Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy
Nan Yang, He Liu, Yao Jiang, Ji Zheng, Dong-mei Li, Chao Ji, Yan-yong Liu, Ping-ping Zuo
Department of Pharmacology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & School of Basic Medicine, Peking Union Medical College, Beijing, China
Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy
Nan Yang, He Liu, Yao Jiang, Ji Zheng, Dong-mei Li, Chao Ji, Yan-yong Liu*, Ping-ping Zuo*
Department of Pharmacology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & School of Basic Medicine, Peking Union Medical College, Beijing, China
Lactulose is known to improve cognitive function in patients with early hepatic encephalopathy; however, the underlying mechanism remains poorly understood. In the present study, we investigated the behavioral and neurochemical eff ects of lactulose in a rat model of early hepatic encephalopathy induced by carbon tetrachloride. Immunohistochemistry showed that lactulose treatment promoted neurogenesis and increased the number of neurons and astrocytes in the hippocampus. Moreover, lactulose-treated rats showed shorter escape latencies than model rats in the Morris water maze, indicating that lactulose improved the cognitive impairments caused by hepatic encephalopathy. The present fi ndings suggest that lactulose eff ectively improves cognitive function by enhancing neuroplasticity in a rat model of early hepatic encephalopathy.
nerve regeneration; brain injury; hepatic encephalopathy; lactulose; neuroplasticity; neurogenesis; Morris water maze; cognition; rats; neuronal nuclei; glial fi brillary acidic protein; NSFC grants; neural regeneration
Funding: This study was supported by a grant from the National Natural Science Foundation of China, No. 30873390.
Yang N, Liu H, Jiang Y, Zheng J, Li DM, Ji C, Liu YY, Zuo PP (2015) Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy. Neural Regen Res 10(9):1457-1462.
Introduction
Hepatic encephalopathy is a common complication of chronic liver disease, and is associated with a poor prognosis. It manifests clinically as a spectrum of neuropsychiatric disturbances encompassing cognitive, intellectual, motor, and psychomotor functions, from subtle changes in personality or sleep-wake cycle to major disturbances in cognitive function, motor activity and coordination (Ferenci et al., 2002; Gorg et al., 2010). Hepatic encephalopathy is least severe in its early stages, with few recognizable clinical symptoms other than mild cognitive and psychomotor defi cits. Nevertheless, early hepatic encephalopathy impairs a patient’s ability to perform certain tasks, such as driving, and reduces their quality of life, in addition to predisposing to full hepatic encephalopathy and reducing the patient’s lifespan, making early hepatic encephalopathy a serious health, social and economic burden. Early diagnosis and treatment initiation would improve patients’ quality of life and prevent the progression of neurological impairments (Felipo, 2013).
Blood levels of ammonia are often elevated in patients with hepatic encephalopathy, so current therapies are based on lowering ammonia. Non-absorbable disaccharides are the fi rst-line drug treatment for lowering the production and absorption of ammonia (Riordan and Williams, 1997; Blei and Cordoba, 2001). Lactulose, a synthetic disaccharide, is the most commonly used non-absorbable disaccharide in the treatment of hepatic encephalopathy (Sharma et al., 2013; Sharma and Sharma, 2013; Ziada et al., 2013). It comprises the monosaccharides lactose and galactose, and is administered as syrup. Doses are generally titrated to achieve two to four semi-soft stools daily, with typical doses of 20 g/30 mL orally three to four times per day. Lactulose signifi cantly improves cognition in patients with early hepatic encephalopathy (Prasad et al., 2007; Luo et al., 2011).
Neuroplasticity can be defi ned as the ability of the nervous system to respond to intrinsic or extrinsic stimuli by reorganizing its structure, function and connections. The process is essential to normal cognitive function and is widely implicated in disease processes (McEwen, 2006). Neurogenesis in the hippocampal dentate gyrus contributes signifi cantly to central neuroplasticity mechanisms such as long-term potentiation, learning and memory (Massa et al., 2011). Additionally, astrocytes have the ability to eliminate ammonia, and play an important role in the pathogenesis of hepatic encephalopathy (Lockwood et al., 1991; Haussinger et al., 2000).
Lactulose is known to improve cognitive impairment; however, few studies have addressed its eff ect on neuroplasticity. Here, we observed the eff ect of lactulose treatment on behavior and cognitive function in a rat model of early hepatic encephalopathy, and investigated its eff ects on neuroplasticity to elucidate the mechanisms underlying its cognition enhancing
eff ects.
