Neurotropic effects of aspartame, stevia and sucralose on memory retention and on the histology of the hippocampus of the ICR mice (Mus musculus)
Lejan Miguel Alabastro Villareal, Rachelle Anne Montes Cruz, Michael Bagui Ples,Rodel Jonathan Santos Vitor IIBiology Department, College of Science, De La Salle University, 2401 Taft Avenue, 0922 Manila, Philippines
Neurotropic effects of aspartame, stevia and sucralose on memory retention and on the histology of the hippocampus of the ICR mice (Mus musculus)
Lejan Miguel Alabastro Villareal, Rachelle Anne Montes Cruz, Michael Bagui Ples,
Rodel Jonathan Santos Vitor II*
Biology Department, College of Science, De La Salle University, 2401 Taft Avenue, 0922 Manila, Philippines
Original article http://dx.doi.org/10.1016/j.apjtb.2015.11.001
Tel: +63 2 524 4611 ext. 460, +63 917 803 2687
Fax: +63 2 536 0228
E-mail: rodel.vitor@dlsu.edu.ph
All experimental procedures involving animals were conducted in accordance to PALAS Code of Practice for the Care and Use of Laboratory Animals in the Philippines and approved by the Institutional Animal Care and Use Committee of De La Salle University.
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 13 Apr 2015
Receivedinrevisedform26 Oct2015
Accepted 10 Nov 2015
Available online 10 Dec 2015
Keywords:
Aspartame
Hippocampus
Learning
Memory
Mus musculus
Stevia
Sucralose
ABSTRACT
Objective: To identify the effects of the consumption of non-nutritive sweeteners on memory retention and on the histology of the hippocampus.
Methods: In this study, 20 mice were used to determine if there is an effect of consuming the maximum allowable dose of the non-nutritive sweeteners on the memory retention and on the histology of the hippocampus. The mice were distributed into four groups and the treatments were given via oral gavage: Group 1 (water), Group 2 (aspartame: 1000 mg/kg), Group 3 (stevia: 1000 mg/kg) and Group 4 (sucralose: 16000 mg/kg). Treatments were administered to the different experimental groups for 32 days, after which memory retention was tested using the two-day water maze protocol. After the tests, the mice were sacrificed and the brain was analyzed histologically for neurotrophic effects.
Results: Based on the results of the two-day water maze protocol, there were no differences between the non-nutritive sweeteners and the control group. However, stevia showed high cellular apoptosis followed by aspartame, sucralose and control group.
Conclusions: There was no significant effect on the memory of the mice. It showed histologically however, that stevia had a significant neurotropic effect compared to the other sweeteners.
1. Introduction
There are two kinds of sweeteners, namely, nutritive and non-nutritive sweeteners [1]. Nutritive sweeteners are naturally occurring like sucrose and fructose [2]. On the other hand, non-nutritive sweeteners are synthetically made like aspartame, stevia and sucralose [3]. Non-nutritive sweeteners, also referred to as high intensity sweeteners, are typically used in small amounts to reduce the caloric intake while sustaining the desired taste in many food products [4].
According to the study done by Romano et al., some of the adverse effects on the central nervous system caused by the intake of aspartame are headaches, mood changes, insomnia and seizures [5]. In addition, other effects include confusion, personality disorders, dizziness and visual difficulty [6]. Recently, it has also been attributed to obesity [7,8]. Chronic aspartame consumption resulted to the longer time for the mice to locate the reward within the T-maze, which showed impaired long-term memory retention [9–11].
Stevia as sweeteners has been used for a long time in South America without any reported adverse effects. Nonetheless, its safety has been the subject of controversy for many years [12]. The metabolically activated steviol has been found to be mutagenic in mice but its activation in humans has not yet been established with further work needed to establish its toxicity in humans[13].
Sucralose is one of the newest non-nutritive sweeteners and its safety has been established by different experiments [14–17]. However, according to Schiffman and Rother, experiments in rat models showed that consumption of foods and fluids containing high-potency sweeteners was interfered with the ability to detect sweet tastants, thereby affecting energy regulation[17].
Despite the adverse effects caused by some artificial sweeteners, they are still widely used and accepted all over the world [18]. Results of this study can be used to reevaluate the maximum daily dose of artificial sweeteners and for further studies on the toxicity of aspartame, stevia and sucralose.
