贝伐珠单抗联合化疗治疗晚期结直肠癌的疗效观察
焦 婉,肖菊香,锁爱莉,赵晓艾,朱 娇
(1. 西安交通大学医学部,陕西西安 710061;2. 西安交通大学第一附属医院肿瘤内科,陕西西安 710061)
贝伐珠单抗联合化疗治疗晚期结直肠癌的疗效观察
焦 婉1,肖菊香2,锁爱莉2,赵晓艾2,朱 娇1
(1. 西安交通大学医学部,陕西西安 710061;2. 西安交通大学第一附属医院肿瘤内科,陕西西安 710061)
目的 观察贝伐珠单抗联合化疗治疗晚期结直肠癌的近期疗效、安全性及远期生存期。方法 回顾性分析2008年6月~2013年12月应用贝伐珠单抗联合以奥沙利铂、伊立替康、氟尿嘧啶及其衍生物为基础的化疗方案,治疗51例晚期结直肠癌的临床疗效、生存分析以及贝伐珠单抗的不良反应。结果 完全缓解(CR)4例,部分缓解(PR)13例,疾病稳定(SD) 15例,疾病进展(PD)19例,有效率(ER)为33.33%,疾病控制率(DCR)为62.75%。一线治疗32例,ER为40.63%,DCR为75%;二线治疗19例,ER为21.05%,DCR为42.11%。贝伐珠单抗联合一线、二线化疗患者的ER差异无统计学意义(P>0.05),但DCR差异有统计学意义(P=0.019),贝伐珠单抗联合不同化疗方案及分别治疗结、直肠癌患者的ER和DCR差异均无统计学意义(P>0.05)。贝伐珠单抗联合一线化疗的无进展生存时间(PFS)明显优于联合二线化疗(P<0.001),但总生存期(OS)差异无统计学意义(P=0.08);贝伐珠单抗联合FOLFIRI、FOLFOX、XELOX及XELIRI方案的中位PFS及OS差异有统计学意义(P<0.001),且以贝伐珠单抗联合以伊立替康为基础的化疗方案疗效更佳。贝伐珠单抗的不良反应主要有高血压7例(13.73%)、皮疹6例(11.76%)、蛋白尿2例(3.92%)、出血2例(3.92%),绝大多数是可控制的。结论 贝伐珠单抗联合化疗治疗晚期结直肠癌患者的疗效是肯定的,贝伐珠单抗一线治疗的DCR及PFS均优于二线,且以贝伐珠单抗联合伊立替康为基础的化疗方案疗效更佳。
贝伐珠单抗;FOLFOX;FOLFIRI;XELOX;XELIRI;转移性结直肠癌;化疗
结直肠癌发病率位于人类最常见恶性肿瘤的第四位,且在癌症死亡率中占据第二位[1-2]。随着社会发展和人们饮食结构的改变,中国近10年来结直肠癌的发病率日益上升[3],尽管其间氟尿嘧啶及其衍生物、奥沙利铂、伊立替康仍是治疗晚期结直肠癌的基本化疗药物,且取得了较大进步[4-6],但是其长期生存仍有待进一步提高。目前,贝伐珠单抗联合化疗已被确立为转移性结直肠癌的一线标准治疗方案[7],然而贝伐珠单抗联合化疗的最佳方案仍需在临床中不断摸索。本文对2008年6月至2013年12月在西安交通大学第一附属医院收治的51例应用贝伐珠单抗联合化疗的晚期结直肠癌患者的临床资料进行回顾性分析,现将治疗效果和不良反应总结如下。
