Upgrading the definition of early gastric cancer: better staging means more appropriate treatment
Luca Saragoni
Department of Pathology, G.B. Morgagni-L. Pierantoni Hospital, Forlì 47121, Italy
Upgrading the definition of early gastric cancer: better staging means more appropriate treatment
Luca Saragoni
Department of Pathology, G.B. Morgagni-L. Pierantoni Hospital, Forlì 47121, Italy
Since Murakami defined early gastric cancer (EGC) as a “carcinoma limited to the gastric mucosa and/or submucosa regardless of the lymph node status”, several authors have focused on the most infuential histopathological parameters for predicting the development of lymph node metastases by considering the lymph node status as an important prognostic factor. A few authors have also considered the depth of invasion as one of the keys to explaining the existence of subgroups of patients afected by EGC with poor prognoses. In any case, EGC is still considered an initial phase of tumor progression with good prognosis. Te introduction of modern endoscopic devices has allowed a precise diagnosis of early lesions, which can lead to improved defnitions of tumors that can be radically treated with endoscopic mucosal resection or endoscopic submucosal dissection (ESD). Given the widespread use of these techniques, the Japanese Gastric Cancer Association (JGCA) identifed in 2011 the standard criteria that should exclude the presence of lymph node metastases. At that time, EGCs with nodal involvement should have been asserted as no longer fting the defnition of an early tumor. Some authors have also demonstrated that the morphological growth patern of a tumor, according to Kodama’s classifcation, is one of the most important prognostic factors, thereby suggesting the need to report it in histopathological drafs. Notwithstanding the acquired knowledge regarding the clinical behavior of EGC, Murakami’s defnition is still being used. Tis defnition needs to be upgraded according to the modern staging of the disease so that the appropriate treatment would be selected.
Early gastric cancer (EGC); defnition; diagnosis; prognosis; treatment
Introduction
Early gastric cancer (EGC) is commonly reported in Japan1,2because of the widespread use of organized screening programs. However, the number of EGC cases is apparently rising in other parts of the world3-6. At the same time, minimally invasive treatments of EGC have been emerging. In particular, therapeutic endoscopic submucosal dissection (ESD) has been introduced as a treatment option7,8. Although excellent results have been reported, concerns have been expressed about the increased rates of local recurrence and early cancer-related deaths after therapeutic endoscopy9-11. Patients selected for ESD should have no lymph node metastases and involved cut ends12,13. However, the current definition of EGC does not consider lymph node status14-16. This review aims to provide an upgraded definition of EGC in the era of minimally invasive treatments, including endoscopy, by critically examining the data from the literature focusing on tumor-specific factors associated with the risk of lymph node metastases.
EGC: challenges
The term EGC, defined in 1971 by the Japanese Society of Gastroenterology and Endoscopy17as a carcinoma limited to the mucosa and/or submucosa regardless of the lymph node status, has continued to trigger a controversy over the years18-20. Numerous studies have focused on the key parameters that can be associated with the risk of lymph node metastases or treatment failure in EGC21-25. The major problem inmicrostaging is represented by the lack of agreement regarding the characteristics that truly classify nonthreatening EGCs, which have excellent prognosis with survival rates of 98%-100%, compared with threatening tumors that have an increased incidence of lymph node metastases (15%-20%) and