Synthesis and Application of Polyurethane Modified Organic Silicone Wet Rubbing Fastness Improver
TONG Dong-feng (仝东凤),LIU Xue (刘 学),2* ,DONG Chao-hong (董朝红)* ,LIU Jie (刘 杰)
1 College of Chemical and Environmental Engineering,Qingdao University,Qingdao 266071,China
2 Key Laboratory of Special Textile Processing Technology of Shandong Province,Qingdao 266032,China
Introduction
Reactive dyes contain groups that react with the hydroxyl(OH)groups in cellulose.Reactive dyes for protein fibers and nylon have also been offered by dye manufactures,but dyeing of cellulose is the major use for dyes in the reactive classification.However,the dyes suffer the disadvantage that dye fiber reaction is not 100% efficient[1].The reaction between the dye and the fiber is a nucleophilic displacement.Since the by-product of the reaction is an acid and because alkali increases the negative nature of the oxygen atom on cellulose,the above reactions are catalyzed by alkali.Dye molecules,which react with the fiber and become fixed,have excellent fastness to washing because of the high strength of the covalent bonds.But dye molecules,which are hydrolyzed may be weakly attached to the fiber and if not washed out at the end of the dye cycle,have very poor washfastness.Especially when the fabric is dyed to a deep color,a great amounts of dye molecules that cannot react with fiber gather together on the surface of the fibers,which results in a very poor wet rubbing fastness of reactive dyes[2].Several kinds of color fastness improver agents have been reported on the application in improving the wet rubbing fastness of dyed fabrics,however,the hand feeling of the treated fabric would be influenced greatly[3-4].Therefore,a multi-functional agent which can improve both color fastness of dyed fabrics and fabric handle feeling should be developed.
Polyurethanes have been gaining importance in a wide range of applications such as foam materials,water proof materials,paints,and adhesives because of their good physical and mechanical properties.And it is easy to control the surface properties,such as toughness,flexibility,adhesion on substrate,and anti-abrasion resistance[5-7].Aqueous polyurethane could form 3D mesh film on the surface of fiber and encapsulate dyes,by which the dyes were protected from hydrolyzing.Furthermore,aqueous polyurethane could react with reactive groups of dyes and fibers,by which the wet rubbing fastness of dyed cotton fabric could be effectively improved[3].The application of silicone softeners turns hard and rough fabric into a soft pleasant textile with which the buyer can expect a high degree of wearing comfort.Silicone have wide spread application in the textile industry from fiber,yarn and fabric production to final product finishing[8].The friction between fibers is decreased significantly due to organic silicone molecules attached onto fiber,thus the softness of fabric is improved.In order to combine the valuable properties of the two polymers, cross-linking structure of organic siliconepolyurethane systems were often designed[9].For these reasons,the experiment employed hydroxyl-terminated polyethermodified silicone,and toluene-2,4-diisocyanate (TDI)as raw materials,and synthesized polyurethane-modified silicone with sodium bisulfate capped.The chemical structure of the polyurethane modified organic silicone was also investigated.In addition,the application properties of the polyurethane modified organic silicone were also studied.
1 Materials and Methods
1.1 Materials
Hydroxyl-terminated polyether modified silicone,TDI,NaHSO3,Drimaren Red K-2G,Drimaren Yellow K-6G,Drimaren Blue K-GR (these dyes were provided by Clariant)were applied in this study.The hydroxyl-terminated polyether modified silicone used in this study were dried at 100 ℃ in vacuum for 4 -5 h in advance to remove all air bubbles and water vapors.All of the reagents used in this study were analytical grade.
1.2 Synthesis of the polyurethane modified organic silicone
First of all,hydroxyl-terminated polyether modified silicone(Fig.1)was charged into a four-necked round bottom flask equipped with a mechanical stirrer,a thermometer,a reflux condenser,heating oil bath,and a nitrogen gas inlet system.The temperature of the oil bath was increased to a certain temperature.Then some TDI were added to the reaction vessel.It took some time to obtain NCO terminated.The NCO contents of polymer were determined and found close to the theoretical value.At last,the temperature of the reaction vessel was decreased to certain temperature and some NaHSO3were introduced into the reaction mixture.It took some time to form yellow transparent liquid in the reaction vessel which was the indication of the formation of polyurethane modified organic silicone (Fig.2).