Materials and Methods
Animals
Thirty adult male Wistar rats, initially weighing 240.38 ± 1.79 g, were supplied by Peking University Health Science Animal Center (Beijing, China; license No. SCXK (Jing) 2007-0001). The rats were housed in polypropylene cages in a temperature (22 ± 1°C) and humidity (60 ± 10%) controlled environment, under a 12-hour light-dark cycle (lights on at 7:00 a.m.). The animals had free access to food and water throughout the experiments. Animal maintenance and experimental protocols were carried out in accordance with guidelines approved by the Animal Care Committee of the Peking Union Medical College and Chinese Academy of Medical Sciences in China.
Animal grouping and early hepatic encephalopathy model establishment
The rats were randomly divided into three groups: control, model and lactulose (n = 10 rats per group). To induce hepatic encephalopathy, rats in the model and lactulose groups received a mixture of carbon tetrachloride (CCl4) and olive oil (1:1 v/v; National Pharmaceutical Group Chemical Reagent Co., Ltd., Beijing, China) at a dose of 1 mL/kg by gavage, in the morning, twice a week (Mondays and Thursdays) for 12 weeks (Tsai et al., 2009). Control animals received normal saline. Animals in the lactulose group also received intragastric lactulose (Solvay Pharmaceutical Co., Ltd., Brussels, Belgium), 6 g/kg, in the afternoon, fi ve times a week (Monday to Friday) for 12 weeks (Ferenci et al., 2002).
Morris water maze
Hepatic encephalopathy rats were evaluated for spatial learning and memory capabilities using a Morris water maze as described previously (Ji et al., 2009; Liu et al., 2012). Two training trials a day were conducted on 3 consecutive days during the 8thweek. The experimental apparatus consisted of a cylindrical water tank (145 cm diameter, 60 cm high) fi lled with water maintained at 21 ± 1°C. The water was made opaque with black ink. A platform (10 cm in diameter) was submerged 2 cm below the water surface and placed at the midpoint of one quadrant. Room lights illuminated the pool, and visual cues around the room (window, cabinets, furniture) were kept consistent. A video camera was placed above the center of the pool and connected to a video tracking system. During each training session, the rats were placed in the pool at a specifi ed starting position and allowed to swim freely until they found the platform. The time required to escape (escape latency) was recorded. Rats that found the platform within 120 seconds were allowed to remain on it for 20 seconds and were then returned to the home cage. If a rat did not reach the platform within 120 seconds, it was gently guided there by the experimenter, and allowed to stay on it for 20 seconds. The test was performed again at 12 weeks to assess spatial cognitive function.
5-Bromo-2′-deoxyuridine (BrdU) injection
After completion of the Morris water maze test, neurogenesis was studied in the granular cell layer of the dentate gyrus. BrdU (Sigma, St. Louis, MO, USA) was dissolved in 0.9% NaCl at 20 mg/mL. Four rats in each group each received three intraperitoneal injections of BrdU (50 mg/kg) at 12 hour intervals (total dose of 2.5 mL/kg) and were sacrifi ced 24 hours after the last injection (Stefovska et al., 2008). The brains were prepared for immunohistochemical staining with BrdU, described below.
Tissue processing and blood sampling
Body weight was monitored weekly. After completion of the behavioral tests, six rats in each group were decapitated and blood samples were collected for analysis of serum levels of ammonia, and activities of alanine aminotransferase and aspartate aminotransferase. Brains were immediately removed and washed in ice-cold isotonic saline. The remaining rats were anesthetized with an overdose of sodium pentobarbital and perfused transcardially with 0.1 M PBS followed by 4% paraformaldehyde (pH 7.4). The brains were removed and postfi xed in 4% paraformaldehyde at 4°C, and cryoprotected in a graded sucrose series (15%, 20% and 30% sucrose in 0.1 M PBS) at 4°C. Coronal sections (35 μm thick) were cut using a cryostat.
Liver tissues were taken from the left lobe of the liver of each rat, fi xed in 15% buff ered paraformaldehyde, and dehydrated through a graded alcohol series. Specimens were embedded in paraffi n blocks, cut into 5 μm thick sections and placed on glass slides, before staining with hematoxylin-eosin to assess liver damage (Yang et al., 2010). Fibrosis was graded (Scheuer, 1991) by a pathologist blinded to experimental grouping.
Serum levels of ammonia, and alanine aminotransferase and aspartate aminotransferase activities, were measured using commercially available kits (Jiancheng Biotechnology Institute, Nanjing, Jiangsu Province, China) according to the manufacturer’s instructions.
