HPLC-MS/MS测定城市废水中的10种药物与个人护理用品
卢红选,刘卫国,
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安710061;2. 西安交通大学 人居环境与建筑工程学院,西安 710049)
HPLC-MS/MS测定城市废水中的10种药物与个人护理用品
卢红选1,刘卫国1,2
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安710061;2. 西安交通大学 人居环境与建筑工程学院,西安 710049)
采用固相萃取技术对水样进行预处理,结合液相色谱-串联质谱分析方法(HPLC-MS/ MS),建立了同时检测城市废水中包括扑热息痛、萘普生、磺胺甲恶唑、磺胺二甲嘧啶、三氯生、双氯芬酸钠、三氯卡班、盐酸四环素、盐酸土霉素、吉非罗平在内共计10种药物与个人护理用品(PPCPs)的分析检测方法。采用中性条件萃取水样,控制上样流速为3 — 5 mL · min–1,用甲醇溶液洗脱。纯水的平均加标回收率为40.8% — 104.5%,相对标准偏差为5.0% — 25.5%(n = 3)。应用所建立的分析方法,对西安浐河表层水进行了分析。结果表明:该方法可用于城市废水PPCPs的检测。10种目标物质中,共检测到4种,其含量为1.4 — 15.0 ng · L–1。
固相萃取;高效液相色谱-串联质谱;药物与个人护理品;城市废水
药物与个人护理品(pharmaceuticals and personal care products,PPCPs)是一类新型的环境污染物,主要是指各种药物(如抗生素、类固醇、消炎药、镇静剂、抗癫痫药、避孕药、神经兴奋剂等)以及个人护理品(如化妆品中的合成香料、显影剂、遮光剂、驱蚊剂、消毒杀菌剂等)等。研究认为:水环境中残留的PPCPs可能会导致人类致癌或过敏性反应,并可能使细菌产生耐药性及抗生素抗性基因的传递和扩散,干扰天然细菌的生态系统,从而威胁人类健康(Daughton and Ternes,1999)。一系列监测研究表明:药物与个人护理品已经成为环境中广泛存在的一类新兴污染物,在 河 流(Glen et al,2003;Tixier et al,2003;Yu and Chu,2009;Zhang et al,2011;Wu et al,2014)、 湖 泊(Buser and Theobald,1998;Glen et al,2003;Tixier et al,2003;Blair et al,2013;Fergusonet al,2013;Zhu et al,2013)、海洋(Weigel et al,2002;Del Rosario et al,2014)、地下水(Barnes et al,2008)、城市污水(Carmona et al,2014)、饮用水(Carmona et al,2014)、食品(Wu et al,2012;Baron et al,2014)等样品中被检出。由于其本身的特殊性质,它们在环境中的残留及潜在风险引起了人们越来越多的忧虑。