1.3 Dyeing process
The pre-wetted fabrics by distilled water were dyed in a dye bath with liquor ratio 50 ∶1.The dyeing process employed is shown in Fig.3.After being dyed,the dyed sample was rinsed thoroughly in cold water and then hot water.At last,the dyed sample was rinsed in cold water and allowed to dry in the open air.
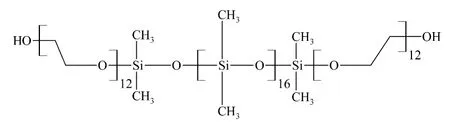
Fig.1 Hydroxyl-terminated polyether modified silicone

Fig.2 Polyurethane modified organic silione

Fig.3 Dyeing process
1.4 Treatment of cotton fabrics with the polymer
The cotton fabrics were immersed in a solution of the polyurethane modified organic silicone at 30 ℃for 5 min.The wet fabrics were squeezed using a laboratory mangle with a pick-up 65%.The fabrics were then dried at 85 ℃for 3 min and cured at 160 ℃for 90 s with a preheated laboratory tenter to fix polyurethane modified organic silicone on the cotton fabrics.
1.5 Characterization of the polymer
The polyurethane modified organic silicone was determined by FT-IR spectra and1H-NMR.
Physical parameters such as solid contents (%),emulsion stability, emulsion appearance, and PH value of the polyurethane modified organic silicone were shown.
Emulsion stability was evaluated by storage time.The emulsion state remains the same under the certain storage time.
1.6 Characterization of cotton fabric with the polyurethane modified organic silicone
The surface morphology of the fabric was determined by scanning electron microscopy (SEM).
The chemical groups present on cotton surfaces were analyzed by FT-IR.
The XRD patternswere determined by powder X-ray diffraction measurements.
1.7 Testing methods
The wet rubbing fastness properties of the fabrics dyed with different dyes such as Red K-2G,Blue K-GR,and Yellow K-6G were tested through GB/T3920—1997 standard methods.
The softness was evaluated by hands feel method,which needed at least three people subjective assessment.
2 Results and Discussion
2.1 Molecular structural characterization
Figure 4 (a)showed the FT-IR spectrum of the polyether modified organic silicone.The bands at 2 900 and 3 475 cm-1were assigned to C—H and O—H bonds which appeared on polyether modified silicone.The strong absorption at 1 250 cm-1was due to Si—CH[10].The bands in a range of 1 150-1 100 cm-1were due to C—O—C and Si—O—Si[10-13].The peak at 835 cm-1was assigned to Si—CH3bond[13-14].Figure 4(b)showed the FT-IR spectrum of the polyurethane modified organic silicone.The peak at 835 cm-1was assigned to Si—CH3rocking[13-14].The band at 3 475 cm-1assigned to O—H stretching disappeared.There were no absorption peaks at 3 475 cm-1,which indicated that no O—H bond existed in the polyurethane modified organic silicone the monomers were polymerized.There was the peak at 1 725 cm-1=of C O of ester and 1 530 cm-1was attributed to N—H bond vibration and C—N symmetry stretch vibration[12,14].The band at 2 270 cm-1was NCO characteristic absorption peak.So the carbamate had been generated.These showed that the polyurethane modified organic silicone had been modified successfully.
1H-NMR is the most useful for the measurement of hydrogen chemical environment because of its high sensitivity to hydrogen bond strength,while the area of apex also evidently reflects the abundance of hydrogen in different chemical shift.[8]Figure 5 showed the1H-NMR spectrum of the polyurethane modified organic silicone.Observed peaks at 0.029-0.066 mg/L were attributed to methyl groups of the organic silicone segment structure.Methylene groups of polyether segment appeared at 3.552-3.647 mg/L.A peak at 7.26 mg/L was assigned to C—H bond of benzene ring in TDI.Small peak appearing at 2.16 mg/L was attributed to methyl groups of benzene ring in TDI.Also weak peak appearing at 2.017 mg/L was due to N—H bond of carbamate.

