS=f(D,Φ)[35].Thus,changes in the backscattering profile of the mayonnaise sample are related to droplet size changes and migration processes.Droplet aggregation phenomena (coalescence/flocculation) are observed at the middle of the tube(zone 1)whereas creaming phenomena(evidenced by a positiveΔBS value)take place at the top of the tube(zone 2).A decrease inΔBS%was observed at the bottom of the tube along with a concomitant increase inΔBS%in the upper zone, due to the formation of a creaming layer [36].Fig.3A is indicative of the creaming process as a function of time for zone 2.The peak thickness of the mayonnaise samples followed the order C1>B>C2>M>R.Fig.3B shows theΔBS% as a function of time for zone 1 and is indicative of droplet aggregation.According to the results C2 was the least affected sample by droplet aggregation phenomena followed by C1, M, B and R.A comparison of the evolution of the particle size variation(zone 1)is presented in Fig.3C.The mean particle size of the mayonnaise samples varied between 3.9 μm (C1) and 5.2 μm(C2),which is in agreement with previous studies[37].The particle size variation observed during the 3 h monitoring process was relatively small (~0.2 μm) for all samples and creaming was identified as the major source of destabilization.Furthermore,the turbiscan stability index(TSI)was calculated for the mayonnaise samples.TSI is a parameter,which can be used for estimation of the emulsion stability and is obtained as the sum of all destabilization phenomena taking place during the monitoring process.High TSI values indicate decreased stability of the system.In the present study,the TSI values followed the order C1(0.70±0.08),M(0.93±0.21),B(0.97±0.21),R(1.0±0.08)and C2(1.10±0.16).Turbiscan analysis suggests that the physical stability of mayonnaise was not significantly affected by the addition of beetroot.On the contrary, C2 was more susceptible to destabilization phenomena manifested as creaming.The addition of beetroot confers protection against particle migration thanks to the increased viscosity of the samples (Table 2).All beetroot-containing samples were more viscous than C1,with M and B having significantly higher values,which might account for their low TSI indices.C2 had the lowest viscosity of all samples but this was not reflected on the physical stability thanks to the presence of stabilisers(i.e.xanthan gum).The increased viscosity of the beetroot-containing mayonnaise samples affected all the textural parameters[38].The properties measured provide an indication of the force required to shear the mayonnaise with a spoon or knife (hardness), the energy required spooning the mayonnaise from the bottle or container(work done),the force required overcoming the