传统的PPCPs的检测方法有微生物检测法,分光光度检测法、气相色谱、液相色谱、电泳分析法等。自20世纪90年代以来,随着新型接口技术,如电喷雾离子化(ESI)、大气压化学电离(APCI)、大气压光电离(APPI)等的成熟,液相色谱-质谱(HPLC-MS)技术在PPCPs检测研究中得到了广泛的应用(Tixier et al,2003;Barnes et al,2008;Blair et al,2013;Zhu et al,2013;Carmona et al,2014)。高分辨率或串联质谱(MS-MS)可提供目标物质结构的详细信息,具有高选择性和高灵敏性,非常适用于环境样品中痕量药物的分析检测,已成为环境样品中PPCPs化合物的分析检测的一个强有力的工具。据文献报道,我国水环境PPCPs化合物在环境水样中检出的质量浓度通常是ng · L–1到μg · L–1级水平(Yang et al,2011;王丹等,2014),目前报道出来的环境中检测到的PPCPs的种类多达160多种,因此,开展快速、灵敏、可靠的PPCPs化合物集成定量检测方法尤为重要。不同于以往文献中报道的药物检测方法多数仅适用于同类药物,本文选取包括抗生素类(磺胺甲恶唑、磺胺二甲嘧啶、盐酸四环素、盐酸土霉素),消炎止痛类(扑热息痛、萘普生、三氯生、双氯芬酸钠、三氯卡班)以及血脂调节剂(吉非罗平)等在内的共10种药物与个人护理用品为目标物,基于高效液相色谱串联质谱联用技术,采用固相萃取技术对样品进行前处理,建立了城市废水中PPCPs的分析方法,并以西安一城市废水为代表,对这一新方法进行了考察。
1 实验部分
1.1 仪器与试剂
PPCPs标准品(扑热息痛(ACT)、萘普生(NPX)、吉非罗平(GF)、磺胺甲恶唑(SMX)、磺胺二甲嘧啶(SMT)、三氯生(TCS)、双氯芬酸钠(DF)、三氯卡班(TCC)、盐酸四环素(TC)、盐酸土霉素(OTC(购自Dr. Ehrenstorfer公司(德国);罗红霉素-d7(RTM-d7)购自于terc公司。甲醇、甲酸为HPLC级,其余药品或试剂均为分析纯。
1.2 样品前处理