Fig.4 FT-IR spectra of (a)the polyurethane modified organic silicone and (b)the polyether modified silicone

Fig.5 1H-NMR spectrum of the polyurethane modified organic silicone
2.2 Physical characterization
In this work,the polyurethane modified organic silicone emulsion of different dosage of NCO was prepared.Table 1 showed physical parameters such as solid contents (%),emulsion stability,emulsion appearance and pH value of the polyurethane modified organic silicone.These characteristics are important and helpful for the further application of the emulsions.It could be seen that pH value was 6-7.Emulsion appearance was almost the same in all studied samples.Solid content of the synthesized material was in the range of 20%-30%.And the solid content of the polyurethane modified organic silicone decreased with the increase of different NCO contents.The reason of this phenomenon may be that the increase of NCO contents contribute to form cross-linking.So the viscosity of polyurethane modified silicone has increased.It clearly indicated that emulsion stability of No.5 was only 1 week.Over time, a part of the emulsion gelled.The phenomenon may be due to the free isocyanate groups that can crosslink with each other in the emulsion.

Table 1 Physical characterization of the polyurethane modified organic silicone
2.3 SEM images of the polymer
Figure 6 showed the SEM analyses of surface changes of cotton fabric with the polyurethane modified organic silicone.Figure 6 (a)showed the rough surface of cotton fabric.There were many tiny grooves.When SEM images in Figs.6 (a)and(b)were compared,it was clear that the cotton fiber of Fig.6(b)were covered with the polyurethane modified organic.Because of the higher film-form ability of polyurethane,it could form a layer of protective film on the surface of fiber.It was considered that the polyurethane modified organic silicone had been attached to the fiber surface.Figure 6 (c)showed the cotton fabric with the polyurethane modified organic silicon treatment after 20 times washing.It was found that the film on the surface of fabrics were damaged to a certain extent,but it still existed on the fiber surface.The reason of the phenomenon may be that polyurethane modified organic silicone finishing agent end capped with sodium bisulfite released NCO at a certain temperature.Then the covalent cross-linking structures could be formed between NCO and OH of cellulose fibers.

Fig.6 SEM of (a)cotton fabric,(b)cotton fabric with polyurethane modified organic silicone treatment,and (c)cotton fabric with the polyurethane modified organic silicon treatment after being washed 20 times
2.4 FT-IR spectra of cotton fabric studies
Figure 7 showed the FT-IR spectra of untreated cotton and fabric with the polyurethane modified organic silicone.Figure 7 (b)showed the FT-IR spectra of cotton fabric with the polyurethane modified organic silicone treatment compared with the untreated cotton shown in Fig.7 (a).The band at 3 450 cm-1was assigned to O—H bonds,which normally appeared on cotton surface,had lower intensity than the untreated surface.This was because the covalent cross-linking structures could be formed between NCO and OH of cellulose fibers.The band at 2 900 cm-1assigned to C—H stretching had higher intensity than the untreated surface due to Si—CH3groups.The absorption at 1 725 cm-1=reflected C O stretching of ester group in polyurethane modified organic.The peak at 1 530 cm-1was attributed to N—H bond vibration and C—N symmetry stretch vibration.The absorption at 1 250 cm-1was due to Si—CH.The peak at 850 cm-1was assigned to Si—CH3rocking.From these analyses,it was clear that polyurethane modified organic silicone had reacted with cellulose fibers.
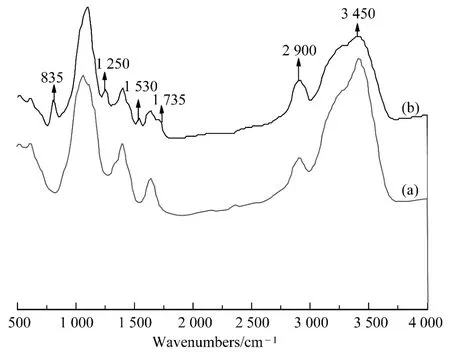
Fig.7 FT-IR spectra of (a)cotton fabric and (b)cotton fabric with polyurethane modified organic silicone treatment
2.5 XRD of cotton fabric studies
Figure 8 showed the XRD of cotton fabrics of untreated or treated with the polyurethane modified organic silicone.It was found that there were the same strong diffraction peaks at 14.8°,16.7°,22.8°,and 34.3°.It was indicated that the crystalline regions of cotton fibers did not change after finishing.So the main reaction between cotton and polyurethane modified organic silicone occurred in the amorphous areas and the surface of cotton fibers.