survival rates of approximately 70%. Over the past years, the most important factor has seemed to be the depth of invasion. In 1991, Inoue et al.26reported that the 5-year survival rate is 100% in patients with mucosal lesions and 90% in those with submucosal invasion. They also demonstrated that lymph node metastases exerted a major effect on prognoses ranging from an overall survival rate of 99%, if N0, to 73%, if N1. In the following years, other authors demonstrated that the tumors infiltrating the submucosa of a stomach are associated with a significant increase in the incidence of lymph node metastases8,27-30and a poor prognosis31. In particular, mucosal tumors have a 6% mean incidence of lymph node metastases versus 28% of submucosal EGCs. However, the depth of infiltration into the wall is not the only prognostic factor and/or predictive parameter of the increased risk of lymph node metastases. In fact, the morphological growth paterns of the lesions should be assessed by pathologists because of their important prognostic meaning. Since Kodama described the morphological growth patterns of EGC in 198332by combining them with the size of the lesions, only few Western authors have considered Kodama’s classification (Table 1)32, thereby showing its important prognostic meaning6,23-25. In particular, we have demonstrated in our previous studies that lymph node metastases and Kodama’s PEN A types are the only independent negative prognostic factors in EGCs. In our experience, Kodama’s PEN A type has an incidence of lymph node metastases of 31.7% and a death risk nearly four times higher (hazard ratio =3.91) than those of all the other Kodama types. Tus, we suggested using Kodama’s classification in ESD and surgical specimens to advise an appropriate treatment to clinicians. When a lymph node status was considered by itself, a few authors determined that patients with EGC who have three or more positive lymph nodes have a signifcantly poor prognosis33-35. In our series, we showed that patients with EGC who have more than three positive nodes have a signifcantly poor prognosis similar to that of patients with advanced disease with a death risk approximately 13 times higher than that of patients with negative lymph nodes (hazard ratio =12.78), as demonstrated by the multivariable analysis6. If lymph node metastases are present, patients must be treated surgically by adding chemotherapy in the adjuvant setting according to the recommendations of the European Society of Medical Oncology36, given the obvious benefits of adjuvant therapy in these patients37-42. Other important risk factors for lymph node metastases are the presence of lymphovascular invasion and tumor dedifferentiation5,9,27-29,33,35,43-54. Moreover, histologic difuse type of Lauren and size larger than 2 cm are signifcantly linked with a higher incidence of lymph node involvement55-77.
In particular, the mean incidence of lymph node metastases is 9% in the absence of lymphovascular invasion vs. 53% in the opposite situation. Well-differentiated tumors have a lymph node involvement in 13% of the cases vs. 34% of poorly differentiated ones, whereas tumors smaller than or equal to 2 cm present nodal metastases in 8% of the patients vs. 25% of those larger than 2 cm.
According to the same literature data, the likelihood of lymph node metastases is 7.1, 6.2, and 3.8 times more probable if the tumor infiltrates the submucosa (vs. mucosa), if a lymphovascular invasion exists (vs. not), and if it was Lauren’s difuse type (vs. intestinal type), respectively.
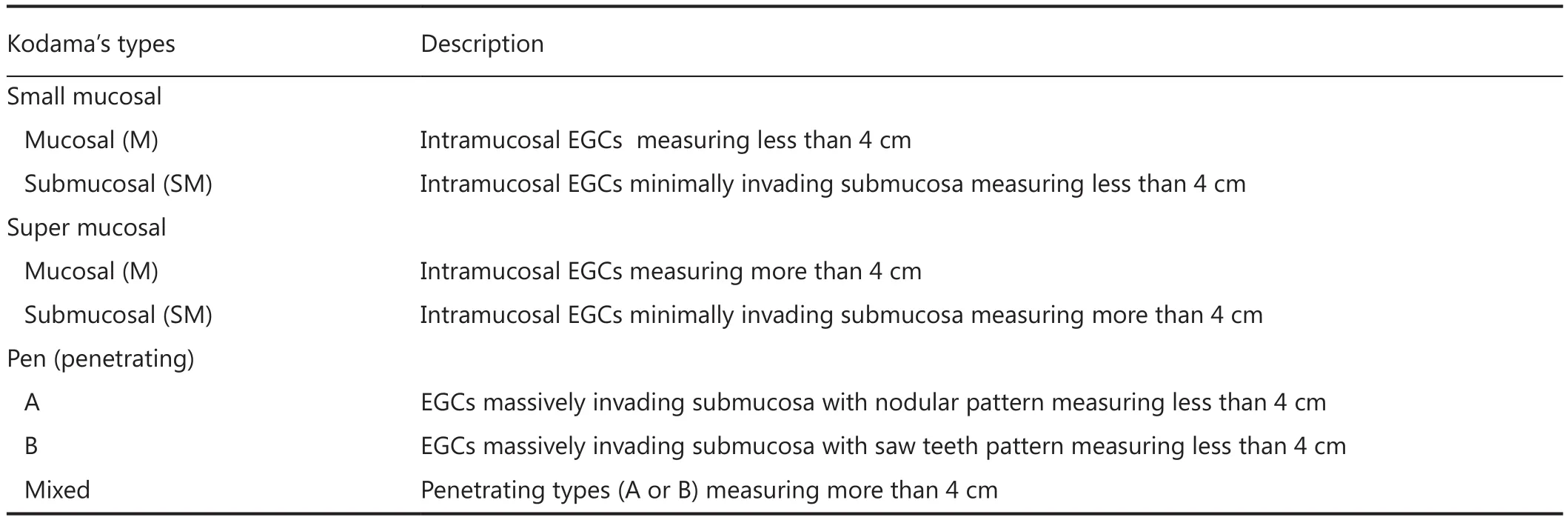
Table 1 Kodama’s classification

Table 2 Macroscopic classification of EGC
According to the macroscopic types (Table 2) defined by Paris classifcation78, the probability of lymph node metastases is 2.3 times higher for depressed lesions (vs. elevated EGCs).