用47 mm的 玻 璃 纤 维 滤 纸(GF/F,Whatman)过滤水样(400 mL),过滤后的水样,加入200 μL内标混合液,调节pH = 7.0。固相萃取时,先用3×5 mL高纯水通过SPE小柱(PolyseryHLB,6 mL / 500 mg,CNW)进行活化和平衡,而后将含有内标的过滤液以5 —10 mL · min–1的流速通过SPE小柱。接着将5 mL高纯水通过SPE小柱,以清洗小柱,并继续抽真空30 min,除去水分。再以1 mL · min–1的流速用甲醇洗脱小柱,洗脱液收集于10 mL具塞玻璃刻度离心管中。最后以高纯氮吹扫洗脱液(水浴温度35℃)至刚好吹干,移入自动进样样品瓶并用甲醇定容至0.5 mL,待色谱分析(流程见图1)。
2 结果与讨论
2.1 HPLC-MS/MS条件优化
根据目标化合物的理化性质,选择ESI为离子源。首先配制1 μg · L–1的10种PPCPs化合物标准液,之后根据化合物性质,结合文献报道,选取ESI+或者ESI–模式,以流动注射分析(Flow Injection Analysis,FIA)的方法分别确定各目标抗生素的特征离子对。利用获取的全部特征离子及离子间的丰度比进行定性分析,以丰度最高的特征离子响应与浓度的关系进行定量分析。10种目标化合物优化的质谱参数见表1。

图1 水中PPCPs 分析方法流程Fig.1 Flow chart for determining the PPCPs in water
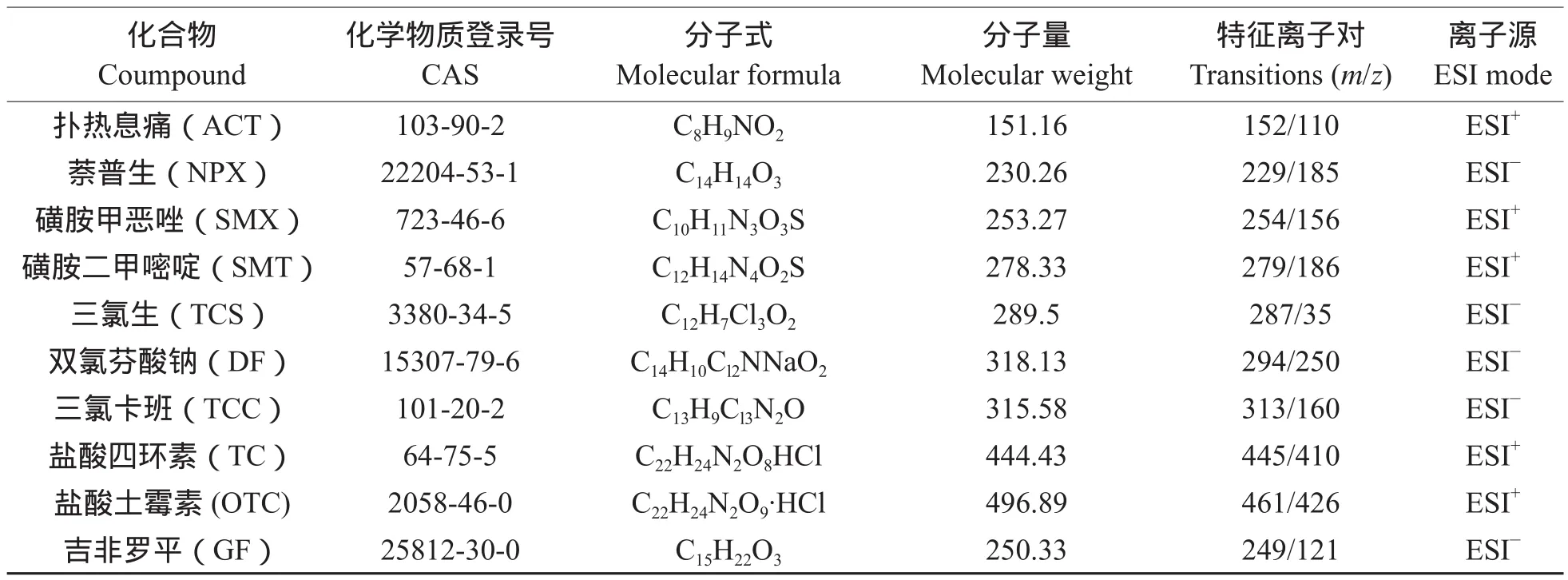
表1 10种目标化合物的详细信息及HPLC-MS/MS参数Tab.1 Details of the 10 target compounds and operating parameters of HPLC-MS/MS
2.2 HPLC-MS/MS测试分析
采用高效液相色谱-串联质谱(岛津HPLCESI-MS/MS 8030)对样品进行测定。流动相A为甲醇溶液,B为含0.01%甲酸的高纯水溶液,流速为0.35 mL · min–1。梯度洗脱顺序为:0 — 10 min,20% A和80% B;10 — 35 min,90% A和10% B。柱温40℃。进样量10 μL。10种PPCPs化合物的总离子流色谱图见图2。
配制1—250 μg · L–16个不同浓度的标准溶液,以目标化合物的浓度为横坐标,不同浓度目标化合物的峰面积为纵坐标,做线性回归分析。10种PPCPs化合物的工作曲线、相关系数和检出限(3倍信噪比)见表2。结果表明:目标化合物在两个数量级浓度范围内具有很好的线性关系。与文献报道相比,PPCPs化合物的线性范围以及检出限均在合理范围内,考虑到目前我国水环境PPCPs化合物在环境水样中检出的质量浓度通常是ng · L–1到μg · L–1级水平,本方法可实际应用于环境中水样的检测。