Brain immunohistochemistry
For BrdU immunohistochemistry, free-floating brain sections (35 μm thick) were incubated overnight at 4°C in primary antibody solution containing mouse monoclonal anti-BrdU antibody (1:100; Chemicon, Temecula, CA, USA). Sections were then washed in PBS and incubated in secondary antibody solution containing biotinylated goat polyclonal anti-mouse antibody I (SP immunohistochemical staining kit, Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China) for 30 minutes at room temperature, washed as before, and incubated in horseradish peroxidase-streptavidin solution for 30 minutes at room temperature. Immunoreactivity was visualized using 0.2% 3,3′-diaminobenzidine (Sigma) in PBS containing 0.025% hydrogen peroxide for approximately 5 minutes, and washed in PBS. The sections were mounted and dried (Ngwenya et al., 2005), and viewed under an optical microscope (Olympus, Tokyo, Japan, 400× magnifi cation). BrdU-labeled cells in the subgranular zone
were counted in every 10thsection, and multiplied by 10 to estimate the total number of BrdU-labeled cells.
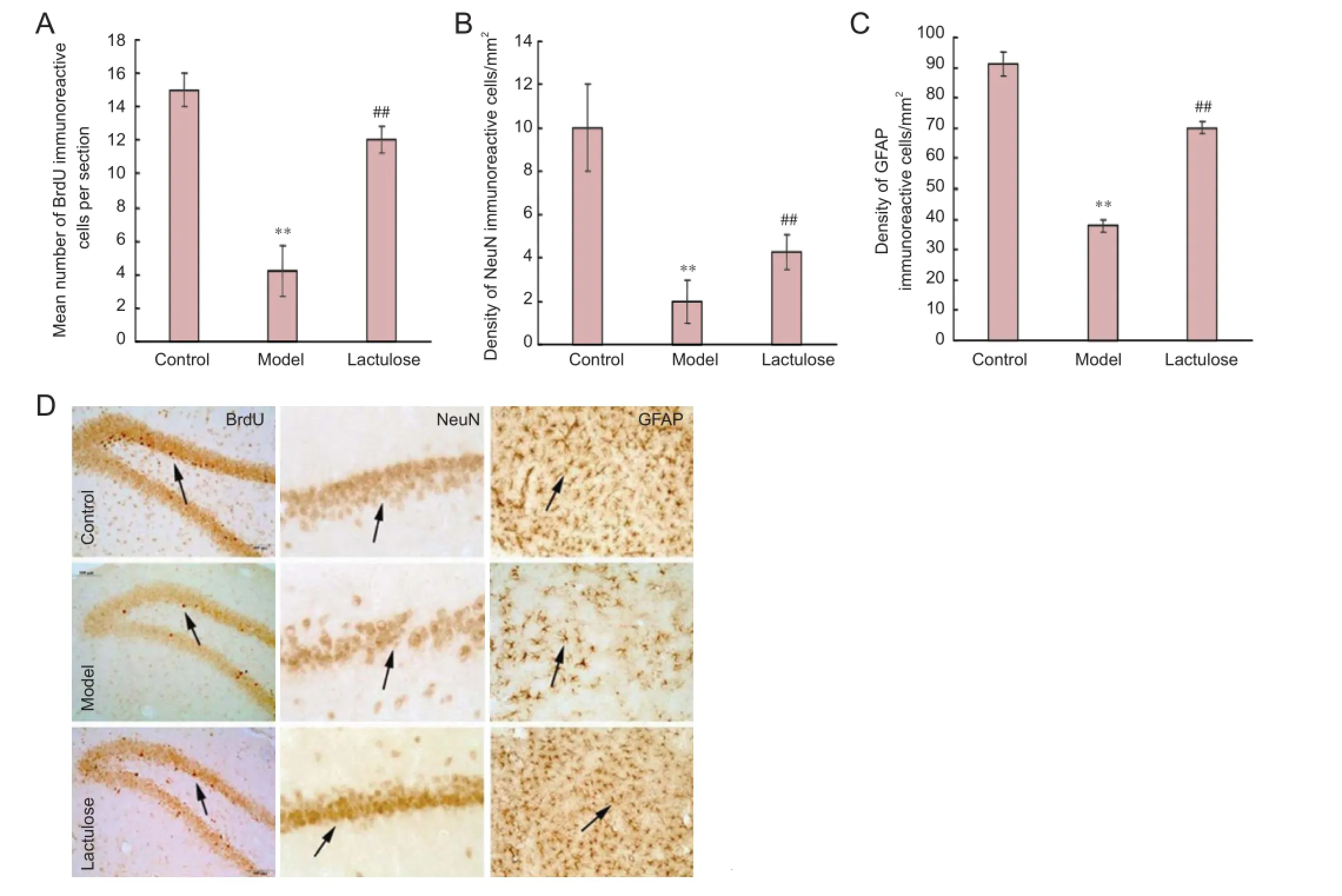
Figure 1 Eff ects of lactulose administration on neurogenesis and on numbers of neurons and astrocytes in the hippocampus of rats with hepatic encephalopathy (imunohistochemical staining).
