1MTA2基因沉默对人乳腺癌MCF-7/ADR多药耐药的逆转作用
严梅娣
浙江省宁波市第七医院普外科,浙江宁波 315202
1MTA2基因沉默对人乳腺癌MCF-7/ADR多药耐药的逆转作用
严梅娣
浙江省宁波市第七医院普外科,浙江宁波 315202
目的乳腺癌是女性中最常见的恶性肿瘤,本研究探讨MTA2对乳腺癌耐药的作用及机制。方法CCK-8检测MCF-7/ADR和MCF-7细胞株对阿霉素的耐药情况。体外化学合成MTA2序列特异性的shRNA,经慢病毒转染人乳腺癌细胞系,Western blot检测慢病毒介导的MTA2敲减效率以及PI3K/AKT/ NF-κB信号通路蛋白。Annexin V-FITC和DAPI染色检测细胞凋亡情况。结果MCF-7/ADR细胞中MTA2表达量显著高于MCF-7细胞株,慢病毒介导的基因沉默显著的敲减了MCF-7/ADR细胞中的MTA2。敲除MTA2逆转MCF-7/ADR细胞的耐药作用并促进细胞凋亡。敲除MTA2通过抑制PI3K/AKT通路抑制下游NF-κB通路活化,并下调p-gp蛋白表达而逆转耐药性。结论敲除MTA2可以逆转MCF-7/ADR细胞的耐药作用,表明MTA2可能参与调节乳腺癌多药耐药作用。
MTA2;乳腺癌;耐药
乳腺癌是女性最常见的恶性肿瘤之一[1],化疗是乳腺癌重要的治疗手段,然而化疗过程乳腺癌容易对药物产生耐受,严重影响了乳腺癌化疗的效果。随着近年来对肿瘤分子生物学和基因功能学研究的深入,人们逐渐认识到某些肿瘤产生耐药的内在原因。因此,寻找调控乳腺癌耐药的关键基因,明确其在乳腺癌耐药机制产生过程中的病理机制,是乳腺癌治疗领域重要的研究方向。
MTA2基因存在于大多数人体组织和器官,特别是在脑组织中的含量,肝脏和睾丸丰富[2-6]。目前研究发现MTA2主要是通过调节雌激素通路,细胞凋亡和细胞骨架形成,共同促进肿瘤的侵袭和转移的过程[2,7,8]。相关的研究表明组织中MTA2过表达与人类恶性肿瘤的发展有密切关系,如在乳腺癌、结肠癌、肺癌、食管癌、胃癌等肿瘤中[9-15],MTA2均成过度表达。然而MTA2与肿瘤耐药的相关研究较少,本研究拟通过检测耐药细胞株MCF-7/ADR中MTA2的表达情况以及沉默MTA2后MCF-7/ ADR对阿霉素的耐药情况,探讨MTA2在乳腺癌耐药中的作用及机制。
1 材料与方法
1.1 材料