以高纯水为介质,分别做3组平行试验,利用回收率和空白实验保证实验的准确性。取400 mL高纯水样,定量加入500 μL浓度为25 μg· L–1的PPCPs混标溶液和200 μL内标溶液,按照图1所示流程对样品进行前处理和仪器分析。结果表明:纯水中的平均加标回收率为40.8% — 104.5%,相对标准偏差为5.0% — 25.5%(n=3)。
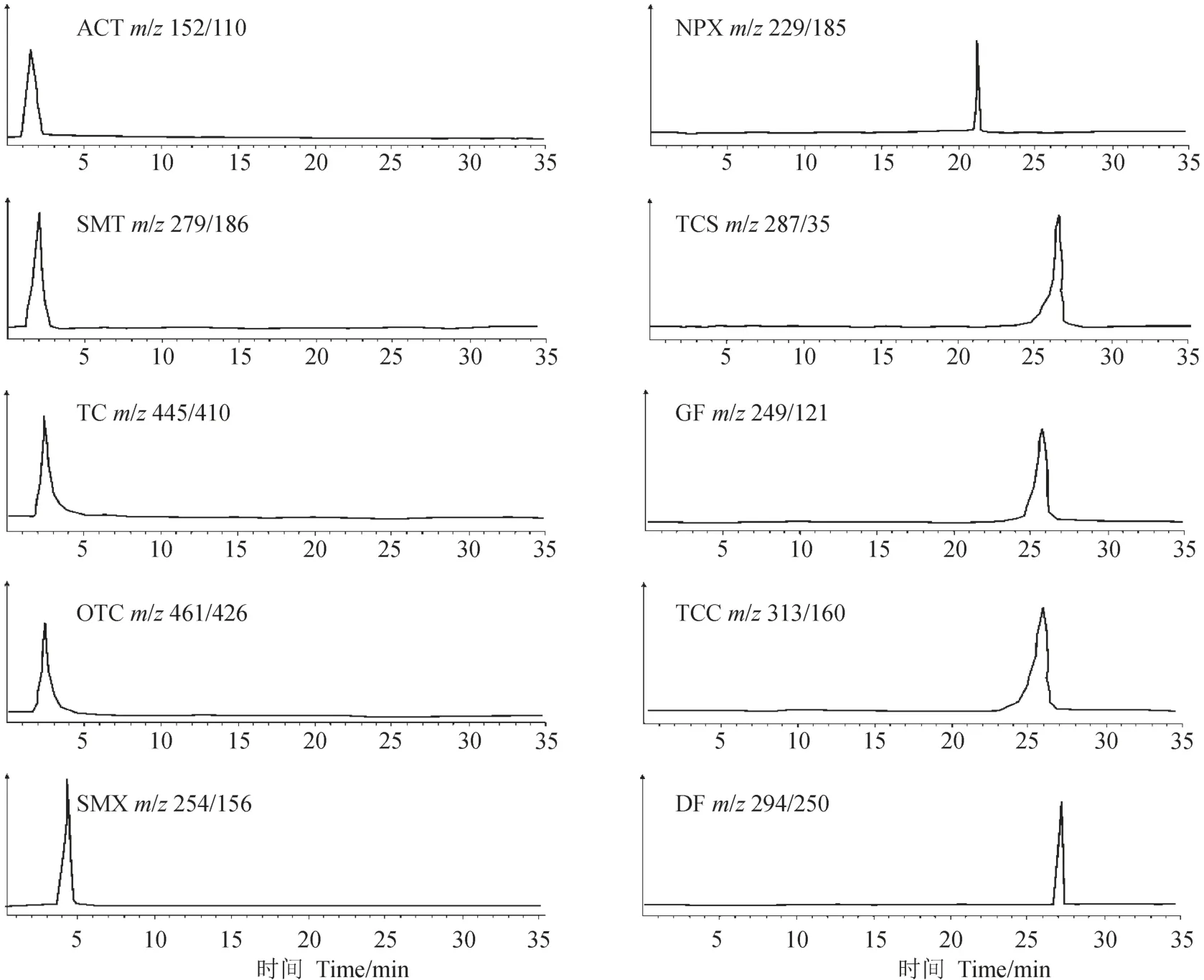
图2 10种PPCPs 的总离子流色谱Fig.2 Total ion chromatograms of the 10 PPCPs
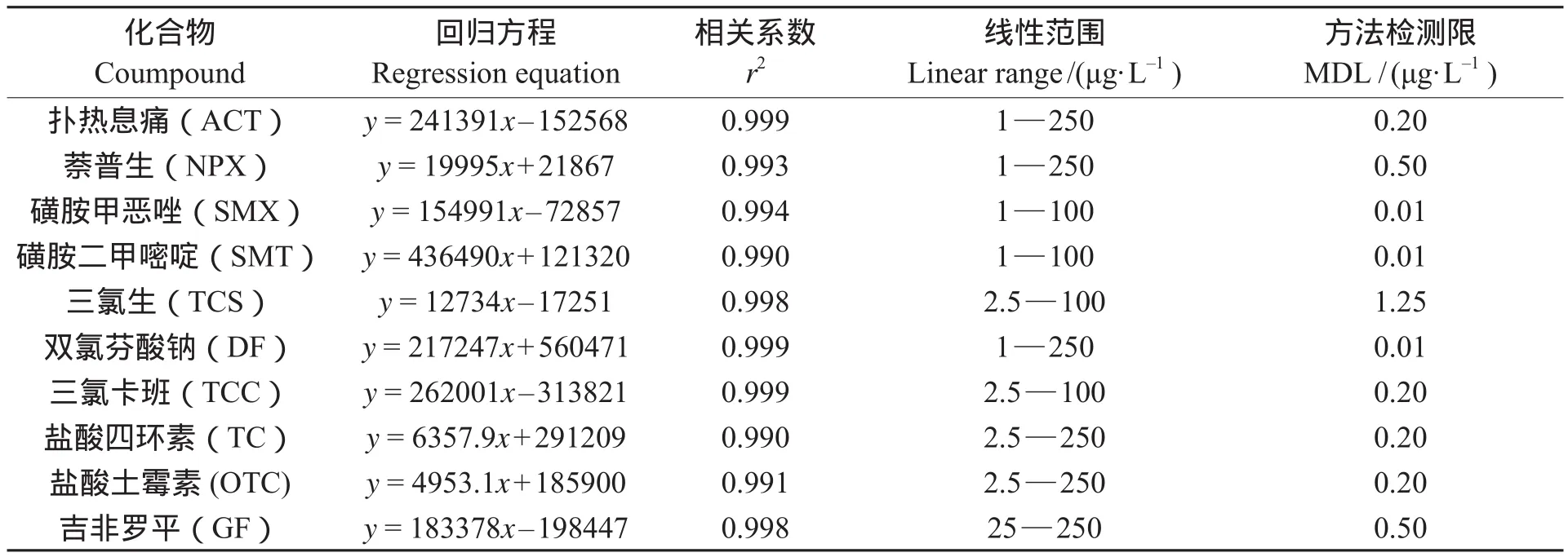
表2 10种PPCPs化合物的线性回归方程、相关系数及检出限Tab.2 Regression equations, correlation coeffi cients (r2) and method detection limits of the 10 PPCPs
2.3 实际水样分析
采集西安市区浐河表层水,采集时间为2015年10月。按照图1所示流程进行样品处理和HPLCMS/MS分析,结果表明:10种PPCPs样品中,共检测到4种PPCPs,分别为ACT(1.4 ng · L–1),NPX(1.4 ng· L–1),TCC(4.8 ng · L–1)以及GF(15.0 ng · L–1),其他6种PPCPs未检测到或者浓度低于方法检测限。
3 结论
结合文献报道以及实验室实测结果,本文建立了利用HPLC-MS/MS分析水样中10种痕量PPCPs化合物的检测分析方法,并利用该方法对西安浐河水样进行了PPCPs化合物的检测分析。由于环境中的PPCPs化合物种类繁多,各类化合物的性质差别较大,对样品前处理以及仪器检测方法均提出了更多的挑战,仅用一种方法无法实现所有PPCPs化合物的同时检测分析。因此,今后将进一步优化前处理条件,建立更多类别的PPCPs化合物检测分析方法,并进一步加强注重迁移转化规律及环境风险方面的研究。
王 丹, 隋 倩, 赵文涛, 等. 2014. 中国地表水环境中药物和个人护理品的研究进展[J]. 科学通报, 59 (9): 743 - 751. [Wang D, Sui Q, Zhao W T, et al. 2014. Pharmaceutical and personal care products in the surface water of China: A review [J]. Chinese Science Bulletin, 59(9): 743 - 751.]
Barnes K K, Kolpin D W, Furlong E T, et al. 2008. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—ⅠGroundwater [J]. Science of the Total Environment, 402(2 / 3): 192 - 200.
Baron P A, Love D C, Nachman K E. 2014. Pharmaceuticals and personal care products in chicken meat and other food animal products: A market-basket pilot study [J]. Science of the Total Environment, 490: 296 - 300.
Blair B D, Crago J P, Hedman C J, et al. 2013. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern [J]. Chemosphere, 93: 2116 - 2123.
Buser H R, Theobald M D. 1998. Occurrence of the pharmaceutical drug clofibric acid and the herbicide mecoprop in various Swiss lakes and in the North Sea [J]. Environmental Science and Technology, 32(1): 188 - 192.
Carmona E, Andreu V, Picó Y. 2014. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water [J]. Science of the Total Environment, 484: 53 - 63.
Daughton C G, Ternes T A. 1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? [J]. Environmental Health Perspectives, 107: 907 - 938.
Del Rosario K L, Mitra S, Humphrey C, et al. 2014. Detection of pharmaceuticals and other personal care products in groundwater beneath and adjacent to onsite wastewater treatment systems in a coastal plain shallow aquifer [J]. Science of the Total Environment, 487: 216 - 223.