1 资料与方法
1.1 一般资料
收集具有较完整临床资料的51例经病理组织学证实的转移性结直肠癌患者,其中男性34例,女性17例,年龄27~78岁,中位年龄为57岁;直肠癌31例(60.78%),结肠癌20例(39.22%);仅有1个器官转移者24例,其中肝转移11例,肺转移8例,盆腔转移3例,肾上腺转移1例,阴道转移1例,2个及以上器官转移者27例。高分化腺癌9例(17.65%),中分化腺癌36例(70.59%),低分化腺癌6例(11.76%)。入组标准:51例患者均至少具有1个影像学可测量病灶;血常规、肝肾功、凝血指标无明显异常;ECOG评分0~2分;无化疗禁忌症及使用贝伐珠单抗禁忌症。
1.2 治疗方案
51例患者均接受了贝伐珠单抗的治疗。27例使用贝伐珠单抗的剂量为5 mg/kg,每2周用药1次,其中有17例联合FOLFIRI方案(伊立替康+亚叶酸钙+氟尿嘧啶)化疗,10例联合FOLFOX方案(奥沙利铂+亚叶酸钙+氟尿嘧啶)化疗;24例患者使用贝伐珠单抗的剂量为7.5 mg/kg,每3周用药1次,其中13例联合XELOX方案(奥沙利铂+希罗达)化疗,11例联合XELIRI方案(伊立替康+希罗达)化疗。一线治疗者(首次化疗联合贝伐珠单抗治疗)32例(62.75%),二线治疗者(曾接受过一线化疗失败后而选择的其他化疗方案联合贝伐珠单抗治疗)19例(37.25%)。
1.3 疗效评价
按实体瘤疗效评价标准RECIST 1.1版分为:完全缓解(complete response, CR)、部分缓解(partial response, PR)、疾病稳定(stable disease, SD)和疾病进展(progressive disease, PD)。有效率(ER)=CR+PR,疾病控制率(DCR)=CR+RP+SD。不良反应评判标准按美国癌症研究所常见毒性评判标准NCI-CTC3.0版进行评价。
1.4 随访
末次随访时间截至2015年3月10日。无进展生存时间(PFS)为自治疗开始至疾病进展的时间,总生存期(OS)为自开始治疗至死亡或最后随访时间。随访时间为5~51月,中位随访时间为21.3月。随访率98%。并观察贝伐珠单抗的主要不良反应如高血压、皮疹等。
1.5 统计学处理
采用SPSS 16.0版统计软件进行统计学处理,计数资料用卡方检验(确切概率法),生存分析用Kaplan-Meier法,并用Log-rank法检验。以P<0.05为差异有统计学意义。
2 结 果
2.1 近期疗效
截至随访结束时间,全组CR 4例,PR 13例,SD 15例,PD 19例,ER为33.33%,DCR为62.75%。贝伐珠单抗联合一线、二线治疗患者ER差异无统计学意义,但DCR差异有统计学意义(P=0.019,表1);贝伐珠单抗联合化疗治疗直肠癌、结肠癌患者的ER和DCR差异无统计学意义(表2);贝伐珠单抗联合不同化疗方案治疗的患者ER和DCR差异无统计学意义(表3)。贝伐珠单抗联合化疗一线治疗结、直肠癌患者其DCR优于二线治疗者(P=0.019)。