Some authors also determined other important predictive factors of lymph node metastases when lymph node involvement is considered, and/or the significant role of a few parameters previously mentioned in predicting nodal positivity is reinforced. In particular, Fukuhara et al.79noted that both lymphovascular invasion and young age are significant predictive factors of lymph node metastases in EGCs for non-Asian ethnic groups. Yang et al.80determined deep submucosal invasion, antral location, and venous invasion as the predictive parameters of lymph node involvement. Gotoda et al.44showed that the incidence of nodal metastases increases (from 9% to 24%) as the depth of tumor infltration increases within the submucosal layer. Shida et al.81demonstrated that lymphatic and venous invasions are independent predictive factors of lymph node metastases.
Considering all these data, some atempts exist to update the definition of EGC and to determine the risk factors associated with the likelihood of lymph node metastases by improving the diagnostic ability. Based on the current definition, the risk of lymph node metastases in patients with EGC is 15%-24%2,71,82. Tis fnding cannot be ignored, and the case for upgrading the definition of EGC should be based on the exclusion of lymph node involvement. Tis observation is particularly true in an era of endoscopic treatment for “fting” patients with EGC.
The indications for endoscopic treatment of EGC have undergone changes while the pioneering techniques of the Japanese were evolving30,43,44,83-85. When the National Cancer Center Hospital of Tokyo (Japan) first introduced the indications for mucosal endoscopic mucosal resection in 198786, the lesions had to be smaller than 15 mm. Current indications for therapeutic endoscopic resection are referred to ESD according to the Japanese Gastric Cancer Association (JGCA)12, and the following “standard” criteria should be fulflled:
(I) Intramucosal tumor;
(II) Well-differentiated intestinal tumor type according to Lauren;
(III) Tumor size <2 cm;
(IV) Absence of neoplastic ulcer;
(V) Absence of lymphovascular invasion;
(VI) Negative horizontal and deep margins.
If all these parameters are satisfied, the ESD diagnostic procedure can be also considered therapeutic.
JGCA has recently provided the “expanded” indications for ESD12for investigative purposes. Tese expanded indications43,44include tumors T1a(mucosal only) exhibiting the following:
(I) Differentiated type, ulcerative findings (ULs) (−), but >2 cm in diameter;
(II) Diferentiated type, ULs (+) and not more than 3 cm in diameter;
(III) Undifferentiated type, ULs (−) and not more than 2 cm in diameter.
The “standard” criteria are strongly recommended because they are considered safe because of the decreased risk of lymph node metastases. This consideration translates into the presence of lymph node metastases as an absolute contraindication to therapeutic endoscopy. Hence, the current definition of EGC, which does not consider the lymph node status, must be upgraded. However, a standardized upgraded definition of EGC shared by both Western and Eastern pathologists cannot be easily found.
First, we should try to standardize the diagnosis of EGC. In fact, Japanese pathologists base their diagnoses of EGC only on cytological atypia and architectural distortion, whereas their Western colleagues define EGCs as tumors that invade the proper lamina. The classification of Vienna87apparently solved the problem. However, after Stolte88performed the revision, the differences could not be bypassed. The 7th edition of the classification by the World Health Organization (WHO)89, shared by Western and Eastern pathologists, defined only invasive tumors as EGCs. Low- and high-grade intraepithelial lesions were also considered depending on cytological atypia as noninvasive counterparts. In addition, according to WHO, the criteria of invasiveness are sometimes not easily defined. They are also apparently poorly reproducible, depending on a subjective evaluation.
Some Western and Eastern authors showed that some lesions defined as high-grade dysplasia by Western pathologists hid submucosal EGCs in ESD specimens90.