Ferguson P J, Bernot M J, Doll J C, et al. 2013. Detection of pharmaceuticals and personal care products (PPCPs) in near-shore habitats of southern Lake Michigan [J]. Science of the Total Environment, 458: 187 - 196.
Glen R B, Helge R, Deborah A G, et al. 2003. Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada [J]. Science of the Total Environment, 311(1/2/3): 135 - 139.
Tixier C, Singer H P, Oellers S, et al. 2003. Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofenand naproxen in surface waters [J]. Environmental Science and Technology, 37(6): 1061 - 1068.
Weigel S, Kuhlmann J, Huhnerfuss H. 2002. Drugs and personal care products as ubiquitous pollutants: occurrence and distribution of clofibric acid, caffeine and DEET in the North Sea [J]. Science of the Total Environment, 295(1/2/3):131 - 141.
Wu C X, Huang X L, Witter J D, et al. 2014. Occurrence of pharmaceuticals and personal care products and associated environmental risks in the central and lower Yangtze River, China [J]. Ecotoxicology and Environmental Safety, 106: 19 - 26.
Wu X Q, Conkle J L, Gan J. 2012. Multi-residue determination of pharmaceutical and personal care products in vegetables [J]. Journal of Chromatography A, 1254: 78 - 86.
Yang J F, Ying G G, Zhao J L, et al. 2011. Spatial and seasonal distribution of selected antibiotics in surface waters of the Pearl Rivers, China [J]. Journal of Environmental Science and Health Part B: Pesticides Food Contaminants and Agricultural Wastes, 46: 272 - 280.
Yu C P, Chu K H. 2009. Occurrence of pharmaceuticals and personal care products along the West Prong Little Pigeon River in east Tennessee, USA [J]. Chemosphere, 75: 1281 - 1286.
Zhang D D, Lin L F, Luo Z X, et al. 2011. Occurrence of selected antibiotics in Jiulongjiang River in various seasons, South China [J]. Journal of Environmental Monitoring, 13: 1953 - 1960.
Zhu S C, Chen H, Li J N. 2013. Sources, distribution and potential risks of pharmaceuticals and personal care products in Qingshan Lake basin, Eastern China [J]. Ecotoxicology and Environmental Safety, 96: 154 - 159.
Determination of 10 pharmaceuticals and personal care products in waste water by HPLC-MS/MS
LU Hongxuan1, LIU Weiguo1,2
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China; 2. School of Human Settlements and Civil Engineering, Xi’an Jiaotong University, Xi’an 710049, China)
Background, aim, and scope Pharmaceuticals and personal care products (PPCPs) represent a variety of chemical, widely used by consumers on a daily basis which include prescription and non-prescription drugs, cosmetics, cleansers, detergents and fragrance produces. PPCPs are considered potentially hazardous compounds because some are ubiquitous, persistent, and biologically active compounds with recognized endocrine disrupting functions (Daughton and Ternes, 1999). These compounds have been widely detected in various environmental matrices throughout the world including rivers (Glen et al, 2003; Tixier et al, 2003; Yu and Chu, 2009; Zhang et al, 2011; Wu et al, 2014), lakes (Buser and Theobald, 1998; Glen et al, 2003; Tixier et al, 2003; Blair et al, 2013; Ferguson et al, 2013; Zhu et al, 2013), oceans (Weigel et al, 2002; Del Rosario et al, 2014), groundwater (Barnes et al, 2008), waste and drinking water (Carmona et al, 2014) and food (Wu et al, 2012; Baron et al, 2014). Detection methods of PPCP include spectrophotometry, gas chromatography, liquid chromatography and electrophoresis. The recent advances in analytical instrumentation have allowed the unequivocal identifi cation and confi rmation of the presence of any compound at very low levels using LC-MS2.The multiple reaction monitoring (MRM) allows monitoring two transitions between precursor and product ions. It is possible to quantify and confi rm the presence of PPCPs at very low concentration levels. However, due to the absence of offi cial monitoring protocols, there is an increasing demand of analytical methods that allow the determination of those compounds in order to obtain more information regarding their behavior and fate in the environments. Therefore, we proposed here a method for the determination of ten