2.2 远期疗效
截至随访结束时间,全组患者PFS的范围为4.1~19.1月,OS的范围为10.5~36.5月。中位PFS及中位OS分别为9.9月及18.4月。一线治疗与二线治疗的中位PFS分别为12.75月及8.7月,差异具有统计学意义(P<0.001)。贝伐珠单抗联合化疗一线治疗的PFS明显优于二线;一线治疗与二线治疗的中位OS分别为19.95月及17.6月,差异无统计学意义(P=0.08,图1)。
表1 贝伐珠单抗联合一线、二线治疗的近期疗效
Tab.1 Comparison of short-term efficacy of bevacizumab combined with chemotherapy as first-and second-1ine treatment

贝伐珠单抗联合化疗方案总例数(n)CR(n)PR(n)SD(n)PD(n)ER(%)DCR(%)联合一线化疗3231011840.6375.00联合二线化疗191341121.0542.11*合计51413151933.3362.75
与一线化疗组比较,*P<0.05(χ2=5.52,P=0.019)。
表2 贝伐珠单抗联合化疗治疗不同部位肠癌的近期疗效
Tab.2 Comparison of short-term efficacy of bevacizumab combined with chemotherapy for colon cancer and rectal cancer
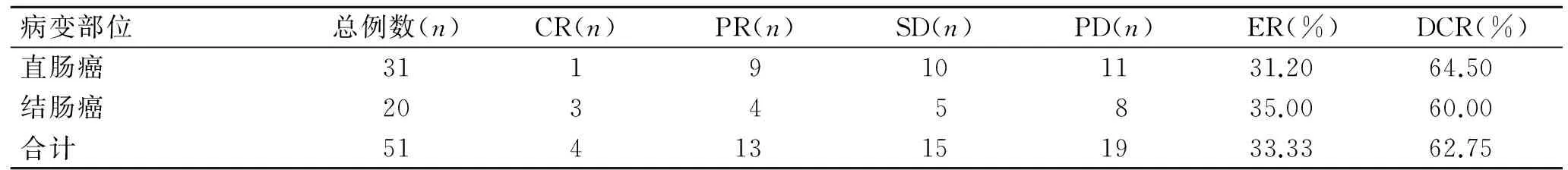
病变部位总例数(n)CR(n)PR(n)SD(n)PD(n)ER(%)DCR(%)直肠癌3119101131.2064.50结肠癌20345835.0060.00合计51413151933.3362.75
表3 贝伐珠单抗联合不同化疗方案的近期疗效
Tab.3 Comparison of short-term efficacy of bevacizumab combined with different chemotherapy regimens
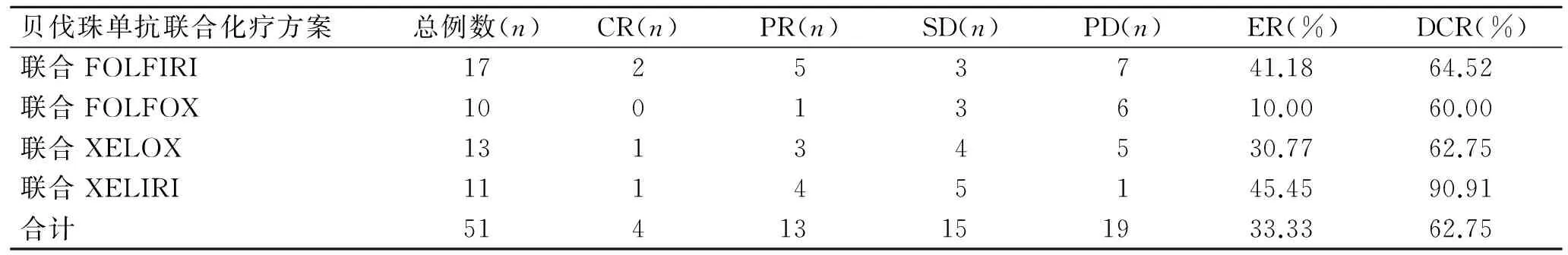
贝伐珠单抗联合化疗方案总例数(n)CR(n)PR(n)SD(n)PD(n)ER(%)DCR(%)联合FOLFIRI17253741.1864.52联合FOLFOX10013610.0060.00联合XELOX13134530.7762.75联合XELIRI11145145.4590.91合计51413151933.3362.75
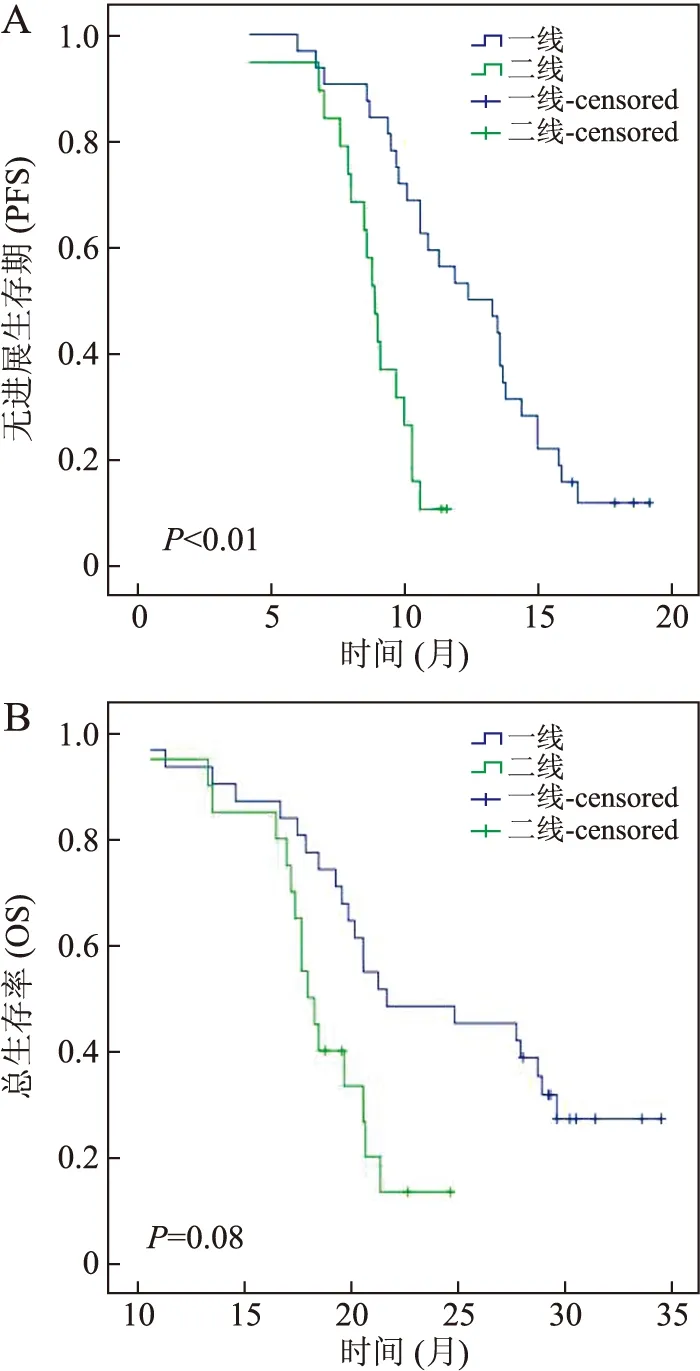
图1 贝伐珠单抗联合一线、二线化疗PFS(A)与OS(B)的比较