人乳腺癌细胞株MCF-7购自于中科院细胞库,MCF-7/ADR购自于上海博谷生物科技有限公司。细胞培养于37℃,5%CO2,含10%胎牛血清的RPM1640培养基(培养基和血清均购于Gibco)。兔抗AKT、P-AKT、g-pg抗体(Epitomics),鼠抗I κBα、NF-κB、cleaved-caspased-3、GAPDH抗体(ProteinTech),羊抗兔HRP抗体,羊抗鼠HRP抗体(Santa Cruz Biotechnology),CCK-8试剂盒购自Dojindo,Annexin V/PI凋亡试剂盒购自Invitrogen。MTA2敲减慢病毒购自上海ji'man吉满生物科技公司。
1.2 方法
1.2.1 蛋白免疫印迹杂交(Western blot) 乳腺癌细胞用阿霉素处理48h后,收集细胞沉淀,提取细胞总蛋白,BCA法测定蛋白浓度后,每个样品上样40g,进行蛋白电泳,电转移到PVDF膜(美国MiLLipore公司)上,5%牛奶封闭后顺序加一抗(1∶1000),二抗(1∶5000),进行杂交,ECL化学发光试剂盒(美国MiLLipore公司)检测杂交信号。
1.2.2 细胞活性实验 将MCF-7和MCF-7/ADR细胞消化后,分别以每孔10 000个细胞的密度接种于96孔培养板,每个时间点做5个平行样本,将培养板在37℃,5%CO2的条件下培养。12h细胞加入阿霉素(0、20、40、80、160μg/mL),处理48h,向每孔加入10μL CCK-8检测试剂,在培养箱内孵育2h。用酶标仪测定在450nm处的吸光度,计算每5孔的吸光度值的平均值和标准差,计算细胞的活性。
1.2.3 慢病毒感染乳腺癌细胞沉默MTA2 乳腺癌细胞MCF-7/ADR铺于6孔板,24h后等细胞贴壁后,加入慢病毒5μL MOI为10,同时加入4μg PB。4h后换成新鲜的完全培养基。48h后收集细胞,Western blot检测MTA2敲减效率,并进行后续试验。
1.2.4 细胞周期与凋亡检测 阿霉素处理48h后的细胞,用胰酶消化收集细胞(培养基上清一起收集),1000rpm,5min离心,然后,细胞用预冷的PBS洗两遍。用1倍结合缓冲液重悬细胞,调节细胞浓度到1×106Cells/mL。接着,加入5μL Annexin V-FITC及1μL 100μg/mL的PI工作液,轻轻混匀,避光室温反应15min。最后加入1倍结合缓冲液400L,轻轻混匀,用流式细胞检测仪器(贝克曼)检测细胞凋亡。
1.2.5 DAPI染色 MCF-7/ADR细胞用阿霉素处理48h收,PBS清洗3遍,4%多聚甲醛固定15min,再用PBS清洗3遍,DAPI染液染色5min,PBS清洗3遍,荧光显微镜下拍照,并统计凋亡细胞数目。
2 实验结果
2.1 多耐药乳腺癌细胞株MCF-7/ADR中的MTA2表达显著高于MCF-7
