替格瑞洛与氯吡格雷的药效观察及与CYP2C19基因多态性的关系
王 彬,薛玉生,倪四峰,于 亮,尚福军
替格瑞洛与氯吡格雷的药效观察及与CYP2C19基因多态性的关系
王 彬,薛玉生*,倪四峰,于 亮,尚福军
(第四军医大学附属唐都医院心内科,西安 710038)
观察替格瑞洛和氯吡格雷的临床疗效,并探讨其与CYP2C19 基因多态性的关系。入选2013年3月至2014年3月在西安市唐都医院心内科接受住院治疗的急性冠脉综合征患者450例。根据拟选取的治疗方案,将所有患者随机分为两组:替格瑞洛组(=227)和氯吡格雷组(=223)。随访6个月,观察两组患者主要心脏不良事件(MACE)发生率。根据是否携带失功基因将两组患者再分别分为两亚组:携带组和非携带组。分别观察两亚组患者的MACE发生率和治疗期间出血并发症发生情况。替格瑞洛组与氯吡格雷组中:非携带组患者MACE的发生率分别为8.4%和8.2%,差异无统计学意义(>0.05);携带组患者MACE的发生率分别为9.1%和15.2%,差异有统计学意义(<0.01)。各组内及组间出血并发症差异无统计学意义(>0.05)。与氯吡格雷相比,应用替格瑞洛治疗急性冠脉综合征,可进一步减少患者心血管事件风险,其机制与是否携带CYP2C19 功能丧失性等位基因有关。
急性冠脉综合征;基因多态性;替格瑞洛;氯吡格雷
急性冠脉综合征介入治疗患者的主要心脏不良事件(major adverse cardiac events,MACE)包括心源性死亡、心肌梗死以及支架再狭窄。为了预防其发生,目前指南建议使用双重抗血小板聚集药物治疗,包括阿司匹林(aspirin)和氯吡格雷(clopidogrel)[1]。氯吡格雷为一种前体药物,需经肝脏CYP2C19酶代谢才能发挥其抗血小板聚集的作用,但是由于肝脏CYP2C19酶存在基因多态性,可能会造成氯吡格雷抵抗,从而降低其抗血小板作用、增加经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)术后患者临床不良事件的发生[2]。替格瑞洛(ticagrelor)是一种新型的口服抗血小板活性药物,能够可逆性结合磷酸腺苷受体,无需经过体内代谢即可发挥抗血小板作用[3,4]。本文通过分别使用替格瑞洛和氯吡格雷治疗急性冠脉综合征,对比观察两者的临床疗效,并进一步探讨两者的药效及其与CYP2C19基因多态性的关系。
1 对象与方法
1.1 研究对象
入选2013年3月至2014年3月在西安市唐都医院心内科接受住院治疗的急性冠脉综合征患者450例。入选标准:(1)年龄18~80岁;(2)行冠状动脉造影术确诊为急性冠脉综合征,包括不稳定型心绞痛、非ST段抬高型急性心肌梗死(non-ST elevation mycarial infaration,NSTEMI)、ST段抬高型急性心肌梗死(ST segment elevation mycardial infarction,STEMI);(3)行PCI术;(4)签署知情同意书。排除标准:(1)未行冠状动脉支架植入;(2)血小板计数<100×109/L;(3)妊娠期、哺乳期、月经期女性;(4)对研究药物过敏或可疑碘造影剂过敏;(5)明显肝肾功能异常;(6)大便潜血持续阳性等其他不宜行PCI治疗的情况。
1.2 研究方法
根据拟选取的治疗方案,将所有患者随机分为两组:替格瑞洛组(=227)和氯吡格雷组(=223)。两组患者术前均给予阿司匹林(300mg,1次/d)口服治疗。替格瑞洛组:术前给予替格瑞洛负荷剂量(180mg);术后口服替格瑞洛(90mg,2次/d)+阿司匹林(100mg,1次/d)。氯吡格雷组:术前给予氯吡格雷负荷剂量(300mg);术后口服氯吡格雷(75mg,1次/d)+阿司匹林(100mg,1次/d)。随访6个月,观察两组患者MACE发生率。
