Fresh-Keeping Technology of Zander Fillet with Vacuum Packaging and Partial Freezing
, , *
1. Institute of Agro-Products Processing Science and Technology, Xinjiang Academy of Agricultural and Reclamation Science, Shihezi 832000, China; 2. College of Food Science, Hainan University, Haikou 570228, China
Fresh-KeepingTechnologyofZanderFilletwithVacuumPackagingandPartialFreezing
QiangZHANG1,WentingDAI2,XinwenJIN1*
1. Institute of Agro-Products Processing Science and Technology, Xinjiang Academy of Agricultural and Reclamation Science, Shihezi 832000, China; 2. College of Food Science, Hainan University, Haikou 570228, China
In order to explore the fresh-keeping methods for zander fillet, we use vacuum packaging and partial freezing to process zander fillet, conduct a comparative analysis of sensory characteristics, percentage of water loss, stiffness period, muscle stiffness, TVB-N and water activity during the storage, and assess the fresh-keeping effect of zander fillet under the conditions of vacuum packaging and partial freezing. The results show that zander can be dead after being placed into the 2℃ water for 30 min, and the stiffness period can be extended about 100 min compared with natural death; the percentage of water loss under the conditions of vacuum packaging before partial freezing is 2.6% lower than under the conditions of partial freezing before vacuum packaging; after being stored at -7℃ and -4℃ for 15 d, TVB-N of fillet ≤15 mg/kg; TVB-N of fillet at -7℃ is 25% lower than at -4℃.
Zander fillet, Partial freezing, Vacuum packaging, Freshness
1 Introduction
Zander is a species of fish from freshwater and brackish habitats in western Eurasia. It is closely related to perch. Zanders often called pike-perch as they resemble the pike with their elongated body and head, and the perch with their spiny dorsal fin. In China, it is only distributed in Ili River and Irtysh River. Zander has a strong disease-resistant ability, and it grows fast. It has tender meat, and few inter-muscular bones. The nutritional value is very high, with the protein content of more than 20.53%. It tastes better than the famous mandarin fish, and it is known as "the "king of freshwater fish". Zander is high oxygen-consuming fish, easy to die after being caught. It is directly frozen in -18℃ cold storage warehouse for storage and transportation after its death. However, both the fish color and texture quality of zander after freezing decline, and the fish body fluid loss and fibrosis is severe after thawing, so that the taste is significantly affected, resulting in low commercial value. The fresh-keeping technology of Xinjiang fish is backward, and the fish quality decline and perishing problem are prominent. Zander is suitable for making fillet, which helps to improve the value and grade of goods; meanwhile, the storage, transportation and consumption are also convenient. Partial freezing refers to a fresh-keeping storage method of reducing the temperature of the material with certain moisture content 1-3℃ below the freezing point of the tissue fluid. In the partial freezing state, part of the water inside the fish is frozen, and the water and enzyme activity is decreased, which can inhibit the growth of microorganism, and reduce fat oxidation. The fish body fluid loss is small and the surface color of fish is good during thawing. The vacuum packaging is used to control the oxygen volume to be less than 1% of bag air volume, thereby making the microbial growth and reproduction rate decline sharply. Therefore, combining vacuum packaging and partial freezing is of great practical significance and economic value to maintaining the freshness and flavor of zander fillet, and improving the existing fresh-keeping technology of zander.
2 Materials and methods
2.1MaterialsandreagentsZander, fresh and alive, is bought from Shihezi zander breeding base, with the average weight of 850g per fish. Magnesia suspension; boric acid absorption solution; hydrochloric acid standard titration solution; methyl red-ethanol indicator; bromocresol green-ethanol indicator. These reagents and drugs were purchased from Tianjin Damao Chemical Reagents Plant.
2.2InstrumentsandequipmentsLG partial freezing refrigerator (Siemens); BS124 electronic balance (Sartorius); HygroPalm portable water activity meter (Rotronic); precision pH meter; the lowest mark thermometer; semi-micro Kjeldahl device; microburette; hardness tester.