首先用不同浓度的阿霉素处理MCF-7和 MCF-7/ADR细胞,检测两组细胞的耐药情况,结果显示,不同浓度的阿霉素对两组细胞的活性均有抑制作用,且呈浓度依赖性(图1A)。其中阿霉素对MCF-7/ADR的IC50为(1567±45)μg/mL,MCF-7为(120±5)μg/mL。耐药倍数为13倍,说明该耐药株MCF-7/ADR的耐药作用显著。Western blot检测MCF-7和MCF-7/ADR细胞中MTA2表达情况,发现MCF-7/ADR细胞中MTA2表达显著高于MCF-7细胞株(图1B)。
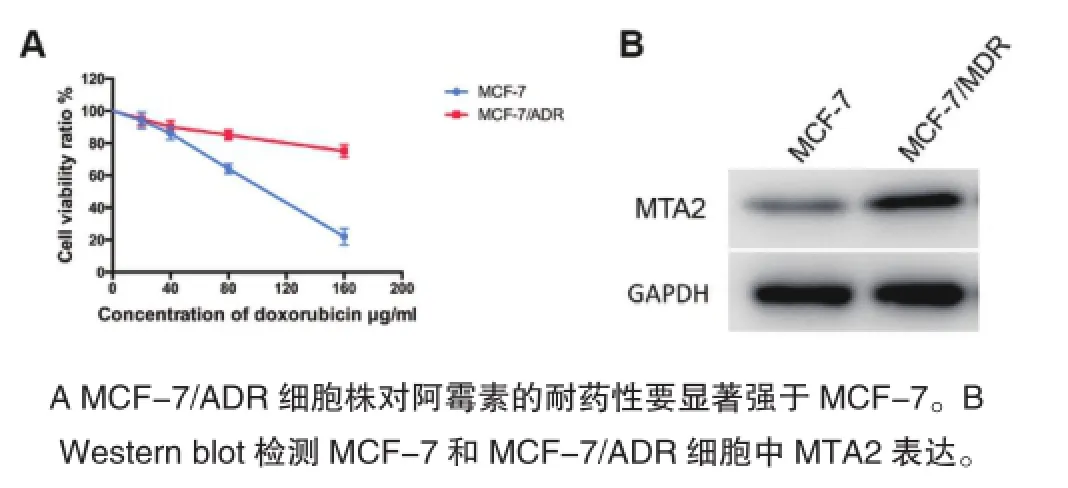
图1 MCF-7/ADR中的MTA2表达显著高于MCF-7
2.2 慢病毒介导的shRNA显著的下调了乳腺癌耐药细胞株MCF-7/ADR中MTA2
分别用对照组病毒(NC)和MTA2敲减病毒(shRNA)感染MCF-7/ADR细胞,48h后在荧光显微镜下观察:计数100个细胞,其中有绿色荧光的占90%以上,如图2A。同时收集细胞沉淀,提取总蛋白,进行Western blot检测。Western blot结果显示,shRNA组的MTA2水平显著低于对照组(图2B)。由此可见,MTA2在人乳腺癌细胞MCF-7/ ADR细胞内被成功敲除。
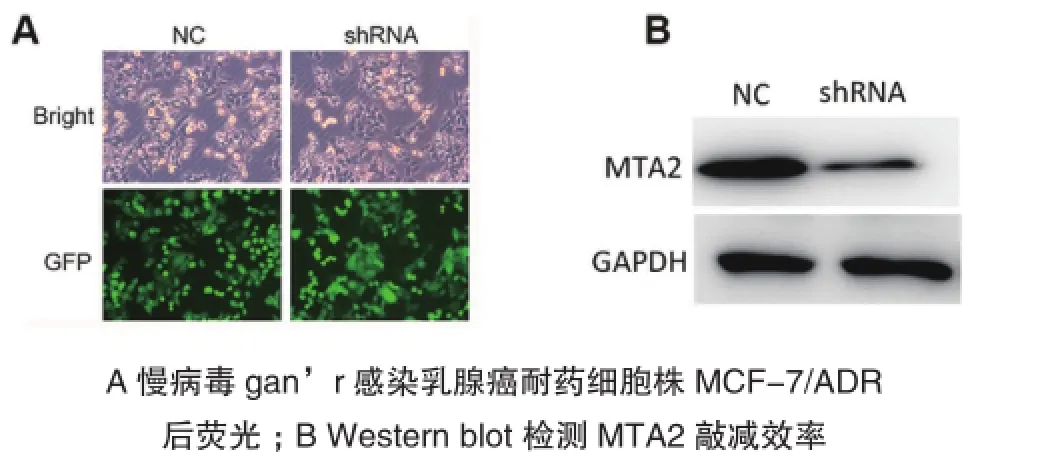
图2 慢病毒敲减乳腺癌耐药细胞株MCF-7/ADR中MTA2
2.3 敲除MTA2逆转MCF-7/ADR细胞的耐药作用并促进细胞凋亡
通过慢病毒介导的MTA2基因敲除后,MCF-7/ADR细胞对阿霉素的耐药作用大大降低,IC50为(120±21)μg/mL,在同样阿霉素浓度处理下,敲除MTA2的MCF-7/ADR细胞活性显著低于MCF-7/ADR对照组细胞。同时在基因沉默MTA2的MCF-7/ADR细胞中加入阿霉素处理48h可以显著促进MCF-7/ADR细胞的凋亡(图3B,C)。
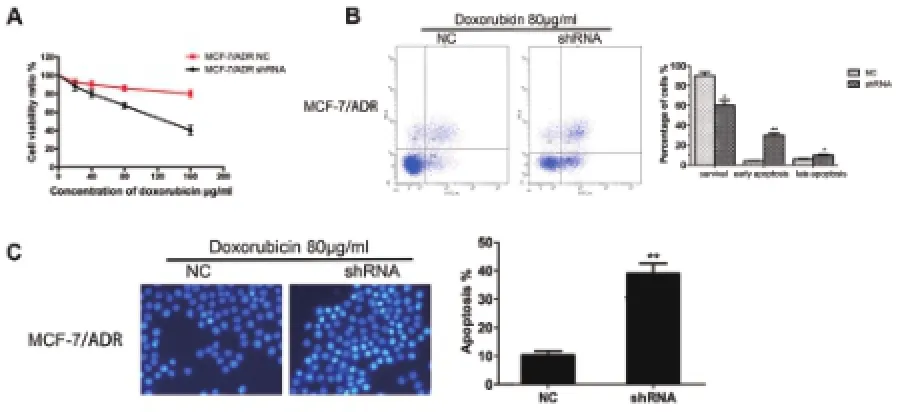
图3 敲除MTA2逆转MCF-7/ADR细胞的耐药作用并促进细胞凋亡A MCF-7/ADR细胞中MTA2被敲除后,耐药性下降。B,C 敲除MTA2后,加入阿霉素处理细胞48h,Annexin V-FITC和DAPI检测细胞凋亡qing’l情况
2.4 敲除MTA2通过抑制PI3K/AKT通路抑制下游NF-κB通路活化,并下调p-gp蛋白表达而逆转耐药性
Western blot 检测发现,敲除MTA2后,MCF-7/ADR细胞中的p-AKT水平下调,IκBα表达上调,NF-κB表达下调,p-gp蛋白在耐药时处于高表达状态,当敲除MTA2后,其表达水平下降,同时,细胞凋亡蛋白cleaved-caspase-3表达水平也显著增加。