采集患者术前当日的空腹血样本进行CYP2C19基因型分析,采用TaqMan探针技术,根据是否携带失功基因(携带任一*2或*3位点功能丧失等位基因)将两组患者再分别分为两亚组:携带组和非携带组。分别观察两亚组患者的MACE发生率(包括心源性死亡、心肌梗死、支架再狭窄形成)和治疗期间出血并发症发生情况。出血的分级标准如下。(1)轻度出血:血红蛋白下降<50g/L,伴有牙龈出血、消化道出血或肉眼血尿等。(2)重度出血:血红蛋白下降≥50g/L,出现颅内出血或自发性心脏压塞。
1.3 统计学处理
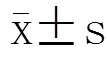
2 结 果
2.1 两组患者一般资料比较
两组患者间的各项基线资料均无统计学差异(>0.05;表1)。

表1 基线资料比较
BMI: body mass index; LVEF: left ventricular ejection fraction; UAP: unstable angina pectoris; NSTEMI: non-ST segment elevation myocardial infarction; STEMI: ST segment elevation myocardial infarction
2.2 两组患者MACE发生率的比较
替格瑞洛组MACE发生数为20例(8.8%),其中心肌梗死4例,支架内血栓15,心力衰竭死亡1例;氯吡格雷组MACE发生数为27例(12.1%),其中心肌梗死7例,支架内血栓19例,心力衰竭死亡1例。两组患者MACE的发生率差异有统计学意义(<0.01)。
2.3 两组患者中非携带组与携带组亚组间MACE发生率的比较
替格瑞洛组与氯吡格雷组中:非携带组患者MACE的发生率分别为8.4%和8.2%,差异无统计学意义(>0.05);携带组患者MACE的发生率分别为9.1%和15.2%,差异有统计学意义(<0.01)。各组内及组间出血并发症差异无统计学意义(>0.05),替格瑞洛组中,携带组有1例脑出血,出血量为10ml,其余均为轻度出血,无致命性出血事件发生(表2)。
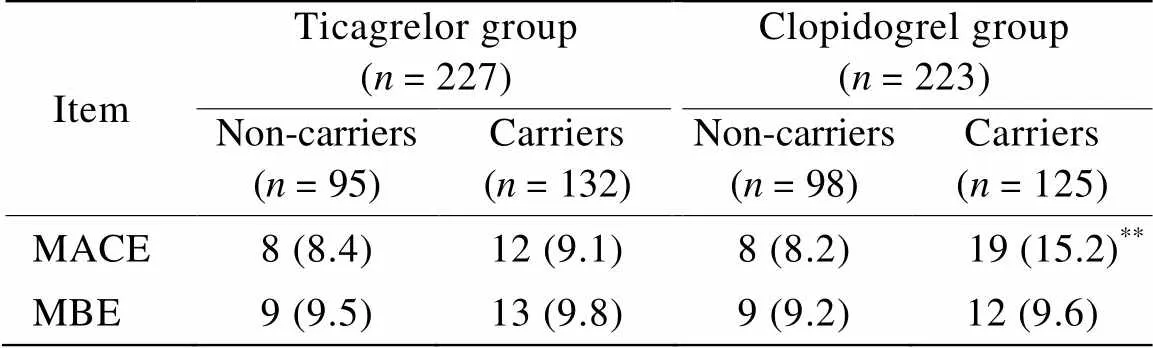
表2 非携带组和携带组间MACE发生率的比较
MACE: major adverse cardiac events; MBE: major bleeding events. Compared with ticagrelor group,**<0.01
3 讨 论
氯吡格雷是目前最广泛使用的抗血小板聚集的药物之一。阿司匹林联合氯吡格雷能显著降低冠状动脉支架术后主要临床不良事件的发生率。但是,氯吡格雷的临床药效存在个体差异,近年研究发现,CYP2C19基因多态性是其抵抗的主要原因[5−7]。CYP2C19代谢酶存在基因多态性,其中可引起氯吡格雷抵抗的*2和*3等位基因在亚裔人群中更为常见,中国人群CYP2C19基因突变频率较高[8−10],由此推论,中国人群发生氯吡格雷抵抗的概率较高。
替格瑞洛是一种新型抗血小板聚集药,不需肝脏激活的活性药物,在对氯吡格雷低反应的患者中替格瑞洛具有一定的有效性[11,12]。文献报道了对18 624例急性冠脉综合征患者的研究,比较了替格瑞洛和氯吡格雷在预防心血管事件发生方面的疗效,结果表明,与氯吡格雷相比,替格瑞洛可使总病死率显著降低22%[13,14]。本研究与PLATO研究的疗效观察结果基本一致,进一步说明了与氯吡格雷相比,替格瑞洛可进一步降低急性冠脉综合征患者MACE的发生率。