2.6 Effect of fabric wet rubbing fastness
Table 2 showed the effect of fabric wet rubbing fastness treated with polyurethane modified organic silicone.There have been reports that polyurethane modified organic silicone containing reactive groups (NCO)could react with reactive groups of dyes and fibers,thus reducing dye hydrolysis.Therefore,the rubbing fastness of deep color cotton fabrics was improved effectively.The rubbing fastness properties of cotton fabric before and after finishing were shown in Table 2.It was revealed that all the wet rubbing fastness was above rating 3.Meanwhile,all the dry rubbing fastness has been improved.And the wet rubbing fastness of different reactive dyed fabric had got enhanced in different degrees.It was not selective for reactive dyes.Wet rubbing fastness of fabric dyed with three kinds of reactive dyes was significantly improved after being treated with the polyurethane modified organic silicone.The organic silicone molecules adsorbed onto the fiber greatly decreased the friction between fibers,thus the softness of cotton fabrics was improved.As can be seen from Table 2,the softness of the cotton fabrics was improved from rating 1 to rating 5.

Fig.8 XRD of (a)cotton fabric and (b)cotton fabric with polyurethane modified organic silicone treatment
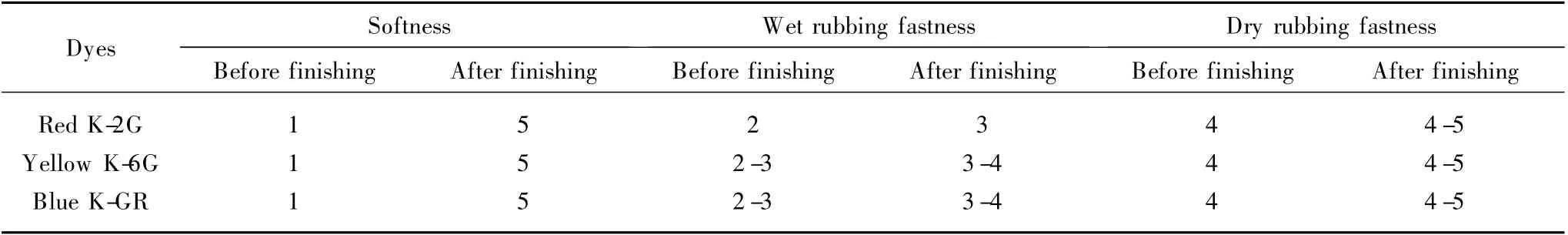
Table 2 Rubbing fastness properties of cotton fabric after finishing
2.7 Color aberration after finishing
Table 3 showed color aberration of the samples after the treatment with polyurethane modified organic silicone.The DL(depth of color)and Dc(vidiness of color)values of the samples decreased after treatment with polyurethane modified organic silicone,the shade becoming darker.This is due to lower refractive index of silicone agent solutions,which reflect less light,thus the silicone agent treated samples show a darker shade[15].The Da(the color of red to green component),Db(the color of yellow to blue component),and Dcvalues of the treated samples changed after treatment.The color of finished fabrics became blue-green.The reason may be that the combination of the polyurethane modified silicone with dye molecules affected the molecular structure of the dye conjugated system.There was little change of DE(the total color difference)for the polyurethane modified organic silicone.