2.3DeterminationofzandermeatfreezingpropertyFreezing curve[1]: kill the fish, take fillet and insert thermometer into the fish muscle; cool it at -21℃ and record the process of change in temperature over time to obtain freezing curve. Freezing point[1-2]: observe the freezing curve. After the temperature drops to 0℃, it is relatively stable within a period of time. This temperature is the zone of maximum ice crystal formation for zander, and most of the water inside the fish has formed ice crystals. This temperature is the freezing point of zander.
2.4Modesofdeathforzander[3]Natural death: catch the fish and place it in containers to wait for its death. Rapid death with ice water: reduce the water temperature to 2-3 ℃ first, and add ice to make water temperature drop to 0-2 ℃; place the live fish into water based on the fish-water mass ratio of 1: 3 and make it die after 20-30 min.
2.5ChangeinthestiffnessperiodofzanderThe test shows that there is also a stiffness period change in the fillet taken from the newly died zander. Referring to fish stiffness index measurement method[2-3], a group of fillet samples under the conditions of natural death and ice water-induced death are selected respectively, with length of 10 cm, width of 3 cm and thickness of 1 cm. The first half of fillet is fixed on the plate and the second half naturally droops. For every 30 min, the average droop length of the endpoint is measured, and the change in droop length of each group of fillets is observed, to get the time when fillets get stiff and the stiffness duration.
2.6VacuumtreatmentandpartialfreezingofzanderfilletThe natural partial freezing, ice-salt partial freezing and cold saline water partial freezing are combined with vacuum processing. Natural partial freezing: place the fillet in -4℃ partial freezing refrigerator. Ice-salt partial freezing: prepare the sodium chloride solution with mass fraction of 1.6%, make it frozen into ice within -4℃ refrigerator, and bury the fillet in cracked ice. Cold saline water partial freezing: prepare the -5℃ saline water with mass fraction of 10%, cool the fillet in the saline water to an internal temperature of -4 to -5 ℃, take out the fillet and place it inside the partial freezing refrigerator for preservation. Vacuum treatment[4-5]: make zander fillets with three kinds of thickness (5, 10, 15 mm) and take the vacuum degree of 0.09, 0.095, 0.1 MPa (Table 1). After treatment, all groups of fillets are stored for 20 d under -4℃ and -7℃, respectively, and the fillet traits are measured, analyzed and compared.
Table1Thetestcombinationofthicknessandvacuumdegreeofdifferentfillets
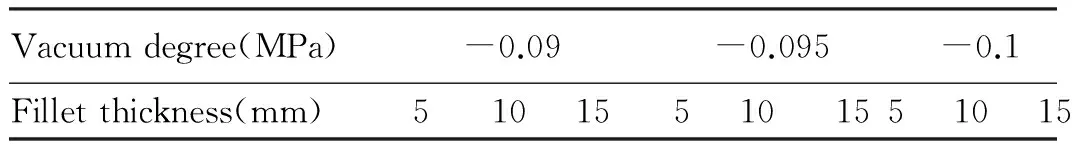
Vacuumdegree(MPa)-0.09-0.095-0.1Filletthickness(mm)5 10 155 10 155 10 15
2.7Determinationoffilletfreshness
2.7.1Fish muscle stiffness. The zander fillets in different storage periods and different conditions are taken respectively, and the durometer is used to measure the stiffness. The measuring head of durometer is used to press the fish muscle, and it is counted as stiffness when 5 mm dent appears. It is measured five times and averaged.
2.7.2Water loss rate[6-11].In different storage periods, the zander fillets under different storage conditions are taken, weighed and recorded, respectively. The weight difference is the fillet water loss.
2.7.3TVB-N[11-12]. The ammonia, amine and other nitrogen-containing alkaline substances, generated from the perishing process of aquatic products, have volatility and low boiling point, and can be distilled in weakly alkaline hydroxide solution. The boric acid is used for absorption to make the absorption solution change from acidity to alkalinity. The standard hydrochloric acid solution is used for titration, and the content is calculated according to the consumption of standard hydrochloric acid solution[6].
2.7.4pH value[12].10 g of minced fish is weighed, and 100 ml of distilled water is added. It is stirred for 30 min using a magnetic stirrer and then filtrated. The filtrate is measured with pH-3C pH meter.