2. Materials and methods
2.1. Animal use and maintenance
A total of 20, 6-week old, male ICR mice (Mus musculus) were procured from the Research Institute for Tropical Medicine, Alabang, Muntinlupa City. They were randomly distributed to the four groups (n = 5), namely, control group (Group 1), aspartame (Group 2), stevia (Group 3) and sucralose (Group 4). The mice were kept in individual cages in the animal house of De La Salle University. All cages were cleaned and sanitized weekly and bedded with husk which was autoclaved. The plates and water bottles were cleaned and dried twice a week. The mice were allowed to acclimatize for one week to adjust to a 12 h light: 12 h dark cycle at 24–26°C. Food and water were given ad libitum. Proper handling and maintenance of the mice were guided by the safety standards set by the Philippine Association of Laboratory Animal Science as approved by the De La Salle University (Institutional Animal Care and Use Committee).
2.2. Treatment preparation and administration
Artificial sweeteners (aspartame, stevia and sucralose) were purchased from local supermarkets and were dissolved in 0.2 mL of distilled water based on the amount to be given into per mouse (Table 1). About 0.2 mL of the different treatments was administered to the mice via gastric gavage for 32 days[19].
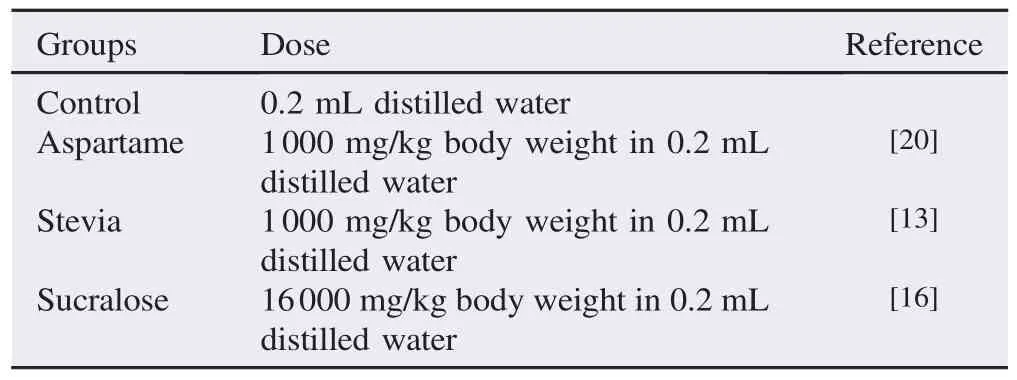
Table 1Treatment given to the different groups for duration of 28 days.
2.3. Two-day water maze protocol
According to Hodges, the water maze, which was used for determining memory retention in mice, was tapped into the cognitive processes that included associative learning, shortterm and long-term non-spatial memory, temporal order and conditional discrimination or anxiety [21].
The water maze pool was 1.8 m in diameter and 6 inches deep. Thewaterwaskeptatroomtemperatureandvisualcueswereplaced on the north, south, east and west sides of the pool that served as “landmarks”for the mice to help it locate the hidden platform. The hiddenglassplatformwas10cmindiameterand6inchesinheight.
The two-day water maze protocol consisted of training phase (Day 1) and test phase (Day 2). This was done two times per week for 32 days for a total of 10 tests. In the experiment, the tests were performed on Thursday and Friday (Test 1) and Saturday and Sunday (Test 2). Monday to Wednesday was the rest days for all the mice. The platform was randomly placed for the Test 1 and then changed for Test 2 [22].
2.3.1. Training phase (Day 1)
The platform was randomly placed within the pool with the pre-release points at north, south, east, or west of the pool. These pre-release points had signs that north, south, east or west were attached to the wall of the pool. In addition, the distance from the pre-release points to the randomly placed platform was measured and recorded. The animals were released facing the wall and a maximum of 60 s was allowed for the mice to locate the platform.
Ifthemicefailedtolocatetheplatformwithintheallottedtime, they were guided to the hidden platform and placed there for 15 s, for them to learn that the safe zone was the platform, before they werereturnedtothecages.Iftheanimalsjumped offthe platform, the animals were guided back towards the platform. The time was recorded when the mouse found the hidden platform and if they failed, the recorded time was 1 min. This was done two times to each mouse (two trials) with intertrial time of 30 s.