本研究还进一步根据患者是否携带CYP2C19功能丧失性等位基因(*2或*3)进行了亚组分析,比较了CYP2C19功能丧失性等位基因对替格瑞洛及氯吡格雷药效学的影响。我们的研究结果发现:两组患者中非携带组患者MACE的发生率差异无统计学意义;相反,携带组患者中MACE的发生率具有显著性差异。所以我们进一步得出结论,CYP2C19基因多态性是氯吡格雷抵抗的主要因素,与氯吡格雷相比,替格瑞洛可进一步降低急性冠脉综合征患者MACE的发生率。同时,结果显示替格瑞洛不增加患者的出血风险。
总之,本研究显示,替格瑞洛是一个具有良好临床效果和发展前景的药物,对急性冠脉综合征的抗血小板治疗将产生很大的影响。对于CYP2C19功能丧失性等位基因携带者及氯吡格雷抵抗患者,使用替格瑞洛是一种可提高患者预后的选择。
[1] Bassand JP, Hamm CW, Ardissino D,. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes[J]. Eur Heart J, 2007, 28(13): 1598−1660.
[2] Niu X, Mao L, Huang Y,. CYP2C19 polymorphism and clinical outcomes among patients of different races treated with clopidogrel: a systematic review and meta-analysis[J]. J Huazhong Univ Sci Technolog Med Sci, 2015, 35(2): 147−156.
[3] Wang XZ, Hui J, Hu Y,. The new drug of anti-platelet aggregation—ticagrelor[J]. Chin J Hospital Pharm, 2013, 33(11): 900−902. [王仙朱, 慧 娟, 胡 燕, 等. 抗血小板聚集新药——替格瑞洛[J]. 中国医院药学杂志, 2013, 33(11): 900−902.]
[4] Wang HY, Su X, Shen CX,. Effectiveness and safety of ticagrelor in acute coronary syndrome[J]. Chin J Evid Based Cardiovasc Med, 2015, 7(4): 468−471. [王贺阳, 苏 晞, 沈成兴, 等. 替格瑞洛在急性冠脉综合征患者中应用的安全性和有效性分析[J]. 中国循证心血管医学杂志, 2015, 7(4): 468−471.]
[5] Sibbing D, Stegherr J, Latz W,. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention[J]. Eur Heart J, 2009, 30(8): 916−922.
[6] Wu LM, Xu W, Tian XL,. Anti-platelet individual treatment guided by CYP2C19 polymorphism[J]. Chin J Evid Based Cardiovasc Med, 2015, 7(2): 207−210. [吴龙梅, 徐 威, 田新利, 等. CYP2C19基因多态性指导下的抗血小板个体化治疗[J]. 中国循证心血管医学杂志, 2015, 7(2): 207−210.]