pharmaceuticals and personal care products, including acetaminophen, naproxen, diclofenac, sulfamethoxazole, sulfadimidine, triclosan, triclocarban, tetracycline hydrochloride, oxytetracycline and gemfi brozile, in waste water using high performance liquid chromatography-tandem mass spectrometry. The aim of this work is to develop an effi cient method for determination of various PPCPs in water. Materials and methods Filtered water samples were extracted using solid-phase extraction cartridges (SPEs) extraction. HLB SPEs (Poly-sery HLB, 6 mL / 500 mg, CNW) were conditioned with 3×5 mL of water. Water samples were allowed to pass through the cartridges at a fl ow rate of approximately 5 — 10 mL · min–1. After sample loading, the cartridges were then subsequently dried for 30 min under full vacuum, and subsequently the ten pharmaceutical compounds were eluted with 10 mL of methanol. The residue was dissolved in 0.5 mL of methanol and transferred into vials for analysis. The PPCPs were analyzed using liquid chromatography-mass spectrometry (LC-MS) with a Shimadzu 8030 system equipped with an autosampler and Labsolutions manager software. Results Electrospray ionization (ESI) was used as LC-MS interfaces since it is the most frequently used ionization mode which is a soft ionization technique, suitable for polar and moderately non–polar compounds. Fragmentor, collision energy, and other source parameters were optimized by injecting individual standard solutions into mass spectrometer by fl ow injection analysis (FIA). After that, two different MRM transitions were selected for each compound: one for quantifi cation and one for qualifi cation. These ions were monitored under time scheduled MRM conditions. The analysis was done with electrospray ionization in negative mode (ESI–) for TCC, NPX, TCS, DF, GF and in positive mode (ESI+) for the ACT, SMX, SMT, TC, OTC. The initial mobile phase proportion was 20% A and 80% B where A=methanol and B = formic acid:water 1:9999, held for 10 min. A was then increased linearly to 90% in 25 min. The MRM transitions for different PPCPs are as follows: ACT m/z 151/110; NPX m/z 229/185; SMX m/z 254/156; SMT m/z 279/186; TCS m/z 287/35; DF m/z 294/250; TCC m/z 313/60; TC m/z 445/410; OTC m/z 461/426; GF m/z 249/121. Discussion The linearity of the MSMS detector was tested with matrix extracts containing PPCPs at concentration between 1 μg · L–1and 250 μg · L–1for ACT, NPX and DF, between 1 μg · L–1and 100 μg · L–1for SMX and SMT, between 2.5 μg · L–1and 100 μg · L–1for TCS and TCC, between 2.5 μg · L–1and 250 μg · L–1for TC, OTC and GF. The average recoveries of the target compounds in the spiked pure water samples ranged from 40.8% — 104.5% with the relative standard deviations ranged from 5.0% — 25.5% (n = 3). The waste water sample collected from Chanhe River in Xi’an was investigated as a case study. Among the 10 PPCPs, 4 PPCPs were detected and the concentrations ranged from 1.4 ng · L–1to 15.0 ng · L–1. Conclusions A method using HPLCMS/MS has been developed and validated for determination of 10 PPCPs (TCC, NPX, TCS, DF, GF, ACT, SMX, SMT, TC and OTC) in water. Subsequently, the method was successfully applied to analysis of the investigated chemicals in water samples collected from Chanhe River in Xi’an. Recommendations and perspectives The complexity of the biological matrices and the low concentration levels of these compounds make necessary the use of advanced sample treatment procedures, sample clean-up, to remove potentially interfering matrix components, as well as the concentration of analytes. Increased attention would have to be paid to metabolites generated in the organisms and released into the environment as well as to metabolites generated in the environment itself by biodegradation, photolytic or oxidation reactions.
solid phase extraction; HPLC-MS/MS; pharmaceuticals and personal care products (PPCPs); waste water
LU Hongxuan, E-mail: luhx@ieecas.cn
10.7515/JEE201604010
2016-03-24;录用日期:2016-06-06
Received Date:2016-03-24;Accepted Date:2016-06-06
国家自然科学基金项目(41572157)
Foundation Item:National Natural Science Foundation of China (41572157)
卢红选,E-mail: luhx@ieecas.cn