2.3.2. Test phase (Day 2)
The platform randomly placed in Day 1 was retained. The animals were still released facing the wall and a maximum of 60 s was allowed for the mice to locate the platform submerged underwater. However, if the animals failed to find the hidden platform within 60 s, they were guided to the platform and placed there for 15 s, before returning them back to the cages. If the animals jumped off the platform, the animals were guided back towards the platform.
2.4. Histological analysis
After 32 days of experiment, the mice were euthanized by cervical dislocation and the brain was extracted and placed in a vial containing 10% buffered formalin. Samples were then brought to the Pathology Department, College of Medicine, University of the Philippines Manila for brain processing and routine hematoxylin and eosin slide preparation.
The processed hippocampal sections of the brain were analyzed by counting the normal cells and the cells that undergone apoptosis per high power objective. The person that counted the neurons was blinded to the experimental group of the particular sample being examined and did all the neuronal counts to avoid Inter-counter variation.
2.5. Statistical analysis
The differences in rate and number of neuronal cells were analyzed using One-way ANOVA and the means were compared using Tukeys test and SPSS version 22 was used to determine significant differences among the treatment groups at P<0.05.
3. Results
The results showed the effect of 4 weeks exposure in maximum concentration of non-nutritive sweetener based on their performance in water maze test and the percentage of cellular apoptosis on the hippocampus (Table 2). The rate (distance/time) of each group performance in training phase and test phase on water maze test was recorded. After the 4 weeks exposure, the percentage of cellular apoptosis on hippocampus was measured to see the effect of exposure on maximum concentration of sweetener.
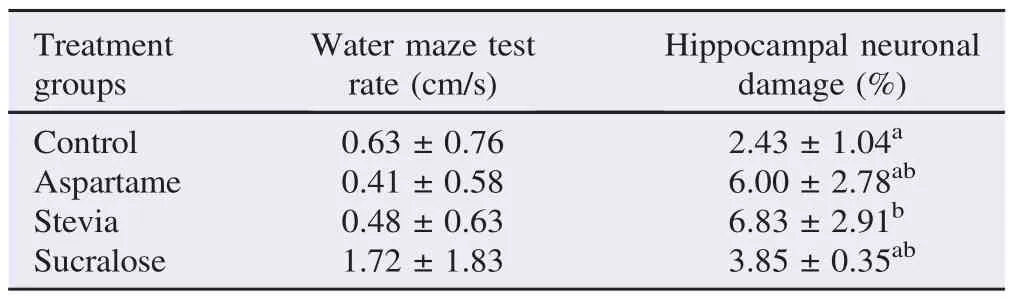
Table 2Effects of the different treatment groups on the water maze test and hippocampal neuronal damage.
3.1. Water maze test
The results showed that the average of the water maze performances of all the groups were not significantly different statistically. Groups 1, 2, 3, and 4 had an average rate 0.63 cm/ s, 0.41 cm/s, 0.48 cm/s and 1.72 cm/s, respectively. With respect to the individual performances of each mouse, 7 out of the 20 mice failed to locate the platform throughout the experiment, 6 out of the 20 mice had an average rate of 1.00–3.00 cm/s and 1 out of the 20 had an average rate of 3.00 cm/s and above.
The number of mice that were able to locate the platform less than 60 s in per test were counted and graphed using a scatter plot (Figure 1). The equation of the line was obtained and revealed that the number of mice that located the platform during the test phase of the experiment increased as the experiment progresses, as showed by the coefficient of correlation of 0.727 36. In other words, there was a positive correlation that as the experiment progresses, the number of mice were able to locate the platform.

Figure 1. The number of mice located the hidden platform during the test phase of the experiment.
Intheexperiment,thebehaviorthatthemicecircledaroundthe pool was observed in the first few tests of the experiment. Na¨ıve animals tended to circle around the pool and appeared a panic response.However,throughtime,themiceknewthatthesafezone, which they could go to, was the platform. The main motivation of the mice in locating the platform was the desire to escape[21].
3.2. Percentage of cellular apoptosis
The number of brain cell damage, specifically cellular apoptosis (Figure 2) in the hippocampus were recorded to determine and compare the effect of the three types of artificial sweetener (aspartame, stevia and sucralose) administered to the mice. Group 3 (stevia) showed the highest percentage damage, followed by Group 2, Group 4 and Group 1.