Nevertheless, we should ask ourselves how to define EGC without considering this topic. Should we simply call “early”those tumors that do not have lymph node metastases or other things are needed? In our previous series of 530 patients with EGC6, we provided new elements for an updated definitionof EGC. In particular, we suggested calling “early” those gastric cancers that are limited to the mucosa, or at least, invade minimally the submucosa, lesions without potential risk of developing lymph node metastases. This study is a retrospective monocentric investigation with a long followup and standardized surgery and histological diagnosis, which showed that tumors with a size of more than 2 cm (66.7%), infltration of the submucosa (74.0%), difuse histological type (29.2%), and Kodama’s PEN A type (42.3%) are important and signifcant risk factors for lymph node metastases. An agreement must be determined on what we mean by and how to measure the minimal invasion of the submucosa. According to the classifcation of Paris78, we should defne those EGCs infltrating the submucosal layer with a maximum depth of 500 micron (SM1 tumors) and minimally invading the submucosa. According to Kodama’s classifcation, we should not use quantitative criteria. However, only minimal invasion of the submucosa should be considered an initial infltration under the muscularis mucosae. According to our previous data, these lesions have the same incidence of lymph node metastases and similar 5- and 10-year survival probability as that of mucosal tumors. In conclusion, we suggest regarding the lesions that are limited to the mucosa or those that invade minimally the submucosa according to Kodama’s criteria and the lesions without lymph node metastases as EGCs. Te size of tumors should be also considered, given the signifcant increased risk of lymph node involvement for lesions larger than 2 cm.
Conflict of interest statement
No potential conficts of interest are disclosed.
1. Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. Tokyo: Kanehara & Co., Ltd., 1995.
2. Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer 1993;72:3174-3178.
3. Green PH, O'Toole KM, Slonim D, Wang T, Weg A. Increasing incidence and excellent survival of patients with early gastric cancer: experience in a United States medical center. Am J Med 1988;85:658-661.
4. Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut 1991;32:1110-1113.
5. Skoropad V, Berdov B, Zagrebin V. Clinicopathological features and outcome of surgical treatment of 149 patients with early (pT1) gastric cancer. Onkologie 2005;28:247-252.
6. Saragoni L, Morgagni P, Gardini A, Marfsi C, Vitimberga G, Garcea D, et al. Early gastric cancer: diagnosis, staging, and clinical impact. Evaluation of 530 patients. New elements for an updated defnition and classifcation. Gastric Cancer 2013;16:549-554.
7. Yada T, Yokoi C, Uemura N. Te current state of diagnosis and treatment for early gastric cancer. Diagn Ter Endosc 2013;2013:241320.
8. Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin N Am 2014;24:213-233.
9. Ishikawa S, Togashi A, Inoue M, Honda S, Nozawa F, Toyama E, et al. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer 2007;10:35-38.
10. Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, et al. Risk factors associated with local recurrence of early gastric cancers afer endoscopic submucosal dissection. Gastrointest Endosc 2008;68:887-894.
11. Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012;76:763-770.
12. Japanese Gastric Cancer Association. Japanese classifcation of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-112.
13. Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol 2005;23:4490-4498.
14. Murakami T. Early cancer of the stomach. World J Surg 1979;3:685-692.
15. Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol 2006;22:561-569.
16. Kitaoka H, Yoshikawa K, Hirota T, Itabashi M. Surgical treatment of early gastric cancer. Jpn J Clin Oncol 1984;14:283-293.
17. Murakami T. Pathomorphological diagnosis, defnition and gross classifcation of early gastric cancer. Gann Monogr Cancer Res 1971;11:53-55.
18. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-2917.
19. Correa P. Gastric cancer: overview. Gastroenterol Clin North Am 2013;42:211-217.
20. Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ter 2014;40:250-260.
21. Abe S, Yoshimura H, Nagaoka S, Monden N, Kinugasa S, Nagasue N, et al. Long-term results of operation for carcinoma of thestomach in T1/T2 stages: critical evaluation of the concept of early carcinoma of the stomach. J Am Coll Surg 1995;181:389-396.
22. Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg 1992;79:241-244.
23. Folli S, Dente M, Dell'Amore D, Gaudio M, Nanni O, Saragoni L, et al. Early gastric cancer: prognostic factors in 223 patients. Br J Surg 1995;82:952-956.