attractive forces between the mayonnaise and the surface of other materials (adhesive force) and the energy required separating the mayonnaise from the spoon or knife (adhesiveness).Hardness, work done, adhesive force and adhesiveness of mayonnaise samples increased with the addition of beetroot.All the textural properties of M were significantly higher compared to C1.Most textural profile indicators of C2 were also higher than C1, but not to a significant level(P>0.05).The exception was adhesiveness which was slightly lower for C2 and could be attributed to microstructural properties and, in particular, to the bigger size of the fat globules in the commercial mayonnaise,which led to a lower contact surface area between droplets and,consequently,a lower degree of interaction[39].
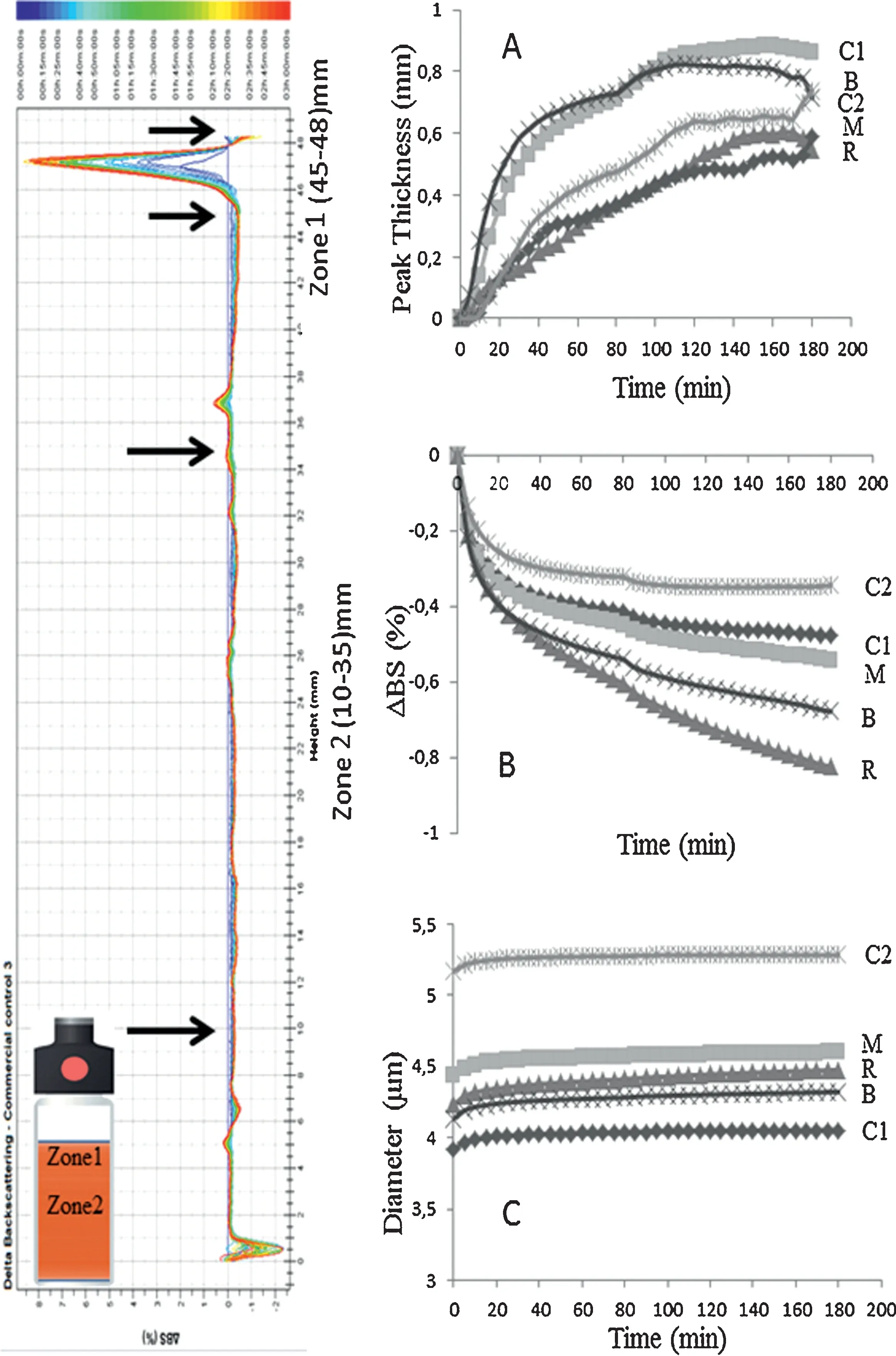
Fig.3.Changes in backscattering(BS)profiles:(A)creaming profiles as a function of time in zone 2(particle migration),(B)%ΔBS profiles as a function of time in zone 1(particle flocculation/coalescence),(C)oil globule diameter profiles as function of time in zone 1(particle size variation).C1(control),M(microwaved),R(roasted),B(boiled),C2(commercial control).
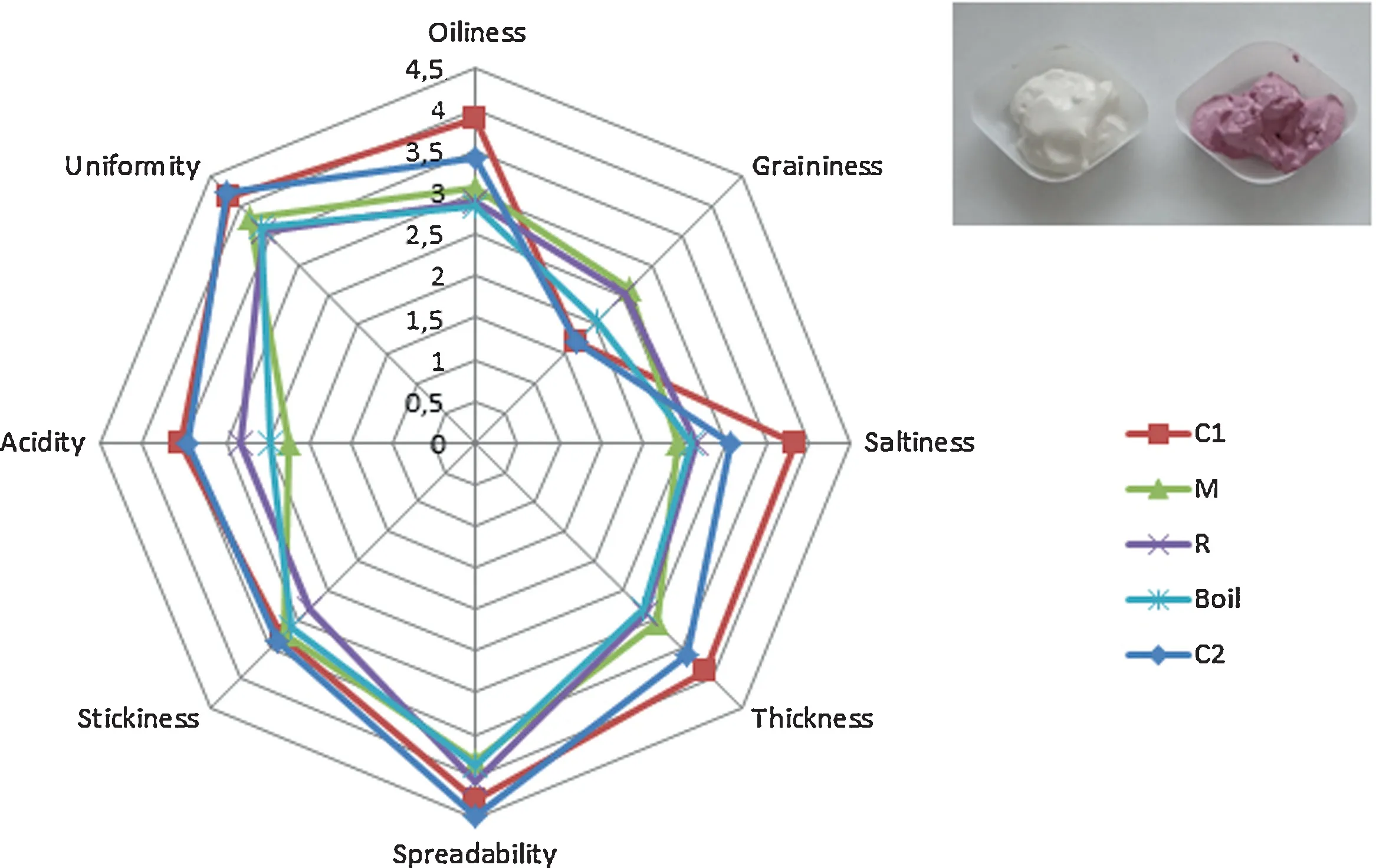
Fig.4.Comparative diagram of specific sensory attributes of the mayonnaise formulations.C1(control),M(microwaved),R(roasted),B(boiled),C2(commercial control).

Table 3 Mean consumer acceptability scores of the mayonnaise formulations(±SD).
3.4.Consumer acceptability evaluation of reformulated mayonnaise
Mean consumer acceptability scores of the 5 mayonnaise formulations are presented in Table 3.The non-specific sensory attributes examined(part I)were not significantly different between samples and ranged from 5.18 to 7.53, which is equivalent to “neither like or dislike” and “like moderately”respectively on the 1–9 hedonic scale.Interestingly, mayonnaise formulated with microwaved beetroot received the highest scores for taste, odour and aftertaste and the second highest scores for texture and appearance.Appearance primarily relates to sensory attributes such as colour, shape, and size, but can also include more complex attributes such as visual surface texture and structural uniformity [40].Unlike the findings of this study, colour attributes impact the overall acceptability scores of reformulated mayonnaise with β-glucan[37].The commercial mayonnaise received the highest score for texture, which reflects the findings of the second part of the sensory analysis(Fig.4).The results obtained are presented in a spider plot that allows the classification of the samples into two groups;mayonnaise without beetroot (C1 and C2) was perceived as more uniform, spreadable, and less grainy than the samples containing