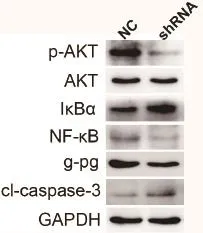
图4 western blot检测敲除MTA2后MCF-7/ADR细胞中AKT,IκBα,NF-κB,p-gp,cleaved-caspase-3蛋白表达情况
3 讨论
乳腺癌是女性最常见的恶性肿瘤之一,在我国位居女性恶性肿瘤发生率的首位。近年来,女性乳腺癌的患病率显著上升,并趋于年轻化。目前,化疗是治疗乳腺癌的重要手段,但是乳腺癌细胞多耐药性的发生是导致化疗失败的主要原因[16]。肿瘤细胞多耐药性产生的原因可能有以下几种:p-pg介导的经典多耐药;多耐药相关蛋白(MRP)产生的耐药机制[17];拓扑异构酶Ⅱ表达水平下降,使肿瘤对抗肿瘤药物敏感性下降,引起耐药[18]。
MTA2基因存在于人体内的大部分组织及器官中,尤其是在脑组织、肝脏及睾丸中的含量较为丰富[13,19]。目前经张顺等分析认为,MTA2主要是通过调节雌激素的通路、细胞凋亡及细胞骨架等路径来共同加速肿瘤的侵袭及转移过程。相关研究现已明确MTA1在组织中的过度表达与多种人类恶性肿瘤的发生发展有着密不可分的关系,如乳腺癌、结直肠癌、胃癌、食道癌以及肝细胞肝癌[7,12,20-22]等。然而MTA2与乳腺癌耐药相关研究未见报道,本研究利用MCF-7/ADR和MCF-7两株细胞,发现耐药细胞株MCF-7/ADR中的MTA2表达水平显著高于MCF-7细胞。通过慢病毒介导的MTA2基因敲除可以逆转MCF-7/ADR细胞的耐药作用并促进细胞凋亡。
PI3K/AKT通路参与许多肿瘤耐药的调控,随着PI3K/AKT信号通路与肿瘤耐药性之间的研究越来越多,PI3K/AKT信号通路的激活被认为使肿瘤耐药产生的新机制。Western blot检测结果发现,MCF-7/ADR细胞中敲除MTA2后,P-AKT表达显著下调。同时NF-κB,g-gp表达也相应的下调。NF-κB是重要的核转录因子,非激活状态下与它的抑制蛋白形成复合物,PI3K/AKT可以通过磷酸化IκB解除对NF-κB的抑制。NF-κB作为重要的核转录因子进入细胞核,调节p-gp的表达,从而影响细胞的耐药性。本研究发现MTA2可以通过PI3K/AKT/ NF-κ通路影响乳腺癌细胞的耐药性。因此通过基因沉默技术敲除MTA2来影响PI3K/AKT/NF-κ通路逆转乳腺癌的多耐药性,为乳腺癌化疗中多见的多药耐药问题提供一个新的治理思路。
[1]Siegel RL,Miller KD,Jemal A.Cancer statistics,2015[J]. CA Cancer J Clin,2015,65(1):5-29.