24. Saragoni L, Gaudio M, Vio A, Folli S, Nanni O, Saragoni A. Early gastric cancer in the province of Forlì: follow-up of 337 patients in a high risk region for gastric cancer. Oncol Rep 1998;5:945-948.
25. Saragoni L, Gaudio M, Morgagni P, Folli S, Vio A, Scarpi E, et al. The role of growth patterns, according to Kodama's classification, and lymph node status, as important prognostic factors in early gastric cancer: analysis of 412 cases. Gastric Cancer 2000;3:134-140.
26. Inoue K, Tobe T, Kan N, Nio Y, Sakai M, Takeuchi E, et al. Problems in the defnition and treatment of early gastric cancer. Br J Surg 1991;78:818-821.
27. Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, Saragoni L, et al. Risk factors for lymph node metastases and their prognostic signifcance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol 2001;31:495-499.
28. Popiela T, Kulig J, Kolodziejczyk P, Sierzega M; Polish Gastric Cancer Study Group. Long-term results of surgery for early gastric cancer. Br J Surg 2002;89:1035-1042.
29. Tachibana M, Takemoto Y, Monden N, Nakashima Y, Kinugasa S, Dhar DK, et al. Clinicopathological features of early gastric cancer: results of 100 cases from a rural general hospital. Eur J Surg 1999;165:319-325.
30. Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg 1992;79:241-244.
31. Shimada S, Yagi Y, Honmyo U, Shiomori K, Yoshida N, Ogawa M. Involvement of three or more lymph nodes predicts poor prognosis in submucosal gastric carcinoma. Gastric Cancer 2001;4:54-59.
32. Kodama Y, Inokuchi K, Soejima K, Matsusaka T, Okamura T. Growth paterns and prognosis in early gastric carcinoma. Superfcially spreading and penetrating growth types. Cancer 1983;51:320-326.
33. Degiuli M, Calvo F. Survival of early gastric cancer in a specialized European center. Which lymphadenectomy is necessary? World J Surg 2006;30:2193-2203.
34. Gertler R, Stein HJ, Schuster T, Rondak IC, Höfer H, Feith M. Prevalence and topography of lymph node metastases in early esophageal and gastric cancer. Ann Surg 2014;259:96-101.
35. An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg 2007;246:749-753.
36. Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi57-63.
37. Macdonald JS, Smalley SR, Benedeti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy afer surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-730.
38. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer afer D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-321.
39. Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-273.
40. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fuoropyrimidine. N Engl J Med 2007;357:1810-1820.
41. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-4393.
42. Smalley SR, Benedeti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Up-dated analysis of SWOG-directed intergroup study 0116 : a phase III trial of adjuvant radiochemotherapy versus observation afer curative gastric cancer resection. J Clin Oncol 2012;30:2327-2333.
43. Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undiferentiated-type early gastric cancer. Gastric Cancer 2009;12:148-152.
44. Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-225.
45. Hayes N, Karat D, Scot DJ, Raimes SA, Grifn SM. Radical lymphadenectomy in the management of early gastric cancer. Br J Surg 1996;83:1421-1423.
46. Koufuji K, Takeda J, Toyonaga A, Yoshihara S, Tanaka Y, Ohta J, et al. Early gastric cancer and lymph node metastasis. Kurume Med J 1997;44:157-164.
47. Bösing N, Verreet PR, Ohmann C, Röher HD. Early stomachcarcinoma--pathologic-anatomic fndings and prognosis. Chirurg 1998;69:259-263; discussion 264.
48. Kitamura K, Yamaguchi T, Taniguchi H, Hagiwara A, Sawai K, Takahashi T. Analysis of lymph node metastasis in early gastric cancer: rationale of limited surgery. J Surg Oncol 1997;64:42-47.
49. Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol 2004;85:181-185; discussion 186.
50. Kim DY, Joo JK, Ryu SY, Kim YJ, Kim SK. Factors related to lymph node metastasis and surgical strategy used to treat early gastric carcinoma. World J Gastroenterol 2004;10:737-740.
51. Borie F, Millat B, Fingerhut A, Hay JM, Fagniez PL, De Saxce B. Lymphatic involvement in early gastric cancer: prevalence and prognosis in France. Arch Surg 2000;135:1218-1223.