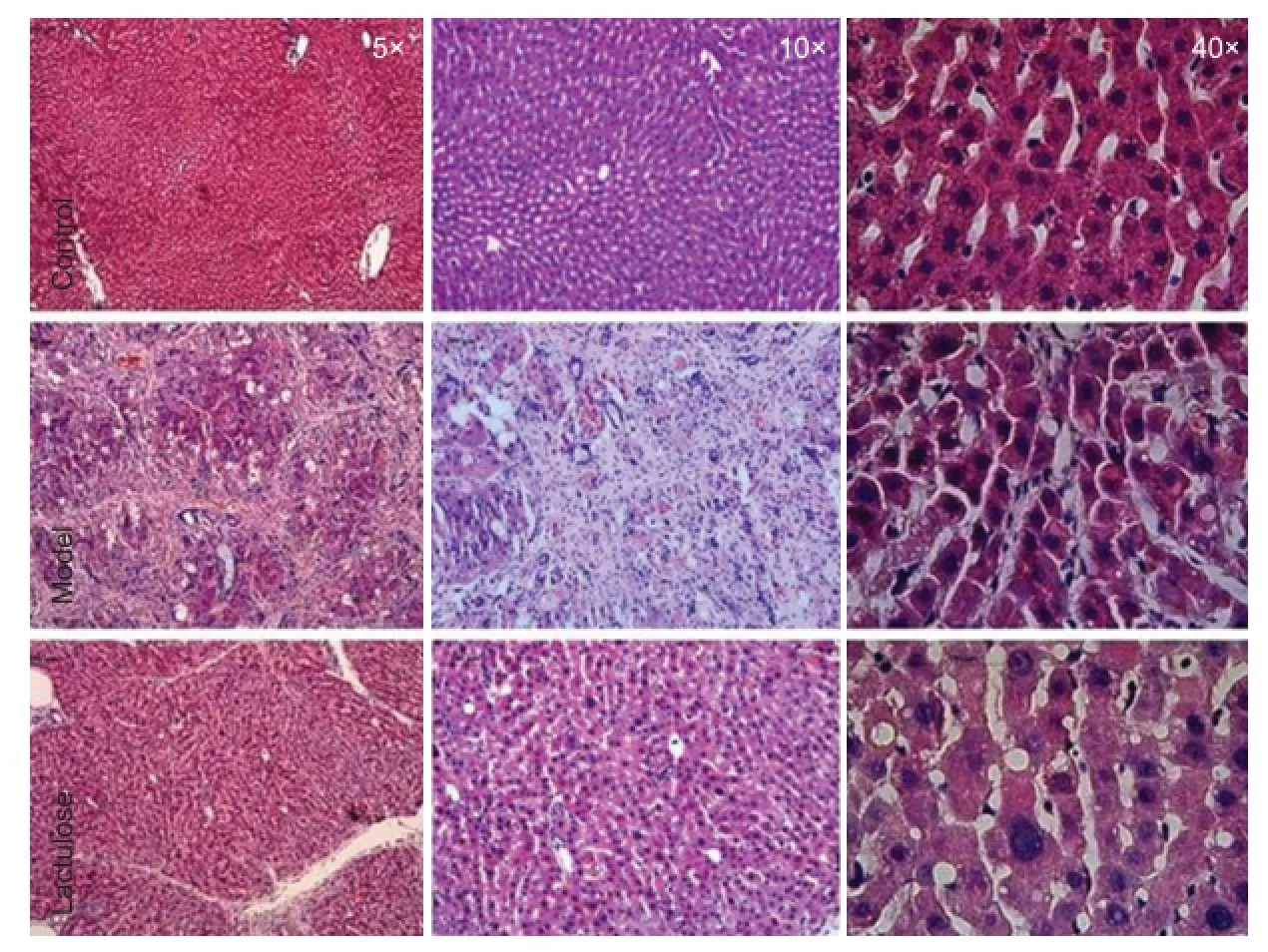
Figure 2 Eff ect of lactulose treatment on liver histology in rats with hepatic encephalopathy.
For immunohistochemistry of neuronal nuclei (NeuN) and glial fi brillary acidic protein (GFAP), free-fl oating brain sections (35 μm thick) were incubated overnight at 4°C in primary antibody solution containing mouse monoclonal anti-NeuN (1:50; Chemicon) or rabbit anti-GFAP (1:50; Beijing Zhongshan Biotechnology Co., Ltd.). Sections were washed in PBS, then incubated in secondary antibody solution containing biotinylated goat anti-mouse or anti-rabbit antibody I (SP immunohistochemical staining kit, Beijing Zhongshan Biotechnology Co., Ltd.) for 30 minutes at room temperature. The sections were washed again and incubated in horseradish peroxidase-streptavidin solution for 30 minutes at room
temperature, before visualization with 0.2% 3,3′-diaminobenzidine (Sigma) in PBS containing 0.025% hydrogen peroxide for approximately 5 minutes. The sections were washed in PBS, mounted on slides, dried, dehydrated through a graded alcohol series, cleared with xylene, and coverslipped using Permount (Fisher Scientifi c, Pittsburgh, PA, USA).