[2]Lu X,Kovalev GI,Chang H,et al.Inactivation of nurd component mta2 causes abnormal t cell activation and lupus-like autoimmune disease in mice[J].The Journal of biological chemistry,2008,283(20):13825-13833.
[3]Ji Y,Zhang P,Lu Y,et al.Expression of mta2 gene in ovarian epithelial cancer and its clinical implication[J]. Journal of Huazhong University of Science and Technology Medical sciences=Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban=Huazhong keji daxue xuebao YixueYingdewen ban,2006,26(3):359-362.
[4]Saito M,Ishikawa F.The mcpg-binding domain of human mbd3 does not bind to mcpg but interacts with nurd/mi2 components hdac1 and mta2[J].The Journal of biological chemistry,2002,277(38):35434-35439.
[5]Xia L,Zhang Y.Sp1 and ets family transcription factors regulate the mouse mta2 gene expression [J].Gene,2001,268(1-2):77-85.
[6]Cui Q,Matsusue K,Toh Y,et al.Assignment of the metastasis-associated gene (mta1) to mouse chromosome band 12f and the metastasis-associated gene 2 (mta2) to mouse chromosome band 19b by fluorescence in situ hybridization[J].Cytogenetics and cell genetics,2001,94(3-4):246-247.
[7]Covington KR,Fuqua SA. Role of mta2 in human cancer[J]. Cancer metastasis reviews,2014,33(4):921-928.
[8]Ma P,Lin S,Bartolomei MS,et al.Metastasis tumor antigen 2 (mta2) is involved in proper imprinted expression of h19 and peg3 during mouse preimplantation development[J]. Biology of reproduction,2010,83(6):1027-1035.
[9]Zhou C,Ji J,Cai Q,et al.Mta2 enhances colony formation and tumor growth of gastric cancer cells through il-11[J]. BMC cancer,2015,15(343):1-2.
[10]Zhang B,Zhang H,Shen G.Metastasis-associated protein 2 (mta2) promotes the metastasis of non-small-cell lung cancer through the inhibition of the cell adhesion molecule ep-cam and e-cadherin[J].Japanese journal of clinical oncology,2015,45(8):755-766.
[11]Zhou J,Zhan S,Tan W,et al.P300 binds to and acetylates mta2 to promote colorectal cancer cells growth[J]. Biochemical and biophysical research communications,2014,444(3):387-390.
[12]Lu J,Jin ML.Short-hairpin rna-mediated mta2 silencing inhibits human breast cancer cell line mda-mb231 proliferation and metastasis[J].Asian Pacific journal of cancer prevention:APJCP,2014,15(14):5577-5582.