52. Nasu J, Nishina T, Hirasaki S, Moriwaki T, Hyodo I, Kurita A, et al. Predictive factors of lymph node metastasis in patients with undiferentiated early gastric cancers. J Clin Gastroenterol 2006;40:412-415.
53. Roviello F, Rossi S, Marrelli D, Pedrazzani C, Corso G, Vindigni C, et al. Number of lymph node metastases and its prognostic signifcance in early gastric cancer: a multicenter Italian study. J Surg Oncol 2006;94:275-280; discussion 274.
54. Xu YY, Huang BJ, Sun Z, Lu C, Liu YP. Risk factors for lymph node metastasis and evaluation of reasonable surgery for early gastric cancer. World J Gastroenterol 2007;13:5133-5138.
55. Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol 2008;15:508-513.
56. Ye BD, Kim SG, Lee JY, Kim JS, Yang HK, Kim WH, et al. Predictive factors for lymph node metastasis and endoscopic treatment strategies for undiferentiated early gastric cancer. J Gastroenterol Hepatol 2008;23:46-50.
57. Haruta H, Hosoya Y, Sakuma K, Shibusawa H, Satoh K, Yamamoto H, et al. Clinicopathological study of lymph-node metastasis in 1,389 patients with early gastric cancer: assessment of indications for endoscopic resection. J Dig Dis 2008;9:213-218.
58. Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly diferentiated adenocarcinoma of the stomach. Endoscopy 2008;40:7-10.
59. Wu ZM, Wu AW, Li ZY, Wu Q, Zhang LH, Wu XJ, et al. Characteristics of lymph node metastasis and prognostic analysis in 157 early gastric cancer patients. Zhonghua Wei Chang Wai Ke Za Zhi 2009;12:350-353.
60. Shen L, Huang Y, Sun M, Xu H, Wei W, Wu W. Clinicopathological features associated with lymph node metastasis in early gastric cancer: analysis of a single-institution experience in China. Can J Gastroenterol 2009;23:353-356.
61. Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, et al. Risk factors for lymph node metastasis in histologically poorly diferentiated type early gastric cancer. Endoscopy 2009;41:498-503.
62. Hölscher AH, Drebber U, Mönig SP, Schulte C, Vallböhmer D, Bollschweiler E. Early gastric cancer: lymph node metastasis starts with deep mucosal infltration. Ann Surg 2009;250:791-797.
63. Kang HJ, Kim DH, Jeon TY, Lee SH, Shin N, Chae SH, et al. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc 2010;72:508-515.
64. Ren G, Cai R, Zhang WJ, Ou JM, Jin YN, Li WH. Prediction of risk factors for lymph node metastasis in early gastric cancer. World J Gastroenterol 2013;19:3096-3107.
65. Li H, Lu P, Lu Y, Liu C, Xu H, Wang S, et al. Predictive factors of lymph node metastasis in undiferentiated early gastric cancers and application of endoscopic mucosal resection. Surg Oncol 2010;19:221-226.
66. Lee JH, Choi IJ, Kook MC, Nam BH, Kim YW, Ryu KW. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg 2010;97:732-736.
67. Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, et al. Risk factors for lymph node metastasis in undiferentiated early gastric cancer. Ann Surg Oncol 2008;15:764-769.
68. Lim MS, Lee HW, Im H, Kim BS, Lee MY, Jeon JY, et al. Predictable factors for lymph node metastasis in early gastric cancer-analysis of single institutional experience. J Gastrointest Surg 2011;15:1783-1788.
69. Wang Z, Ma L, Zhang XM, Zhou ZX. Risk of lymph node metastases from early gastric cancer in relation to depth of invasion: experience in a single institution. Asian Pac J Cancer Prev 2014;15:5371-5375.
70. Wang Z, Zhang X, Hu J, Zeng W, Liang J, Zhou H, et al. Predictive factors for lymph node metastasis in early gastric cancer with signet ring cell histology and their impact on the surgical strategy: analysis of single institutional experience. J Surg Res 2014;191:130-133.
71. Park DJ, Lee HK, Lee HJ, Lee HS, Kim WH, Yang HK, et al. Lymph node metastasis in early gastric cancer with submucosal invasion: feasibility of minimally invasive surgery. World J Gastroenterol 2004;10:3549-3552.