Fig.1 Comparison of PFS (A) and OS (B) of bevacizumab combined with chemotherapy as first- and second-line treatment
贝伐珠单抗联合FOLFIRI、FOLFOX、XELOX及XELIRI方案的中位PFS分别为9.8、9.8、9.7、10.5月,差异有统计学意义(P<0.001);贝伐珠单抗联合FOLFIRI、FOLFOX、XELOX及XELIRI方案的中位OS分别为18.4、18.3、18.2、19.8月,差异有统计学意义(P<0.001,图2)。贝伐珠单抗联合XELIRI方案治疗的PFS及OS均优于其余方案。
2.3 不同临床特征对生存的影响
对患者的不同临床特征进行单因素分析,结果显示PFS及OS与性别、年龄、原发部位之间差异无统计学意义(P>0.05),而与ECOG、分化程度和转移数目之间差异具有统计学意义(P<0.001,表4)。
2.4 不良反应
本组与贝伐珠单抗相关的不良反应:高血压7例(13.73%)、皮疹6例(11.76%)、蛋白尿2例(3.92%)、出血2例(3.92%)。其中,6例皮疹均为Ⅰ~Ⅱ度,停药1~2周后自行消失;高血压7例(其中3人既往有高血压病史,经药物治疗后血压控制良好),1例既往有高血压病史的患者因长期使用贝伐珠单抗(18月)出现脑出血而停用,其余6例经口服降压药后血压控制平稳;2例蛋白尿停药后消失;出血表现为鼻衄及痔疮,各1例,均为轻度,经药物治疗后症状消失。

图2 贝伐珠单抗联合不同化疗方案PFS与OS的比较
Fig.2 Comparison of PFS (A) and OS (B) of bevacizumab combined with different chemotherapy regimens
表4 不同临床特征对晚期结直肠癌患者生存的影响
Tab.4 Impacts of different clinical features on survival patients with advanced colorectal cancer
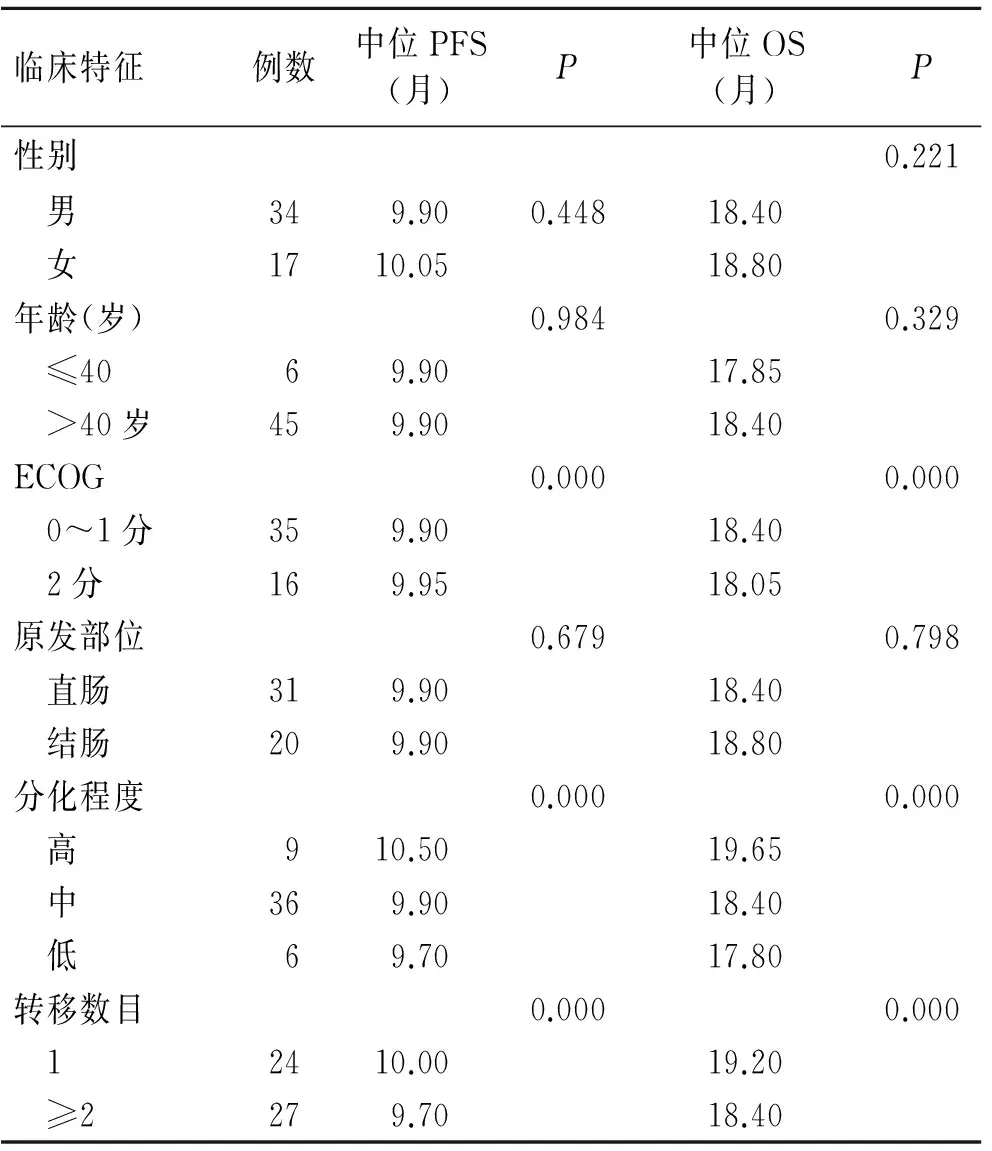
临床特征例数中位PFS(月)P中位OS(月)P性别0.221 男349.900.44818.40 女1710.0518.80年龄(岁)0.9840.329 ≤4069.9017.85 >40岁459.9018.40ECOG0.0000.000 0~1分359.9018.40 2分169.9518.05原发部位0.6790.798 直肠319.9018.40 结肠209.9018.80分化程度0.0000.000 高910.5019.65 中369.9018.40 低69.7017.80转移数目0.0000.000 12410.0019.20 ≥2279.7018.40
3 讨 论