[7] Sinhal AR, Aylward PE. New antiplatelet agents and the role of platelet function testing in acute coronary syndromes[J]. Clin Ther, 2013, 35(8): 1064−1068.
[8] Kang HJ, Clare RM, Gao R,. Ticagrelorclopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) trial[J]. Am Heart J, 2015, 169(6): 899−905.
[9] Levine GN, Jeong YH, Goto S,. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI[J]. Nat Rev Cardiol, 2014, 11(10): 597−606.
[10] Liu R, Shi ST, Suo M,. Clinical effectiveness and safety evaluation for the shift from clopidogrel to ticagrelor in patients with low clopidogrel response[J]. Chin J Intervent Cardiol, 2014, 22(1): 12−17. [刘 然, 师树田, 索 旻, 等. 对氯吡格雷低反应患者换用替格瑞洛后的有效性与安全性评价[J]. 中国介入心脏病学杂志, 2014, 22(1): 12−17.]
[11] Qaderdan K, Ishak M, Heestermans AA,. Ticagrelor or prasugrelclopidogrel in elderly patients with an acute coronary syndrome: optimization of antiplatelet treatment in patients 70 years and older—rationale and design of the POPular AGE study[J]. Am Heart J, 2015, 170(5): 981−985.
[12] Jneid H, Anderson JL, Wright RS,. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines[J]. Circulation, 2012, 126(7): 875−910.
[13] Wang LL, Li Q, Kang L,. Thrombelastography for reviewing antiplatelet efficacy of ticagrelor and clopidogrel in patients with acute coronary syndrome[J]. Chin J Evid Based Cardiovasc Med, 2014, 6(3): 281−284. [王丽丽, 李 群, 康 林, 等. 应用血栓弹力图评估ACS患者替格瑞洛与氯吡格雷抗血小板的疗效[J]. 中国循证心血管医学杂志, 2014, 6(3): 281−284.]
[14] Wallentin L, Becker RC, Budaj A,. Ticagrelorclopidogrel in patients with acute coronary syndromes[J]. N Engl J Med, 2009, 361(11): 1045−1057.
(编辑: 吕青远)
Efficacies of ticagrelor and clopidogrel and their correlation with CYP2C19 gene polymorphisms in patients with acute coronary syndrome
WANG Bin, XUE Yu-Sheng*, NI Si-Feng, YU Liang, SHANG Fu-Jun
(Department of Cardiology, Tangdu Hospital, Fourth Military Medical University, Xi’an 710038, China)
To observe the clinical efficacies of ticagrelor and clopidogrel and investigate their correlation with CYP2C19 gene polymorphisms.A total of 450 hospitalized patients with acute coronary syndrome in our department from March 2013 to March 2014 were recruited in this study. According to their treatment regimes, the patients were randomly divided into the ticagrelor group (=227) and the clopidogrel group (=223). Their incidence of the major adverse cardiac events (MACE) was compared in the 6 months’ follow-up. The patients were also assigned into 2 subgroups according to their carrying CYP2C19*2/*3 loss-of-function allele or not. The incidence of the MACE and bleeding events were compared between the subgroups in the follow-up.Between the ticagrelor group and clopidogrel group, the incidence of MACE was 8.4% and8.2% respectively for the non-carriers of CYP2C19*2/*3 loss-of-function allele, though without significant difference (>0.05), and was 9.1% and 15.2% separately for the carriers, with obvious difference (<0.01). However, there was no statistical difference in the incidence of bleedingevents between the ticagrelor and clopidogrel groups and between the carriers and non-carriers (>0.05).Ticagrelor is superior to clopidogrel in decreasing the risk of MACE in the patients with acute coronary syndrome, which may be associated with carrying CYP2C19 loss-of-function allele or not.
acute coronary syndrome; gene polymorphisms; ticagrelor; clopidogrel
R541; R969
A
10.11915/j.issn.1671-5403.2016.02.031
2015−10−11;
2015−11−30
薛玉生, E-mail: xys978088@163.com