2.7.5Water activity (aw)[13-15].Water activity orawis the partial vapor pressure of water in a substance divided by the standard state partial vapor pressure of water. When the water in some products is frozen, the water activity will be affected. The test of water activity in this experiment is based on the method of GB/T 23490-2009DeterminationofFoodWaterActivity[7], and the instrument of water activity is used to test. At first, the sensor probe and measuring tank are placed in the fillet testing environment to be preheated for 10 min; then the fillet samples are placed in the measuring tank with a probe cover. The instrument of water activity is read after 2 min pass.
3 Results and analysis
3.1ZanderfreezingcurveThe relationship between zander freezing time and temperature is shown in Fig. 1, and the curve shape shows three stages with certain characteristics. At the first stage, the fish temperature decreases rapidly in a relatively short period of time; at the second stage, when the fish temperature drops below a certain value and maintains relatively stable, a lot of water in zander muscle tissue is frozen at this temperature to emit a large amount of latent heat, and it is just the freezing point temperature of zander; at the third stage, with prolonged freezing, the fillet temperature decreases rapidly again, and most of the water in fish has been frozen into ice crystals, mainly releasing the sensible heat. During fish freezing process, most of the heat is released at the second stage, and it can be judged that the zander freezing point is -2.4℃.
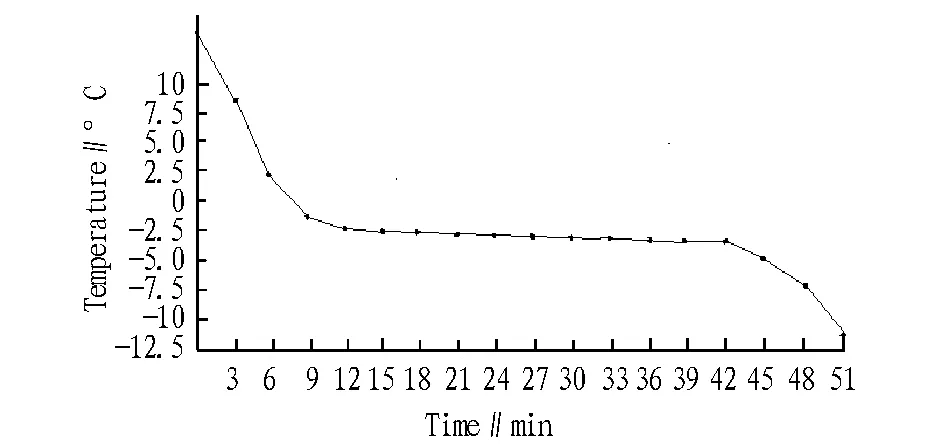
Fig.1 The time-temperature curve at -21℃
3.2ThechangeinzanderfilletstiffnessundertwomodesofdeathIn the process of entering the stiffness period, the droop length of fillet gradually decreases, but gradually increases after the end of stiffness period. As shown in Table 2 and Fig. 2, the stiffness period under ice water-induced death is later than under natural death, and the stiffness speed is slower, so the stiffness period is longer. The ice water-induced death is to quickly kill fish using low temperature, and the fish makes few struggles and consumes less ATP. The process of stiffness is accompanied by the continuous decomposition and reduction of ATP, so the ice water-induced death method can delay the fish’s entry into stiffness period, prolong the duration of stiffness, and postpone the occurrence of autolysis and decay. Therefore, it is considered that the ice water-induced death can better maintain freshness and quality of zander fillet from the raw material stage, which is of certain significance to extending the shelf life of fillet, while natural death is at a disadvantage in this regard.