自从在恶性肿瘤患者体内发现局部血管密度增加往往先于快速进展的转移肿瘤,靶向治疗肿瘤血管的概念就已成为关注焦点。血管内皮生长因子(vascular endothelial growth factor, VEGF)是重要的促成血管生成因子之一,所以对抗VEGF成为控制肿瘤发展的重要靶点之一。贝伐珠单抗是人源化单克隆IgG1抗体,能够抑制肿瘤新生血管生成并影响内皮细胞增殖。以VEGF/VEGFR为代表的靶向治疗联合化疗不仅提高了晚期结直肠癌患者的近期疗效,而且成为延长生存的重要选择。
多项国际临床试验均已表明,贝伐珠单抗联合化疗无论是一线还是二线均可提高晚期结直肠癌患者的疗效,延长生存期。HURWITZ等[7]报道的一项Ⅲ期临床试验结果显示,贝伐珠单抗一线联合化疗治疗组的PFS及OS分别为8.5月和18.3月,而安慰剂联合化疗组则分别为7.2月和15.1月,贝伐珠单抗一线组的疗效明显优于安慰剂组;贝伐珠单抗二线治疗中,COOTENBERG等[8]报道的一项Ⅲ期临床试验结果显示,FOLFOX4组和FOLFOX4+贝伐珠单抗组比较,前者PFS及OS分别为4.7月和10.8月,后者PFS及OS分别为7.3月和12.9月,联合贝伐珠单抗组的疗效明显优于单用化疗组。据此,美国FDA分别于2004年和2006年将贝伐珠单抗+IFL和贝伐珠单抗+FOLFOX治疗批准用于晚期结直肠癌患者的一线和二线治疗[7-8]。另有FIRE3和86406等多项临床研究从不同角度显示贝伐珠单抗联合化疗等靶向治疗提高了晚期结直肠癌患者的长期生存[9-10]。另外,近期有多篇文献报道显示,贝伐珠单抗联合以伊立替康为基础的方案疗效优于以奥沙利铂为基础的方案[8,11-15],其中1例肝、肺多发转移的直肠癌患者,经使用XELIRI+贝伐珠单抗治疗达PR后,分别行肝、肺转移灶切除术,达无疾病生存状态18月[16]。
本研究51例晚期结直肠癌患者经贝伐珠单抗联合化疗后,近期疗效:CR 4例,PR 13例,SD 15例,PD 19例,ER为33.33%,DCR为62.75%。贝伐珠单抗一线、二线联合化疗治疗晚期结直肠癌患者RR差异无统计学意义,但DCR差异有统计学意义;贝伐珠单抗联合不同化疗方案治疗的患者ER和DCR差异无统计学意义。远期疗效观察显示,贝伐珠单抗联合一线化疗的PFS明显优于二线联合化疗,但OS差异无统计学意义;贝伐珠单抗联合FOLFIRI、FOLFOX、XELOX及XELIRI方案的中位PFS及OS差异均有统计学意义,且以贝伐珠单抗联合以伊立替康为基础的化疗方案疗效更佳。与以上文献报道结果一致。更为重要的是,搜索同期单纯化疗治疗晚期结直肠癌患者的相关文献报道,其结果显示,中位PFS及OS分别为4.7~7.2月和9.65~15.1月[7-8,17-19],而本研究的中位PFS及中位OS分别为9.9月及18.4月,二者相比,贝伐珠单抗联合化疗具有明显优势。