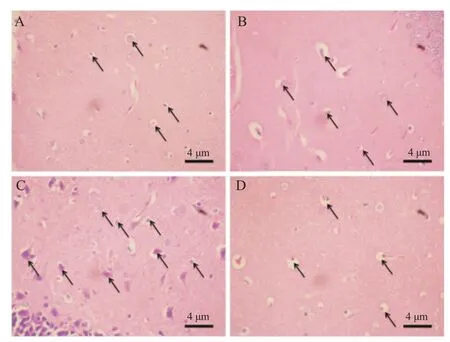
Figure 2. A section of the mice hippocampus showing neuronal damage (arrows) in four groups.A: Control group; B: Aspartame group; C: Stevia group; D: Sucralose group.
The hippocampal area of the brain was analyzed based on the percentage of cellular apoptosis on four groups in the experiment. Recent study showed that excessive intake of artificial sweetener triggered large number of free radical, specifically oxygen and nitrogen, which caused alteration in the antioxidant system. This alteration caused the induction of oxidative stress leading to the cellular level damage of brain cells [23].
A significant percentage of neuronal damage, specifically cellularapoptosis,wasseenontheexperimentalgroupcomparedto the control group, most likely due to the artificial sweetener, specifically aspartame, sucralose and stevia that were administered.
4. Discussion
Aspartame is metabolically broken down into methanol, aspartic acid and phenylalanine [5]. Although there was not significantly different, this group showed the lowest rate in locating the platform for the water maze test. The possible reason for their activity in water maze test may be due to the fact that aspartame has a chemical component that may lead to learning disabilities in mice.
Phenylalanine, one of the products produced, is converted to tyrosine to produce catecholamines [24]. According to Meldrum et al., high concentrations of phenylalanine compete with tyrosine uptake in the brain and inhibit the enzyme tyrosine hydroxylase, which act as a catalyst for the synthesis of (catecholamines) norepinephrine [25]. Tyrosine is an important component of the brain as it is used to synthesize catecholamines (norepinephrine), one of the important neurotransmitters that affect attention [26]. An increase in concentration of phenylalanine in the brain may cause phenylketonuria, a disorder that results from accumulation of unsynthesized phenylalanine in the brain which can cause intellectual disability, seizures and other medical problems [27].
Based on the percentage of cellular apoptosis in the hippocampus, aspartame showed the second highest percentage of celldeath that can be attributed to the effect of the components of aspartame. A study conducted in National Research Center in Egypt by Abdel-Salam et al. confirmed that excessive intake of aspartame for a long period of time will impair the performance of mice on cognitive memory due to increase in oxidative stress on mice brain[19]. Oxidative stress was found to be the primary cause of cellular damage on hippocampal area of the mice because it demands high production of adenosine triphosphate which causes brain to consume O2rapidly[28]. Higher demand of O2causes interference with the function of mitochondria, which results in an increase of O2formation. Aspartame is also capable of damaging the essential cellular components such as nucleic acid lesions, gene damage and gene repair activity that can lead to apoptosis [23].
Stevia is composed of stevioside and rebaudioside A, which is found to be rich in mineral components, such as iron, manganese and cobalt. Minerals serve as some of the important roles in the body such as for maintaining normal heart rhythm, contraction of muscle, conductivity of neurons, regulation of cellular metabolism and also in memory. However, an excess intake of minerals may also lead to worse cases [29].
Stevia showed the second lowest rate in the water maze test and the highest percentage of cellular apoptosis. The possible reason for its rate and percentage of neuronal damage may be due to the mineral components of stevia. Interestingly, although iron is essential for normal functioning of the brain as it is a cofactor in the production of neurotransmitters necessary for brain to function normally, especially in learning and memory, accumulation of this mineral in brain causes brain damage if taken in excess, specifically in learning and memory [30]. Therefore, a high concentration of iron in the brain causes an injury to the brain cells and can lead to oxidative stress via formation of oxygen free radical that causes cellular apoptosis in the brain [31]. Involvement of free radicals with lipid peroxidation of the cell membrane can lead to an increase in membrane fluidity of the cell, which results in the disturbance of calcium homeostasis and finally cell death [32].