Table2Thechangeinfillet’sdrooplengthovertime
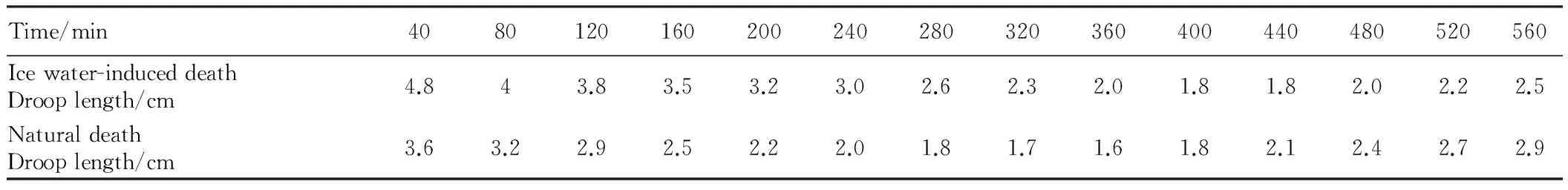
Time/min4080120160200240280320360400440480520560Icewater-induceddeathDrooplength/cm4.843.83.53.23.02.62.32.01.81.82.02.22.5NaturaldeathDrooplength/cm3.63.22.92.52.22.01.81.71.61.82.12.42.72.9
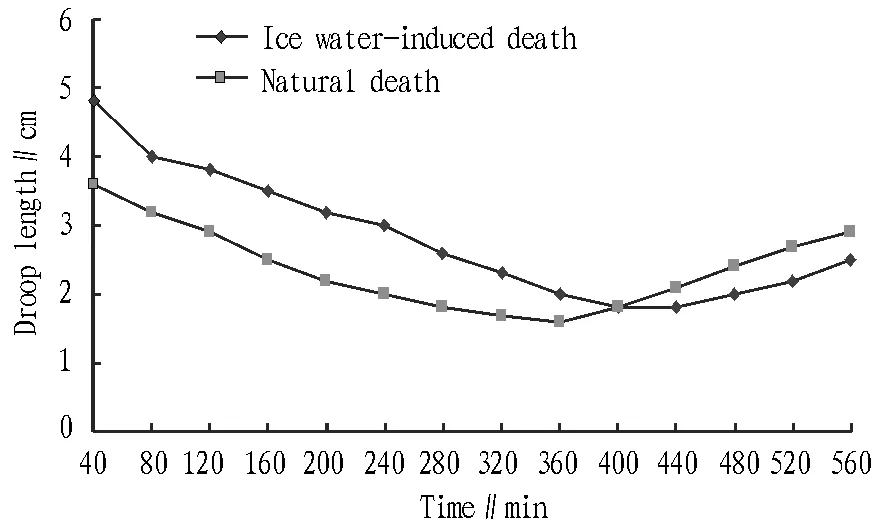
Fig.2 The change in stiffness of zander fillets under ice water-induced death and natural death
3.3PartialfreezingofzanderfilletThe change in percentage of water loss, TVB-N and pH value using three kinds of partial freezing methods is shown in Fig. 3. Fig. 3 (a) shows that there is no significant difference in percentage of fillet water loss among three partial freezing methods, but under low temperature saline water partial freezing, the percentage of water loss rises significantly after 15 d, compared with the other two conditions, which may be due to the fact that the outward migration of water in fillet tissue is increased under infiltration of saline water, and the fillet surface evaporation is increased. From Fig. 3 (b) , (c), it can be found that for the fillets treated with three kinds of partial freezing methods, there are no significant differences in the change of TVB-N and pH values. Overall, the freshness within 15 d is good, and it can still be used to make sashimi.

Fig.3 The change in three indicators about zander fillet under three different partial freezing conditions
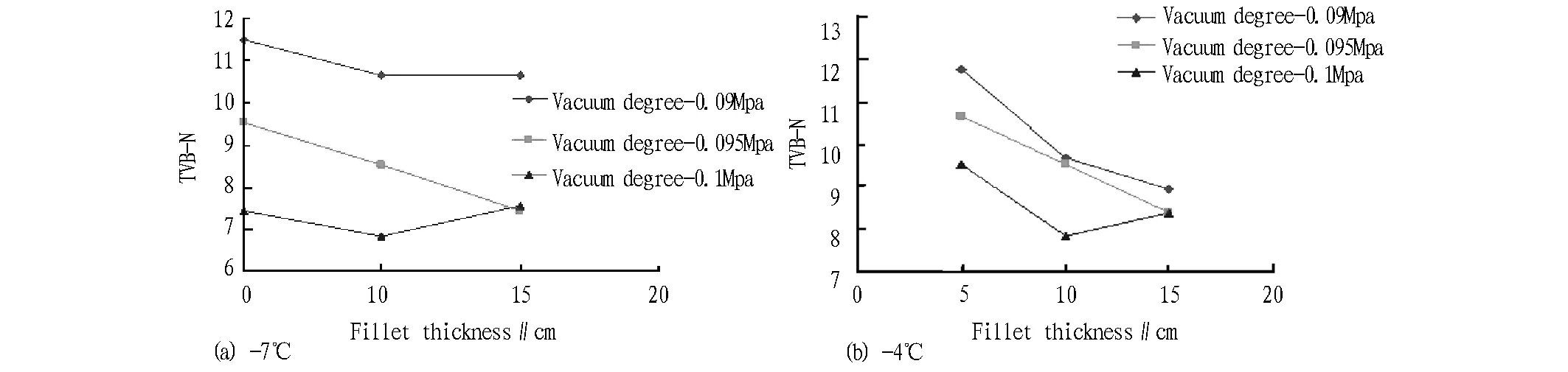
Fig.4 TVB-N change of zander fillet under different temperature vacuum partial freezing
3.4VacuumpartialfreezingofzanderfilletUnder vacuum packaging, the water in fish tissue will migrate outward. In addition, during fillet partial freezing, due to different thickness of fillets, there are differences in the cooling speed of fillets, and these differences will be reflected in the fresh-keeping effect of fillets. For the fillets with different thickness and degrees of vacuum processing, they are stored for 20 d at -4℃ and -7℃, and the TVB-N and pH changes of each group of samples are observed. The initial TVB-N of fresh samples is 4.5 mg/100 g, and the initial pH is 7.32.