本研究贝伐珠单抗的主要不良反应包括:高血压4例(7.84%)、皮疹6例(11.76%)、蛋白尿2例(3.92%)、出血2例(3.92%)。值得注意的是,1例患者在长期使用贝伐珠单抗(27月)的情况下因脑出血而死亡。晚期结直肠癌患者使用贝伐珠单抗是安全的,其相关不良反应均可控制;如果既往有高血压病史的患者在使用贝伐珠单抗时,一定要严密监测血压,根据患者血压控制情况决定是否继续应用。
综上所述,贝伐珠单抗联合化疗治疗晚期结直肠癌患者的疗效是肯定的,不良反应是可耐受的。贝伐珠单抗联合以伊立替康为基础的化疗方案在一线治疗时效果更为突出,值得关注。
[1] AHMEDIN J, FREDDIE B, MELISSA M, et al. Global cancer statistics[J]. CA Cancer J Clin, 2011, 61:69-90.
[2] AHMEDIN JEMAL, FREDDIE BRAY, MELISSA M, et al. Global cancer statistics[J]. CA Cancer J Clin, 2011, 61(2):69-90.
[3] ZHENG ZX, ZHENG SW, CHEN WQ. Colorectal cancer incidence and mortality in China[J]. Asian Pac J Cancer Prev, 2010, 15(19):8455-8460.
[4] JAMES R, JEFFREY M, JAMES C, et al. Outcome and natural history of patients with stage IV colorectal cancer receiving chemotherapy without primary tumor resection[J]. Ann Surg Oncol, 2012, 19(2):379-383.
[5] TOSHIAKI W, MICHIO I, YASUHIRO S, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer[J]. Int J Clin Oncol, 2015, 20(2):207-239.
[6] GIANTONIO BJ, CATALANO PJ, MEROPOL NJ, et al. Eastern Cooperative Ontology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200[J]. J Clin Oncol, 2007, 25(12):1539-1544.
[7] HERBERT I, HURWITZ LF, JOHN D, et al. Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic[J]. J Clin Oncol, 2005, 23:3502-3508.