For NeuN immunostaining, the number of immunoreactive cells in the hippocampal CA1 pyramidal cell layer were evaluated under a light microscope at a magnifi cation of 400 × with the investigator blinded to the experimental grouping. Every 10thsection was quantifi ed and the immunopositive neurons were expressed as the mean number of cells per mm2. For GFAP immunostaining of the CA1, the number of immunoreactive cells of the stratum oriens, stratum pyramidale, and stratum radiatum were analyzed as described for NeuN staining (Liu et al., 2011).
Statistical analysis
Data were expressed as the mean ± SEM and analyzed statistically using one-way analysis of variance with SPSS 13.0 software (SPSS, Chicago, IL, USA). After testing for homogeneity of variances, Tukey’s multiple comparisons method was performed to identify diff erences between experimental groups. Statistical signifi cance was set at P < 0.05.
Results
Quantitative analysis of experimental animals
Thirty rats were randomly assigned to the control, model and lactulose groups (n = 10 per group). No infections were observed during the experimental period, but two rats in the model group died from serious liver damage during the 7thweek. Their data were excluded from the fi nal statistical analysis.
Eff ect of lactulose treatment on neuroplasticity in rats with CCl4-induced hepatic encephalopathy
Neurogenesis was examined by quantifying BrdU-immunoreactive cells in the dentate gyrus (Figure 1A, D). NeuN and GFAP immunoreactivity was used to identify functional neurons in the CA1 (Figure 1B, D) and astrocytes in the dentate gyrus (Figure 1C, D), respectively.
There were signifi cantly fewer BrdU-, NeuN- and GFAP-immunoreactive cells in the model group than in the control group (P < 0.01), and significantly more of all three cell types in the lactulose group compared with the model group (P < 0.01). This suggests that lactulose reversed hepatic encephalopathy-induced cellular alterations.
Eff ect of lactulose treatment on liver histology in rats with CCl4-induced hepatic encephalopathy
In the model group, fi brous tissue substituted most of the portal areas in the liver, and some portal areas were connected to central veins. Moreover, hepatocyte clusters were completely surrounded by fi brous tissue, producing cirrhotic nodules. Livers from lactulose-treated animals had less severe pathology than model animals, indicating that lactulose ameliorated the liver damage induced by CCl4(Figure 2). Eff ect of lactulose treatment on spatial cognitive function in rats with CCl4-induced hepatic encephalopathy
The Morris water maze test is widely used to measure cognitive defi cits in rodent models of neurological disorders. Model rats took notably longer than control rats to fi nd the hidden platform (P < 0.01), indicating that spatial reference memory was impaired in rats with CCl4-induced hepatic encephalopathy. Rats that received lactulose had signifi cantly shorter escape latencies than model rats (P < 0.01), suggesting that lactulose alleviated the spatial memory defi cits induced by CCl4(Figure 3).
Eff ect of lactulose treatment on body weight of rats with CCl4-induced hepatic encephalopathy
Weight loss and cachexia are commonly observed in patients with hepatic encephalopathy (Lockwood et al., 1986). Ammonia stimulation of the hypothalamic satiety centers may suppress appetite, leading to cachexia. We therefore monitored body weight throughout the experimental period (Figure 4). By 3 weeks, the model rats had notably lower body weights than controls (P < 0.01), and lactulose-treated animals weighed signifi cantly more than rats in the model group (P < 0.01).
Eff ect of lactulose treatment on the serological parameters of rats with CCl4-induced hepatic encephalopathy
To investigate the protective effects of lactulose on liver function, we measured serum ammonia level, and alanine aminotransferase and aspartate aminotransferase activities. All three parameters were signifi cantly elevated in the model group (P < 0.01), indicative of severe liver damage. However, rats in the lactulose group had lower serum ammonia (P <0.01) (Figure 5A), alanine aminotransferase activity (P <0.05) and aspartate aminotransferase activity (P < 0.01) than the model rats (Figure 5B).
Discussion
Most patients with cirrhosis will develop early hepatic encephalopathy, which severely aff ects cognitive ability, day-today functioning, and quality of life. The condition is always related to increased mortality and places great demands on the healthcare system. The pathophysiology of the disorder remains poorly understood, meaning that treatment choices are limited. Ammonia is known to have a central role in the development of hepatic encephalopathy, so non-absorbable disaccharides, such as lactulose and lactitol, are the most commonly used pharmacological treatments.