beetroot.Furthermore,the commercial mayonnaise was perceived as significantly more acidic(P=0.040)and salty(P=0.022)compared to the sample with microwaved beetroot.These findings are attributed to formulation differences between thein situsamples and the commercially available mayonnaise and reflect the vinegar and salt levels respectively.The findings of the present study suggest that reformulation with beetroot powder had moderate impact on texture-specific sensory properties suchasuniformity and graininess.Further work isrequired to improve the homogenisation process in order to incorporate beetroot in the recipe.On the other hand, the appearance and granular nature of the beetroot mayonnaise had no significant effect on the acceptability scores,suggesting that the reformulated product can be exploited commercially[41].
Conclusions
In conclusion,minimally processed beetroot can effectively protect mayonnaise from lipid oxidation during storage at 4°C for 28 days.TBARS and Rancimat results showed that the antioxidant capacity of microwaved beetroot conferred a protective effect that was comparable to synthetic antioxidants used in commercial products.The physical stability of the mayonnaise was not impaired by the addition of beetroot.The effects on texture of reformulated mayonnaise had an impact on the sensory properties.No significant differences were detected between acceptability scores of beetroot mayonnaise and the control samples.In order to further elucidate the potential use of beetroot in mayonnaise additional research should be carried out to assess the physical stability and textural attributes of the supplemented mayonnaise following 28 days of storage.In addition,the optimal microwave cooking conditions need to be identified to ensure maximum antioxidant retention in the beetroot.
Conflict of interest
All authors declare that there are no conflicts of interest.
Acknowledgements
Funds for the study were provided by the Scottish Government’s Rural and Environment Science and Analytical Services Division and conducted as part of the Scottish Government Strategic Research programme(Food Land&People).
- 食品科学与人类健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- Effect of Roselle calyces extract on the chemical and sensory properties of functional cupcakes
- Thermo-mechanical and micro-structural properties of xylanase containing whole wheat bread
- Biological activities of silver nanoparticles from Nothapodytes nimmoniana(Graham)Mabb.fruit extracts
- Reducing oxidative stress and hepatoprotective effect of water extracts from Pu-erh tea on rats with high-fat diet
- Coagulase gene polymorphism of Staphylococcus aureus isolates:A study on dairy food products and other foods in Tehran,Iran