[8] DURAN AO, HALIT K, MEHMET B, et al. XELOX plus bevacizumab vs. FOLFIRI plus bevacizumab treatment for first-line hemotherapy in metastatic colon cancer: a retrospective study of the Anatolian Society of Medical Oncology[J]. Asian Pac J Cancer Prev, 2014, 15 (23):10375-10379.
[9] ARIEL H, SARI GD, EREZ B, et al. Thereal-lifeimpact of adding bevacizumab to first-line therapy in metastatic colorectal cancer patients: A large Israeli retrospective cohort study[J]. Acta Oncologica, 2015, 54:164-170.
[10] BAI L, ZHANG DS, WU WJ, et al. Clinical outcomes of Chinese patients with metastatic colorectal cancer receiving first-line bevacizumab-containing treatment[J]. Med Oncol, 2015, 32:27.
[11] ALEXANDROS A, IOANNIS M, SAVVOULA M, et al. Bevacizumab added to the irinotecan and capecitabine combination for advanced colorectal cancer: A well-tolerated, active and convenient regimen[J]. Anticancer Res, 2008, 28:3087-3092.
[12] SUENAGA M, MIZUNUMA N, MATSUSAKA S, et al. A phase I/II study of biweekly capecitabine and irinotecan plus bevacizumab as second-line chemotherapy in patients with metastatic colorectal cancer[J]. Drug Des Dev Ther, 2015:9 1653-1662.
[13] CAO RH, ZHANG S, MA DD, et al. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer[J]. Med Oncol, 2015, 32(1):1-5.