Various experimental approaches, such as the Morris water maze in rodent hepatic encephalopathy models, have been adopted to investigate the neurocognitive eff ects of hepatic encephalopathy (Ortiz et al., 2006; Mendez et al., 2008). In the present study, hepatic encephalopathy model rats took signifi cantly longer time to fi nd the hidden platform than control rats. Lactulose treatment improved this defi cit in cognitive function, consistent with results obtained from clinical trials of lactulose in patients with early hepatic encephalopathy (Prasad et al., 2007; Luo et al., 2011).
Neuroplasticity plays a signifi cant role in cognitive function.
Exploring changes in neuroplasticity can enhance our understanding of disease pathogenesis and improve treatment strategies. One component of neuroplasticity is neurogenesis. Adult neurogenesis predominantly occurs in two restricted regions: the subgranular zone of the dentate gyrus, and the subventricular zone (Cheung et al., 2007; Molnar, 2011). Newborn cells in the subgranular zone diff erentiate into neuronal cells and establish synaptic connections with neighboring cells (Zhao et al., 2008). Adult hippocampal neurogenesis plays a critical role in synaptic plasticity processes such as long-term potentiation and learning and memory (Massa et al., 2011). To date, there have been few studies investigating the eff ect of hepatic encephalopathy on neurogenesis. In the present study, we used BrdU staining to assess the eff ect of lactulose on hippocampal neurogenesis. BrdU is a thymidine analog that becomes incorporated into the DNA of dividing cells during the S-phase of the cell cycle, and as such is widely used to measure cell proliferation and neurogenesis in the adult mammalian brain, including in humans (Taupin, 2007). Our results suggest that lactulose increases the number of new neurons in the hippocampal dentate gyrus. It should be noted that BrdU was injected 24 hours before animals being sacrifi ced in present study, because we intended to assess the proliferation of neural stem cells. In future study, the diff erentiation of neural stem cells should be taken into consideration by using double-label technique for BrdU and GFAP/NeuN.
Chastre et al. (2010) found that ammonia and pro-infl ammatory mediators reduced GFAP expression. Previous studies revealed that GFAP mRNA and protein expression were signifi cantly reduced in animal models of acute liver failure (Belanger et al., 2002) and in cultured astrocytes exposed to ammonia (Norenberg et al., 1990), confi rming the important role of astrocytes in the pathogenesis of hepatic encephalopathy and consequences for neuronal function. Astrocytes eliminate ammonia by converting glutamate to glutamine by amidation, catalyzed by glutamine synthetase (Lockwood et al., 1991; Haussinger et al., 2000). In the present study, lactulose elevated the number of GFAP-immunoreactive cells, which may mediate its neuroprotective eff ect.
Mounting evidence suggests that lactulose is neuroprotective. Chen et al. (2012) proposed that bacterial fermentation in the gastrointestinal tract can produce considerable amounts of hydrogen from lactulose, which was used to explain the protective eff ect of lactulose for ischemic stroke. Our data supports this hypothesis, strongly suggesting that lactulose treatment improves neuroplasticity by enhancing neurogenesis and increasing the number of astrocytes and neurons, eff ectively restoring learning ability in rat models of hepatic encephalopathy.
Author contributions: NY, YYL and PPZ designed the study. HL, NY, YJ, DML and JZ performed the experiment. CJ and NY collected and analyzed the data. NY and YYL wrote the manuscript. PPZ reviewed the paper. All authors approved the fi nal version of the paper.
Confl icts of interest: None declared.
Belanger M, Desjardins P, Chatauret N, Butterworth RF (2002) Loss of expression of glial fibrillary acidic protein in acute hyperammonemia. Neurochem Int 41:155-160.
Blei AT, Cordoba J (2001) Hepatic encephalopathy. Am J Gastroenterol 96:1968-1976.
Chastre A, Jiang W, Desjardins P, Butterworth RF (2010) Ammonia and proinfl ammatory cytokines modify expression of genes coding for astrocytic proteins implicated in brain edema in acute liver failure. Metab Brain Dis 25:17-21.
Chen X, Zhai X, Kang ZM, Sun XJ (2012) Lactulose: an eff ective preventive and therapeutic option for ischemic stroke by production of hydrogen. Med Gas Res 2:3.
Cheung AF, Pollen AA, Tavare A, DeProto J, Molnar Z (2007) Comparative aspects of cortical neurogenesis in vertebrates. J Anat 211:164-176.
Felipo V (2013) Hepatic encephalopathy: eff ects of liver failure on brain function. Nat Neurosci Rev 14:851-858.