[13]Zhou CF,Ji J,Yuan F,et al.[expression of metastasis associated 1 family member 2 (mta2) in gastric cancer and its relationship with transcription factor sp1[J].Zhonghua zhong liu za zhi,Chinese journal of oncology,2012,34(8):592-595.
[14]Liu YP,Shan BE,Wang XL,et al.Correlation between mta2 overexpression and tumour progression in esophageal squamous cell carcinoma[J].Experimental and therapeutic medicine,2012,3(4):745-749.
[15]Wang S,Qi Y,Zhang J,et al.[expression and significance of mta2 in non-small cell lung cancer][J].Zhongguo fei ai za zhi = Chinese journal of lung cancer,2010,13(8):777-780.
[16]Atalay C,Deliloglu Gurhan I,Irkkan C,et al.Multidrug resistance in locally advanced breast cancer [J].Tumour biology:the journal of the International Society for Oncodevelopmental Biology and Medicine,2006,27(6):309-318.
[17]Jordens MS,Keitel V,Karababa A,et al.Multidrug resistance-associated protein 4 expression in ammoniatreated cultured rat astrocytes and cerebral cortex of cirrhotic patients with hepatic encephalopathy[J].Glia,2015.
[18]Montani M,Hermanns T,Muntener M,et al.Multidrug resistance protein 4 (mrp4) expression in prostate cancer is associated with androgen signaling and decreases with tumor progression [J].Virchows Archiv:an international journal of pathology,2013,462(4):437-443.
[19]Zhang S,Li W,Zhu C,et al.Sertoli cell-specific expression of metastasis-associated protein 2 (mta2) is required for transcriptional regulation of the folliclestimulating hormone receptor (fshr) gene during spermatogenesis[J].The Journal of biological chemistry,2012,287(48):40471-40483.
[20]Errico A,Aze A,Costanzo V.Mta2 promotes tipindependent maintenance of replication fork integrity[J].Cell cycle,2014,13(13):2120-2128.
[21]Zhou C,Ji J,Cai Q,et al.Mta2 promotes gastric cancer cells invasion and is transcriptionally regulated by sp1[J]. Molecular cancer,2013,12(1):102.
[22]Chen DW,Fan YF,Li J,et al.Mta2 expression is a novel prognostic marker for pancreatic ductal adenocarcinoma[J].Tumour biology:the journal of the International Society for Oncodevelopmental Biology and Medicine,2013,34(3):1553-1557.
The reversal effect of MAT2 silencing on multidrug-resistant breast cancer cell MCF-7/ADR
YAN Meidi
Department of General Surgery,the Seventh Hospital of Ningbo,Ningbo 315202,China
ObjectiveBreast cancer is the most common malignant tumor in women.To investigate the effect and mechanism of MTA2 on breast cancer drug resistance.MethodsCCK-8 was used to detect the drug resistance of MCF-7/ADR and MCF-7 cells to adriamycin.MTA2 sequence specific shRNA was synthesized in vitro and transfected into human breast cancer cell line by lentivirus.Knockdown efficiency of lentivirus mediated MTA2 and the signaling pathway protein,such as PI3K,AKT and NF-κB,were detected by Western blot.Apoptosis was detected by Annexin V-FITC and DAPI staining.ResultsMTA2 expression in MCF-7/ADR cells was significantly higher than that in MCF-7 cell line.Lentivirus-mediated gene silencing significant knockdown MTA2 in MCF-7/ADR cells.Knockdown of MTA2 inhibited the activation of the downstream NF- kappa B pathway by inhibiting the PI3K or AKT pathway,and reversed the resistance to P-gp protein expression.ConclusionKnockout MTA2 can reverse the MCF-7/ADR cells drug resistant effect and these suggest MTA2 may be involved in regulating the role of breast cancer multidrug resistance.
MTA2;Breast cancer;Drug resistant
R737.9
A
2095-0616(2016)22-22-04
2016-09-11)
浙江省宁波市医学科技计划项目(2014A39)。