3.4.1Change in TVB-N. After the fillets with different degrees of vacuum packaging are stored at -4℃ and -7℃ for 20 d, the TVB-N change is shown in Fig. 4 (a), (b). As can be seen from Fig. 4 (a), (b), at -0.09 MPa and -0.095 MPa, TVB-N value decreases with the increasing thickness of fillets, and at the highest vacuum degree, it shows another variation trend, and TVB-N value of 10 mm fillets is lowest. The preservation effect of 10 mm fillets is good. Under the same vacuum degree, thickness and time, TVB-N value in the -7℃ storage environment is lower than in the -4℃ storage environment.
3.4.2Change in pH. After the fillets with different degrees of vacuum packaging are stored at -4℃ and -7℃ for 20 d, the pH change is shown in Fig. 5. As can be seen from Fig. 5, under different vacuum degrees, pH changes are consistent with the increase in the thickness of the fillets. At -0.09 MPa, pH shows a downward trend; at -0.095 MPa, pH is lowest; at -0.1 MPa, pH shows a rising trend. And the vacuum degree has a great impact on the fillets with the thickness of 5 mm.

Fig.5 pH change of zander fillet under different temperature vacuum partial freezing

Fig.6 aw change of zander fillet under different temperature vacuum partial freezing
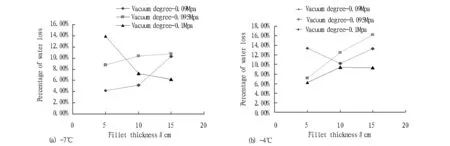
Fig.7 change in percentage of water loss of zander fillet under different temperature vacuum partial freezing
3.4.3Change inaw. As shown in Fig. 6,awvalue is obviously small at -7℃, and low water activity of aquatic products can better inhibit proliferation of microorganisms[8-9]. Affected by the experimental environment, the water activity error is large, and the fluctuation in points of Fig. 6 (a) is obvious, but when the environmental differences are large, the error does not affect the overall analysis and judgment. -4℃ and -7℃ in the experiment show great environmental differences, and can eliminate the interference of experimental errors in analysis and judgment.
3.4.4Change in percentage of water loss. After the fillets with different degrees of vacuum packaging are stored at -4℃ and -7℃ for 20 d, the change in the percentage of water loss is shown in Fig. 7. By comparing Fig. 7 (a) and Fig. 7 (b), it is found that the correlation between vacuum degree and fillet water loss is low, and the fillet water loss is small under -7℃ partial freezing. In addition, the percentage of water loss shows an increasing trend with increasing thickness of the fillets, because the increase of fillet thickness causes uneven freezing inside fillet.