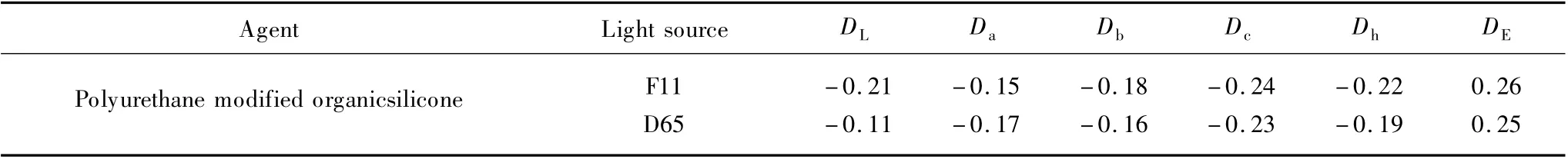
Table 3 Color aberration after finishing
2.8 Performance comparison
Table 4 showed the performance comparison results of fabrics treated with different finishing agent.It was observed that the fabrics treated with polyurethane modified organic silicone had the same softness compared with the fabrics treated by organic silicone softeners.However,there was a remarkable increase in rubbing fastness of treated fabrics by polyurethane modified organic silicone compared with the samples treated by organic silicone.This was because the reactive groups (NCO)of polyurethane modified organic silicone reacted with reactive groups of dyes and fibers.The polyurethane segment of polyurethane modified organic silicone formed 3D mesh film on the surface of fibers and protected the dyes from hydrolyzing.This implies that polyurethane helps to improve the rubbing fastness of the treated fabrics.
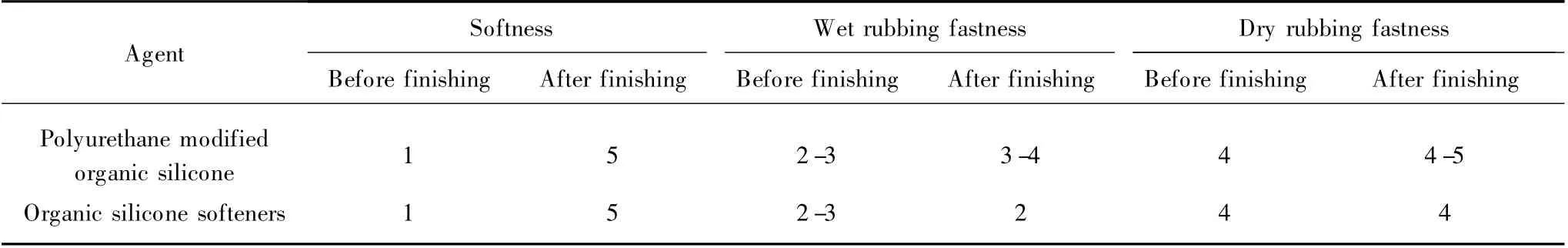
Table 4 Performance comparison of different finishing agent
3 Conclusions
(1)The chemical structure of the polyurethane modified organic silicone was characterized by FT-IR and1H-NMR.It was confirmed that the polyurethane modified silicone polymers were successfully synthesized using TDI and hydroxylterminated polyethers modified organic silicone as raw materials.
(2) The polymer film on the cotton surface was investigated by SEM and the results showed that a thin polymer film was successfully formed.The FT-IR of treated cotton fabrics indicated that the covalent cross-linking system had been formed between OH of cellulose fibers and NCO of the polyurethane modified organic silicone.The polyurethane modified organic silicone finishing agent end capped with sodium bisulfite has released NCO at a certain temperature.XRD analyses revealed that the crystalline region of treated cotton fabrics wasn't changed after finishing,implying the main reaction of cotton and polyurethane modified organic silicone occurred in the amorphous areas of cotton fibers.
(3) Polyurethane modified organic silicone polymer emulsion is a very good wet rubbing fastness agent,which can improve the wet rubbing fastness of the fabric dyed by reactive dyes by rating 1.There was little change of DE values for the polyurethane modified organic silicone.In addition,the softness of treated cotton fabrics was excellent.
[1]Burkinshaw S M,Katsarelias D.A Study of the Wash-off and Aftertreatment of Dichlorotriazinyi Reactive Dyes on Cotton[J].Dyes and Pigments,1995,29(2):139-153.