Felipo V, Butterworth RF (2002) Neurobiology of ammonia. Prog Neurobiol 67:259-279.
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT (2002) Hepatic encephalopathy-defi nition, nomenclature, diagnosis, and quantifi cation: fi nal report of the working party at the 11thWorld Congresses of Gastroenterology, Vienna, 1998. Hepatology 35:716-721.
Gorg B, Qvartskhava N, Bidmon HJ, Palomero-Gallagher N, Kircheis G, Zilles K, Haussinger D (2010) Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology 52:256-265.
Haüssinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S (2000) Hepatic encephalopathy in chronic liver desease: a clinical manifestation of astrocyte swelling and low grade cerebral edema. J Hepatol 32:1035-1038.
Ji C, Li Q, Aisa H, Yang N, Dong YL, Liu YY, Wang T, Hao Q, Zhu HB, Zuo PP (2009) Gossypium herbaceam extracts attenuate ibotenic acid-induced excitotoxicity in rat hippocampus. J Alzheimers Dis 16:331-339.
Liu Y, Yang N, Hao W, Zhao Q, Ying T, Liu S, Li Q, Liang Y, Wang T, Dong Y, Ji C, Zuo P (2011) Dynamic proteomic analysis of protein expression profi les in whole brain of Balb/C mice subjected to unpredictable chronic mild stress: implications for depressive disorders and future therapies. Neurochem Int 58:904-913.
Liu Y, Aisa HA, Ji C, Yang N, Zhu H, Zuo P (2012) Eff ects of Gossypium herbaceam extract administration on the learning and memory function in the naturally aged rats: neuronal niche improvement. J Alzheimers Dis 31:101-111.
Lockwood A, Yap E, Wong W (1991) Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab 11:337-341.
Lockwood AH, Ginsberg MD, Rhoades HM, Gutierrez MT (1986) Cerebral glucose metabolism after portacaval shunting in the rat. Patterns of metabolism and implications for the pathogenesis of hepatic encephalopathy. J Clin Invest 78:86-95.
Luo M, Li L, Lu CZ, Cao WK (2011) Clinical effi cacy and safety of lactulose for minimal hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol 23:1250-1257.
Massa F, Koehl M, Wiesner T, Grosjean N, Revest JM, Piazza PV, Abrous DN, Oliet SH (2011) Conditional reduction of adult neurogenesis impairs bidirectional hippocampal synaptic plasticity. Proc Natl Acad Sci U S A 108:6644-6649.
McEwen BS (2006) Protective and damaging eff ects of stress mediators: central role of the brain. Dialog Clin Neurosci 8:367-381.
Mendez M, Mendez-Lopez M, Lopez L, Aller MA, Arias J, Cimadevilla JM, Arias JL (2008) Spatial memory alterations in three models of hepatic encephalopathy. Behav Brain Res 188:32-40.
Molnar Z (2011) Evolution of cerebral cortical development. Brain Behav Evol 78:94-107.
Neary JT, Whittemore SR, Zhu Q, Norenberg MD (1994) Destabilization of glial fi brillary acidic protein mRNA in astrocytes by ammonia and protection by extracellular ATP. J Neurochem 63:2021-2027.
Ngwenya LB, Peters A, Rosene DL (2005) Light and electron microscopic immunohistochemical detection of bromodeoxyuridine-labeled cells in the brain: diff erent fi xation and processing protocols. J Histochem Cytochem 53:821-832.
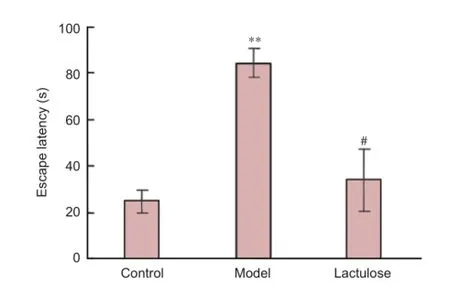
Figure 3 Eff ects of lactulose treatment on escape latency of rats with hepatic encephalopathy in the Morris water maze 12 weeks after receiving carbon tetrachloride.