Manganese in high concentration has been found to cause Parkinson's disease and learning disabilities [33,34]. However, there are still no studies on the specific mechanism and how manganese causes learning disabilities. Lastly, presence of high concentrations of cobalt in the brain stimulates the production of reactive oxygen, which can lead to cellular apoptosis[35].
Sucraloseisahydrolyzedproductof6-chloro-6-deoxyglucose thatentersthe brainthrough the blood byinhibiting the processof D-glucose transportation across the epithelium [15]. It was also found that it competes with the glucose uptake in the brain and causes D-glucose concentration to drop and chlorinated sugar to rise. Since the neurons cannot store glucose, they depend on the concentration of glucose in the blood stream to function properly [17]. However, this group showed the fastest rate among all groups in locating the platform, as their test for memory. The probable reason behind this is that sucralose is poorly absorbed and minimally metabolized in the body. This was demonstrated by Sims et al. that mice given an oral dose of chlorine-36-labeled sucralose had 97% of sucralose excreted via excreta and urine and that 90% of the sucralose excreted were unchanged [36]. This group also showed the lowest percentage of cellular apoptosis in hippocampal area of the brain. The minimal cellular damage of this group was the advantage of its metabolism. Its type of reactivity to the body causes that it cannot show a significant amount of neural damage in mice[36]. In addition to this, the chemical component of sucralose which is 6-chloro-6-deoxyglucose, was found to cause lesion in the nuclei of brainstem, cerebellum and spinal cord which is the most probable reason why there's a minimal percentage of cellular apoptosis found in the hippocampus[17].
In conclusion, the three sweeteners tested did not have any effect on the memory of the mice, as tested in the two-day water maze protocol. On the other hand, in the histological analysis performed, stevia had the highest percentage of cellular apoptosis followed by aspartame and sucralose.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was partially funded by De La Salle University Science Foundation.
References
[1] Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014; 514(7521): 181-6.
[2] Fitch C, Keim KS. Academy of nutrition and dietetics. Position of the academy of nutrition and dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012; 112(5): 739-58.
[3] Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, et al. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2012; 35(8): 1798-808.
[4] Figlewicz DP, Ioannou G, Bennett Jay J, Kittleson S, Savard C, Roth CL. Effect of moderate intake of sweeteners on metabolic health in the rat. Physiol Behav 2009; 98(5): 618-24.
[5] Romano M, Diomede L, Guiso G, Caccia S, Perego C, Salmona M. Plasma and brain kinetics of large neutral amino acids and of striatum monoamines in rats given aspartame. Food Chem Toxicol 1990; 28(5): 317-21.
[6] Pepino MY. Metabolic effects of non-nutritive sweeteners. Physiol Behav 2015; http://dx.doi.org/10.1016/j.physbeh.2015.06.024.
[7] Fernstrom JD. Non-nutritive sweeteners and obesity. Annu Rev Food Sci Technol 2015; 6: 119-36.
[8] Roberts JR. The paradox of artificial sweeteners in managing obesity. Curr Gastroenterol Rep 2015; 17(1): 423.
[9] Christian B, McConnaughey K, Bethea E, Brantley S, Coffey A, Hammond L, et al. Chronic aspartame affects T-maze performance, brain cholinergic receptors and Na+,K+-ATPase in rats. Pharmacol Biochem Behav 2004; 78(1): 121-7.
[10] Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners-a review. J Food Sci Technol 2014; 51(4): 611-21.
[11] Qurrat-ul-Ain, Khan SA. Artificial sweeteners: safe or unsafe? J Pak Med Assoc 2015; 65(2): 225-7.
[12] Brandle JE, Starratt AN, Gijzen M. Stevia rebaudiana: its agricultural, biological, and chemical properties. Can J Plant Sci 1998; 78: 527-36.
[13] Sekihashi K, Saitoh H, Sasaki Y. [Genotoxicity studies of stevia extract and steviol by the comet assay]. J Toxicol Sci 2002; 27: 1-8. Japanese.
[14] Goldsmith LA. Acute and subchronic toxicity of sucralose. Food Chem Toxicol 2000; 38: S53-69.