[2]Zheng Q S,Li L,Wang W,et al.Study on Parameters of Saving Protease for Cashmere Shrink Proof[J].Journal of Xi'an Polytechnic University,2010 (3):279-284.(in Chinese)
[3]Xu J,Zhu Q.Application Process of Improver WPU for Wet Rubbing Fastness[J].Dyeing and Finishing,2006(24):17-19.(in Chinese)
[4]Mao Z P,Lu Q M,Qin D H,et al.The Effect of the Wet Rubbing Fastness Improver ZQ-W on the Properties the Fabrics Dyed with Reactive Dyes[J].Textile Auxiliaries,2005,22(4):20-21.(in Chinese)
[5]Daemia H,Rad R R,Barikani M,et al.Catalytic Activity of Aqueous Cationic Polyurethane Dispersions:a Novel Feature of Polyurethanes[J].Applied Catalysis A:General,2013,468:10-17.
[6]Ge Z,Luo Y J.Synthesis and Characterization of Siloxane-Modified Two-Component Waterborne Polyurethane [J].Progress in Organic Coatings,2013,76(11):1522-1526.
[7]Castagna A M,Fragiadakis D,Lee H K,et al.The Role of Hard Segment Content on the Molecular Dynamics of Poly(tetramethylene oxide)-Based Polyurethane Copolymers [J].Macromolecules,2011,44(19):7831-7836.
[8]Naghash H J,Abili B.Synthesis of a Silicone Containing Allylic Monomer and Its Uses in the Waterborne Polyurethane/Vinyl Acetate-Acrylic Hybrid Emulsion Copolymers[J].Progress in Organic Coatings,2010,69(4):486-494.
[9]Zubera M,Zia K M,Tabassum S,et al.Preparation of Rich Handles Soft Cellulosic Fabric Using Amino Silicone Based Softener,Part II:Colorfastness Properties [J].International Journal of Biological Macromolecules,2011,49(1):1-6.
[10]Akovali G,Rzaev Z M O,Mamedov D G.Plasma Surface Modification of Polyethylene with Organosilicon and Organotin Monomers[J].European Polymer journal.1996,32(3):375-383.
[11]Jia X,Li Y F,Cheng Q,et al.Preparation and Properties of Poly (vinyl alcohol)/Silica Nanocomposites Derived from Copolymerization of Vinyl Silica Nanoparticles and Vinyl Acetate[J].European Polymer Journal,2007,43(4):1123-1131.
[12]Tang E J,Cheng G X,Shang Q,et al.A Novel Approach to the Preparation of Powder Coating-Manufacture of Polyacrylate Powder Coatings via One Step Minisuspension Polymerization[J].Progress in Organic Coatings,2006,57(3):282-287.
[13]Teshima K,Sugimura H,Inoue Y,et al.Wettability of Poly(ethylene terephthalate)Substrates Modified by a Two-Step Plasma Process:Ultra Water Repellent Surface Fabrication[J].Chemical Vapor Deposition,2006,10(6):295-297.
[14]Parvinzadeh M.The Effects of Softeners on the Properties of Sulfur-Dyed Cotton Fibers [J].Journal of Surfactants and Detergents,2007,10(4):219-223.
[15]Daemi H,Barikani M,Barmar M.Compatible Compositions Based on Aqueous Polyurethane Dispersions and Sodium Alginate[J].Carbohydrate Polymers,2013,92(1):490-496.
 Journal of Donghua University(English Edition)2015年3期
Journal of Donghua University(English Edition)2015年3期
- Journal of Donghua University(English Edition)的其它文章
- Group Performance Evaluation in Universities with Entropy Method
- Inactivation of Giardia Intestinalis by Peroxone Process (H2 O2 /O3)and Its Disinfection Mechanisms
- Optical Measurements to Reveal Roles of Slightly Crosslinked Poly(dimethyldiallylammonium chloride)s in Fixing Anionic Dyes on Cotton Fabric
- Portfolio Choice under the Mean-Variance Model with Parameter Uncertainty
- Behavior of Benzene Decomposition by Using Pulse Modulated Power Supply
- Experimental Investigation on Constrained Abrasive Fluid Polishing for Optical Glass