72. Fujii M, Egashira Y, Akutagawa H, Nishida T, Nita T, Edagawa G, et al. Pathological factors related to lymph node metastasis of submucosally invasive gastric cancer: criteria for additional gastrectomy afer endoscopic resection. Gastric Cancer 2013;16:521-530.
73. Nesi G, Basili G, Girardi LR, Maneti A, Bilioti G, Barchielli A. Pathological predictors of lymph node involvement in submucosalgastric carcinoma: a retrospective analysis of long-term outcome. In Vivo 2009;23:337-341.
74. Seto Y, Shimoyama S, Kitayama J, Mafune K, Kaminishi M, Aikou T, et al. Lymph node metastasis and preoperative diagnosis of depth of invasion in early gastric cancer. Gastric Cancer 2001;4:34-38.
75. Son HJ, Song SY, Kim S, Noh JH, Sohn TS, Kim DS, et al. Characteristics of submucosal gastric carcinoma with lymph node metastatic disease. Histopathology 2005;46:158-165.
76. Song SY, Park S, Kim S, Son HJ, Rhee JC. Characteristics of intramucosal gastric carcinoma with lymph node metastatic disease. Histopathology 2004;44:437-444.
77. Ohashi S, Okamura S, Urano F, Maeda M. Clinicopathological variables associated with lymph node metastasis in submucosal invasive gastric cancer. Gastric Cancer 2007;10:241-250.
78. Te Paris endoscopic classifcation of superfcial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-S43.
79. Fukuhara S, Yabe M, Montgomery MM, Itagaki S, Brower ST, Karpeh MS Jr. Race/Ethnicity is predictive of lymph node status in patients with early gastric cancer. J Gastrointest Surg 2014;18:1744-1751.
80. Yang HJ, Kim SG, Lim JH, Choi J, Im JP, Kim JS, et al. Predictors of lymph node metastasis in patients with non-curative endoscopic resection of early gastric cancer. Surg Endosc 2015;29:1145-1155. 81. Shida A, Fujioka S, Kawamura M, Takahashi N, Ishibashi Y, Nakada K, et al. Prediction of lymph node metastasis in patients with submucosa-invading early gastric cancer. Anticancer Res 2014;34:4471-4474.
82. Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General patern in 1931 patients. Ann Surg 1989;210:596-602.
83. Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, et al. Longterm outcomes afer endoscopic mucosal resection for early gastric cancer. Gastric Cancer 2006;9:88-92.
84. Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer 1996;77:602-606.
85. Tsujitani S, Oka S, Saito H, Kondo A, Ikeguchi M, Maeta M, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999;125:148-154.
86. Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225-229.
87. Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. Te Vienna classifcation of gastrointestinal epithelial neoplasia. Gut 2000;47:251-255.
88. Stolte M. Te new Vienna classifcation of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch 2003;442:99-106.
89. World Health Organization. Pathology and Genetics of Tumours of the Digestive System. 4th edition. Lyon : IARC press, 2010.
90. Sakurai U, Lauwers GY, Vieth M, Sawabe M, Arai T, Yoshida T, et al. Gastric high-grade dysplasia can be associated with submucosal invasion: evaluation of its prevalence in a series of 121 endoscopically resected specimens. Am J Surg Pathol 2014;38:1545-1550.
Cite this article as: Saragoni L. Upgrading the definition of early gastric cancer: beter staging means more appropriate treatment. Cancer Biol Med 2015;12:355-361. doi: 10.7497/j.issn.2095-3941.2015.0054
Luca Saragoni
E-mail: luca.saragoni@auslromagna.it
Received July 23, 2015; accepted October 15, 2015.
Available at www.cancerbiomed.org
Copyright © 2015 by Cancer Biology & Medicine
 Cancer Biology & Medicine2015年4期
Cancer Biology & Medicine2015年4期
- Cancer Biology & Medicine的其它文章
- Mechanistic basis and clinical relevance of the role of transforming growth factor-β in cancer
- PI3K/Akt/mTOR inhibitors in breast cancer
- Advances in drug delivery system for platinum agents based combination therapy
- Dermatofibrosarcoma protuberans: from translocation to targeted therapy
- Synergistic suppression of the PI3K inhibitor CAL-101 with bortezomib on mantle cell lymphoma growth
- Pathological clavicular fracture as first presentation of renal cell carcinoma: a case report and literature review