[14] YASUO H, TOMOHIRO N, YAMAGUCHI T, et al. A Phase Ⅰ/Ⅱ study of XELIRI plus bevacizumab as second-line chemotherapy for Japanese patients with metastatic colorectal cancer (BIX Study)[J]. The Oncologist, 2014, 19:1131-1132.
[15] FUCHS CS, MARSHALL J, BARRUECO J, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluompyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study[J]. Clin Oncol, 2008, 26(4):689-690.
[16] AISU NY, YOSHIDA Y, ISHII F, et al. A successfully resected case of recurrent lung and liver metastases of rectal cancer treated with XELIRI + bevacizumab therapy[J]. Case Rep Oncol, 2013, 6:143-147.
[17] 陈栋晖,宋卫峰,王理伟. 伊立替康二线治疗晚期结直肠癌疗效观察[J]. 中国癌症杂志,2008,18(7):542-544.
[18] 金学军,王慧,李卫军. 晚期结直肠癌Folfox6方案33例化疗观察[J]. 中国医药指南,2013, 11(2):143-144.
[19] LIU HY, ZHANG B. Clinical study of oxaliplatin or irinotecan plus capecitabine in the treatment of advanced colorectal cancer[J]. Chin J Clin Pharmacol, 2013, 20(4):243-245.
(编辑 国 荣)
Therapeutic effects of bevacizumab combined with chemotherapy regimens on metastatic colorectal cancer
JIAO Wan1, XIAO Ju-xiang2, SUO Ai-li2, ZHAO Xiao-ai2, ZHU Jiao1
(1. Xi’an Jiaotong University Health Science Center, Xi’an 710061; 2. Department of Oncology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, China)
Objective To investigate the effects of bevaeizumab combined with chemotherapy regimens on short-term efficacy, safety and short-term survival of metastatic colorectal cancer (mCRC). Methods We made a retrospective analysis of efficacy and patient’s survival and adverse events of bevacizumab combined with chemotherapy regimens for 51 mCRC patients treated in the First Affiliated Hospital of Xi'an Jiaotong University from June 2008 to December 2013. Results Of the 51 patients with mCRC, 4 patients achieved complete response, 13 achieved partial response (PR), 15 had stable disease (SD) and 19 had progressive disease (PD), with an effective ness rate (ER) of 33.33% and disease control rate (DCR) of 62.75%. First- and second-line treatment or different chemotherapy regimen combined with bevacizumab did not significantly differ in ER (P>0.05). However, DCR of 32 patients who received first-line treatment of chemotherapy combined with bevacizumab was superior to that of the others who received second-line treatment of chemotherapy combined with bevacizumab (P=0.019). The median PFS of 32 patients who received first-line treatment of chemotherapy combined with bevacizumab was higher than that of the others who received second-line treatment of chemotherapy combined with bevacizumab (P<0.001); however, there were no significant difference in OS (P=0.08). The different chemotherapy of bevacizumab combined with FOLFOX regimen, FOLFIRI regimen, XELOX regimen or XELIRI regimen differed significantly in median PFS and OS (P<0.001). The adverse events related to bevacizumab included hypertension (7 cases, 13.73%), skin eruption (6 cases, 11.76%), proteinuria (2 cases, 3.92%) and bleeding (2 cases, 3.92%), most of which could be alleviated by drug treatment. Conclusion Bevacizumab combined with chemotherapy regimen is effective and well tolerated in patients with metastatic colorectal cancer. The first-line treatment is superior to the second-line one for PFS and DCR, and chemotherapy regimens based on beacizumab combined with irinotecan have a better therapeutic effect.
bevacizumab; FOLFOX; FOLFIRI; XELOX; XELIRI; metastastatic colorectal cancer; chemotherapy
2015-04-18
2015-10-09
肖菊香. E-mail:1983181726@qq.com
R735
A
10.7652/jdyxb201601021
优先出版:http://www.cnki.net/kcms/detail/61.1399.R.20151208.1625.002.html(2015-12-08)