[15] Rodero AB, de Souza Rodero L, Azoubel R. Toxicity of sucralose in humans: a review. Int J Morphol 2009; 27(1): 239-44.
[16] Brusick D, Grotz VL, Slesinski R, Kruger CL, Hayes AW. The absence of genotoxicity of sucralose. Food Chem Toxicol 2010; 48(11): 3067-72.
[17] Schiffman SS, Rother KI. Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J Toxicol Environ Health B Crit Rev 2013; 16(7): 399-451.
[18] Riob´o Serv´an P, Sierra Poyatos R, Soldo Rodríguez J. Low and no calorie sweeteners (LNCS); myths and realities. Nutr Hosp 2014; 30: 49-55.
[19] Abdel-Salam OM, Salem NA, El-Shamarka ME, Hussein JS, Ahmed NA, El-Nagar ME. Studies on the effects of aspartame on memory and oxidative stress in brain of mice. Eur Rev Med Pharmacol Sci 2012; 16(15): 2092-101.
[20] Abhilash M, Paul MV, Varghese MV, Nair RH. Effect of long term intake of aspartame on antioxidant defense status in liver. Food Chem Toxicol 2011; 49(6): 1203-7.
[21] Hodges H. Maze procedures: the radial-arm and water maze compared. Brain Res Cogn Brain Res 1996; 3(3–4): 167-81.
[22] Gulinello M, Gertner M, Mendoza G, Schoenfeld BP, Oddo S, LaFerla F, et al. Validation of a 2-day water maze protocol in mice. Behav Brain Res 2009; 196(2): 220-7.
[23] Ashok I, Sheeladevi R. Biochemical responses and mitochondrial mediated activation of apoptosis on long-term effect of aspartame in rat brain. Redox Biol 2014; 2: 820-31.
[24] Hall JE. Guyton and hall textbook of medical physiology. Philadelphia: Saunders; 2011.
[25] Meldrum BS, Nanji N, Cornell RG. Lack of effect of aspartame or of L-phenylalanine on photically induced myoclonus in the baboon, Papio papio. Epilepsy Res 1989; 4(1): 1-7.
[26] Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol 2000; 405(1–3): 397-406.
[27] Filiano JJ. Neurometabolic diseases in the newborn. Clin Perinatol 2006; 33(2): 411-79.
[28] de Morais H, de Souza CP, da Silva LM, Ferreira DM, Werner MF, Andreatini R, et al. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav Brain Res 2014; 258: 52-64.
[29] McArdle WD, Katch FI, Katch VL. Essentials of exercise physiology. Philadelphia: Lippincott Williams and Wilkins; 2010.
[30] Piñero DJ, Connor JR. Iron in the brain: an important contributor in normal and diseased states. Neuroscientist 2000; 6(6): 435-53.
[31] Piloni NE, Fermandez V, Videla LA, Puntarulo S. Acute iron overload and oxidative stress in brain. Toxicology 2013; 314(1): 174-82.
[32] Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem 1994; 63(3): 793-807.
[33] Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol 2006; 5(8): 677-87.
[34] Collipp PJ, Chen SY, Maitinsky S. Manganese in infant formulas and learning disability. Ann Nutr Metab 1983; 27(6): 488-94.
[35] Kubrak OI, Husak VV, Rovenko BM, Storey JM, Storey KB, Lushchak VI. Cobalt-induced oxidative stress in brain, liver and kidney of goldfish Carassius auratus. Chemosphere 2011; 85(6): 983-9.
[36] Sims J, Roberts A, Daniel JW, Renwick AG. The metabolic fate of sucralose in rats. Food Chem Toxicol 2000; 38: S115-21.
*Corresponding author:Rodel Jonathan Santos Vitor II, DVM, Assistant Professorial Lecturer, Biology Department, De La Salle University, 2401 Taft Avenue, 0922 Manila, Philippines.
 Asian Pacific Journal of Tropical Biomedicine2016年2期
Asian Pacific Journal of Tropical Biomedicine2016年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
- Anti-hyperglycemic effects of aqueous Lenzites betulina extracts from the Philippines on the blood glucose levels of the ICR mice (Mus musculus)
- Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR
- Step-by-step external fixation of unstable pelvis with separate anterior and posterior modules
- Feasibility of using melatonin as a new treatment agent for Ebola virus infection: A gene ontology study
- Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