4 Conclusions
According to the time-temperature freezing curve of zander, it can be found that the zander freezing point is -2.4℃. The stiffness period under ice water-induced death is later than under natural death, and the stiffness speed is slower, so the stiffness period is longer and the decay speed is slower. The ice-salt partial freezing can better maintain the sensory quality of fillets, and the TVB-N value after 20 d is still in line with Class I National Freshness Standard of Aquatic Products. Although the percentage of water loss is slightly high, it does not affect the appearance quality. Under vacuum partial freezing conditions, there are small fluctuations in environmental temperature at -7℃, and various indicators of fish are good, favorable for fish preservation, so -7℃ can be chosen as the partial freezing temperature for preservation.
[1] FENG ZZ. Refrigeration technology of aquatic products[M]. Beijing: China Agriculture Press, 1997:25-30;39-44. (in Chinese).
[2] MI HB, ZHENG XJ, LIU C,etal. Superchilling storage and the quality of superchilled products[J]. Food and Fermentation Industries,2010(9):124-128.(in Chinese).
[3] LI LH, HAO SX, DIAO SQ,etal. Proposed new color retention method for tilapia fillets by euthanatizing with reduced carbon monoxide[J]. Journal of Food Processing and Preservation, 2008, 32(5):729-739.
[4] FENG ZZ. Food cryology[M]. Beijing: China Light Industry Press, 2001:58-63;192-226.(in Chinese).
[5] GAO ZL, XIE J. Research progress on low-temperature preservation of aquatic products[J]. Guangdong Agricultural Sciences, 2012(14): 98-101. (in Chinese).
[6] GAO YJ, XIONG WD. Food packaging[M].Beijing: Chemical Industry Press, 2005:1209-218.(in Chinese).
[7] YU G, ZHANG HJ, YANG SL,etal. Research progress in preservation methods for tuna and its quality changes during storage[J].Science and Technology of Food Industry, 2013, 34(21):381-384.(in Chinese).
[8] Saroat Rawdkuen, Samart Sai-Ut, Saisunee Khamsorn,etal. Biochemical and gelling properties of tilapia surimi and protein recovered using an acid-alkaline process[J]. Food Chemistry, 2009,112(1) :112-119.
[9] Silva Afonson, Sant’ Ana LS. Effects of pretreatment with rosemary (RosmarinusofficinalisL.) in the prevention of lipid oxidation in salted tilapia fillets[J]. Journal of food quality,2008,31(5):586-595.
[10] TAN FL, FOCK SC. Freezing of tilapia fillets in an air blast freezer[J]. International Journal of Food Science and Technology, 2009, 44(8):1619-1625.
[11] SF Biscalchin-Gryschek, M Oetterer, CR Gallo. Characterization and frozen storage stability of minced nile tilapia (Oreochromis niloticus) and red tilapia (Oreochromis spp)[J].Journal of aquatic food product technology,2003,12(3):57-69.
[12] XIA YY. The quality inspection and inspection of food hygiene[M]. Beijing: China Industry Press, 1993: 370-372. (in Chinese).
[13] On the determination of water activity of food, GB/T 23490-2009[S]. (in Chinese).
[14] SCOTT VN, BERNARD DT. Influence of temperature on the measurement of water activity of food and salt systems[J]. Journal of Food Science, 2006, 48(2):552-554.
[15] BIAN K. On the relationship between water activity and food storage stability[J]. Journal of Zhengzhou Institute of Technology, 1997, 18(4): 41-48. (in Chinese).
January 1, 2016 Accepted: March 3, 2016
Supported by Key Science and Technology Project in Hainan Province (090110).
*Corresponding author. E-mail: 821861127@qq.com
 Asian Agricultural Research2016年3期
Asian Agricultural Research2016年3期
- Asian Agricultural Research的其它文章
- Evaluation of Social Vulnerability to Natural Disasters on a County Scale in Henan Province
- An Empirical Analysis of the Export Competitiveness of Agricultural Products in Hubei Province Based on Inter-provincial Comparison
- Changes and Influencing Factors of Maize Production Pattern in China
- The Changes in Guilin Paddy Soil Organic Matter and Rice Yield under Long-Term Fertilization
- Study on the Discipline Reputation of Agricultural Colleges and Universities
- Pricing Power of Agricultural Products under the Background of Small Peasant Management and Information Asymmetry