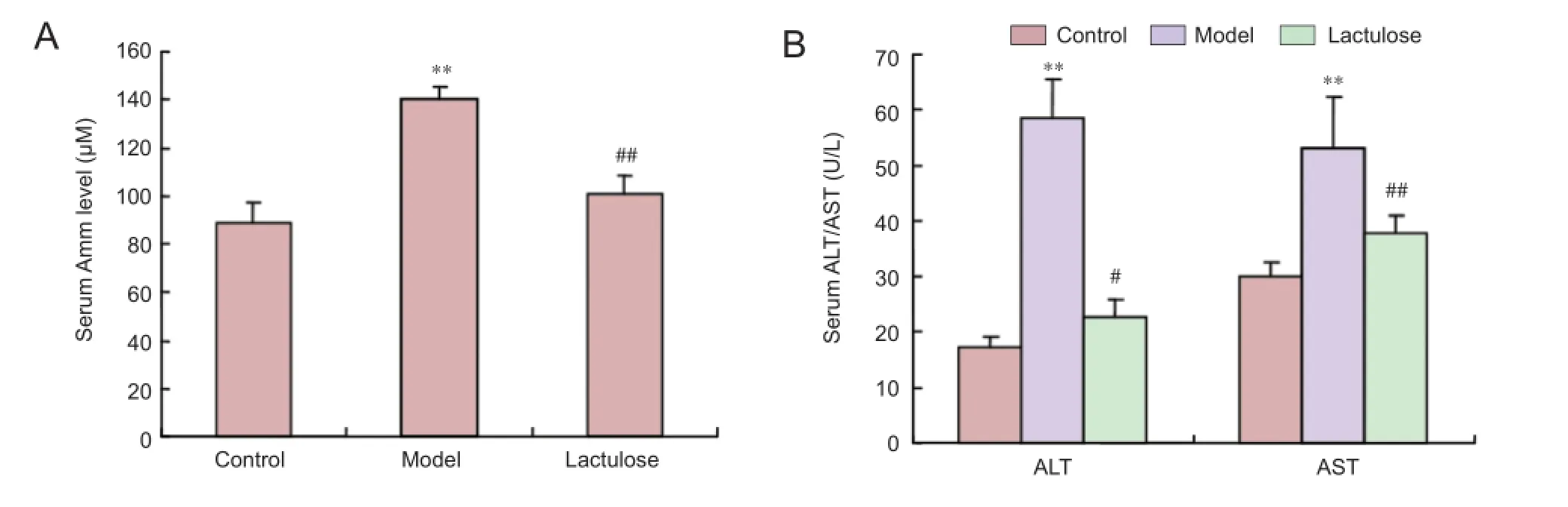
Figure 5 Eff ects of lactulose treatment on serological parameters in rats with carbon tetrachloride-induced hepatic encephalopathy.
Norenberg MD, Neary JT, Norenberg LO, McCarthy M (1990) Ammonia induced decrease in glial fi brillary acidic protein in cultured astrocytes. J Neuropathol Exp Neurol 49:399-405.
Ortiz M, Cordoba J, Jacas C, Flavia M, Esteban R, Guardia J (2006) Neuropsychological abnormalities in cirrhosis include learning impairment. J Hepatol 44:104-110.
Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R (2007) Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 45:549-559.
Riordan SM, Williams R (1997) Treatment of hepatic encephalopathy. N Engl J Med 337:473-479.
Scheuer PJ (1991) Classifi cation of chronic viral hepatitis: a need for reassessment. J Hepatol 13:372-374.
Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK (2013) A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol 108:1458-1463.
Sharma P, Sharma BC (2013) Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis 28:313-320.
Stefovska VG, Uckermann O Czuczwar M Smitka M, Czuczwar P Kis J Kaindl AM Turski L, Turski WA, Ikonomidou C (2008) Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol 64:434-345.
Taupin P (2007) BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev 53:198-214.
Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, Hung SC, Fu YS (2009) The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fi brosis. Liver Transpl 15:484-495.
Yang FR, Fang BW, Lou JS (2010) Eff ects of Haobie Yangyin Ruanjian decoction on hepatic fi brosis induced by carbon tetrachloride in rats. World J Gastroenterol 16:1458-1464.
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645-660.
Ziada DH, Soliman HH, El YSA, Hamisa MF, Hasan AM (2013) Can Lactobacillus acidophilus improve minimal hepatic encephalopathy? A neurometabolite study using magnetic resonance spectroscopy. Arab J Gastroenterol 14:116-122.
Copyedited by Murphy JS, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
*Correspondence to:
Yan-yong Liu, Ph.D. or Ping-ping Zuo,
Ph.D., liuyanyong@126.com or
Pingping_zuo@126.com.
orcid:
0000-0003-2979-6017 (Yan-yong Liu)
0000-0002-2874-8032 (Ping-ping Zuo)
10.4103/1673-5374.165516
http://www.nrronline.org/
Accepted: 2015-07-02
- 中国神经再生研究(英文版)的其它文章
- Elastic modulus aff ects the growth and diff erentiation of neural stem cells
- Optimal concentration and time window for proliferation and diff erentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours
- Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy
- Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells
- Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury
- Transplantation of erythropoietin gene-modifi ed neural stem cells improves the repair of injured spinal cord

