Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy
Jeff rey N. Weiss, Steven Levy, Susan C. Benes
1 Retina Associates of South Florida, Margate, FL, USA
2 MD Stem Cells, Ridgefi eld, CT, USA
3 Wilmer Eye Institute, The Johns Hopkins Hospital, Baltimore, MD, USA
Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy
Jeff rey N. Weiss1, Steven Levy2,*, Susan C. Benes3
1 Retina Associates of South Florida, Margate, FL, USA
2 MD Stem Cells, Ridgefi eld, CT, USA
3 Wilmer Eye Institute, The Johns Hopkins Hospital, Baltimore, MD, USA
We present the results from a patient with relapsing optic neuropathy treated within the Stem Cell Ophthalmology Treatment Study (SCOTS). SCOTS is an Institutional Review Board approved clinical trial and has become the largest ophthalmology stem cell study registered at the National Institutes of Health to date (www.clinicaltrials.gov Identifi er NCT 01920867). SCOTS utilizes autologous bone marrow-derived stem cells (BMSCs) for treatment of retinal and optic nerve diseases. Pre-treatment and post-treatment comprehensive eye exams of a 54 year old female patient were performed both at the Florida Study Center, USA and at The Eye Center of Columbus, USA. As a consequence of a relapsing optic neuritis, the patient’s previously normal visual acuity decreased to between 20/350 and 20/400 in the right eye and to 20/70 in the left eye. Signifi cant visual fi eld loss developed bilaterally. The patient underwent a right eye vitrectomy with injection of BMSCs into the optic nerve of the right eyeand retrobulbar, subtenon and intravitreal injection of BMSCs in the left eye. At 15 months after SCOTS treatment, the patient’s visual acuity had improved to 20/150 in the right eye and 20/20 in the left eye. Bilateral visual fi elds improved markedly. Both macular thickness and fast retinal nerve fi ber layer thickness were maximally improved at 3 and 6 months after SCOTS treatment. The patient also reduced her mycophenylate dose from 1,500 mg per day to 500 mg per day and required no steroid pulse therapy during the 15-month follow up.
nerve regeneration; stem cells; optic nerve; autoimmune; optic neuropathy; ophthalmology; bone marrow-derived stem cells; blindness; visual loss; Stem Cell Ophthalmology Treatment Study; neural regeneration
Weiss JN, Levy S, Benes SC (2015) Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen Res 10(9):1507-1515.
Introduction
SCOTS, the Stem Cell Ophthalmology Treatment Study, is the largest ophthalmology stem cell study registered at the National Institutes of Health (www.clinicaltrials.gov Identifier NCT Number 01920867). SCOTS is an open label, non-randomized, effi cacy study. There is no placebo or sham arm. All patients that meet eligibility criteria and were enrolled in the study received active treatment. Bone marrow aspirated from the posterior iliac crest was separated to provide bone marrow-derived stem cells (BMSCs) within a stem cell concentrate.
Inclusion criteria for SCOTS include:
-Have objective, documented damage to the retina or optic nerve unlikely to improve OR have objective, documented damage to the retina or optic nerve that is progressive.
-Have less than or equal to 20/40 best corrected central visual acuity in one or both eyes AND/OR an abnormal visual fi eld in one or both eyes.
-Be at least 3 months post-surgical treatment intended to treat any ophthalmologic disease and be stable.
-If under current medical therapy (pharmacologic treatment) for a retinal or optic nerve disease be considered stable on that treatment and unlikely to have visual function improvement (for example, glaucoma with intraocular pressure stable on topical medications but visual fi eld damage).
-Have the potential for improvement after BMSCs treatment and be at minimal risk of any potential harm from the procedure.
-Be 18 years of age or older.
-Be medically stable and able to be medically cleared by their primary care physician or a licensed primary care practitioner for the procedure. Medical clearance means that in the estimation of the primary care practitioner, the patient
can reasonably be expected to undergo the procedure without signifi cant medical risk to health.
Exclusion criteria include:
-Patients who are not capable of an adequate ophthalmologic examination or evaluation to document the pathology.
-Patients who are not capable or not willing to undergo follow up eye exams with the principle investigator or their ophthalmologist or optometrist as outlined in the protocol.
-Patients who are not capable of providing informed consent.
-Patients who may be at signifi cant risk to general health or to the eyes and visual function should they undergo the procedure.
There were three arms of SCOTS with the type of treatment chosen based on the degree of visual loss, etiology of visual loss, associated risk factors for the treatment arms and the patient’s medical risk status. Bilateral treatment was provided assuming both eyes meet eligibility requirements. As these were autologous stem cells, no immunosuppression was required. An FDA cleared class 2 medical device was used to separate the bone marrow aspirate into a stem cell concentrate. This concentrate (approximately 14–15 cm3) contained 1.2 billion total nucleated cells including mesenchymal stem cells. 3 cm3of concentrate was used for retrobulbar injection, 0.05 cm3for intravitreal injection, 0.1 cm3for subretinal injection, 0.2 cm3for intraoptic nerve injection, and the remainder of the concentrate for intravenous injection.
Arm 1 consists of retrobulbar and subtenon injection, followed by intravenous injection, of stem cell concentrate. Patients with ophthalmic conditions which preclude safe or eff ective utilization of intravitreal injection of concentrate, such as the presence of silicon oil, may be off ered Arm 1 if meeting inclusion criteria. Arm 2 consists of retrobulbar, subtenon and intravitreal administration, followed by intravenous infusion, of concentrate. Patients meeting inclusion criteria with visual acuity between 20/40 and 20/200 in one or both eyes and/or visual loss may be off ered Arm 2. Arm 3 is reserved for retinal and optic nerve patients with severe visual loss meaning visual acuity of 20/200 or worse in at least one eye. Typically patients admitted to Arm 3 have much poorer vision. Arm 3 consists of the better-seeing eye receiving the same treatment as Arm 1 or more typically, Arm 2, and the eye with more extensive visual acuity loss receiving a core pars plana vitrectomy with subretinal or intra-optic nerve injection of concentrate followed by intravenous infusion of stem cells. Monocular patients are not eligible for Arm 3. Follow up was required at 1, 3, 6 and 12 months after treatment with reporting of the eye exam results to the principal investigator and study director.
The importance of SCOTS rests with its openness to various retinal and optic nerve diseases. SCOTS is focused on the cellular damage to tissue which is causing the visual loss. This allows the study to enroll and assess improvements in a number of diseases including visual loss that may be a result of more than one disease. SCOTS also provides BMSCs in close proximity to the damaged tissue which may maximize paracrine eff ects.
The SCOTS procedure is patient funded and performed under general anesthesia. Treatment is provided in a fully licensed ambulatory surgical center in Coconut Creek, Florida, USA. All human investigations were performed with informed consent. The study was reviewed and approved by our Institutional Review Board. The study was registered at the National Institutes of Health.
Clinical History and Course
The patient is a 54 year old female who developed sudden vision loss in the right eye to 20/400 on June 6, 2010. She was diagnosed with non-arteritic anterior ischemic optic neuropathy (NAION) by her optometrist and ophthalmologist as her attack was sudden, painless, and she had a swollen optic nerve in the right eye. She felt she had lost the whole superior visual fi eld in that attack as well as the central vision in the right eye. At that time, visual acuity in the left eye was 20/30 and the left eye had small inferior and superior arcuate visual fi eld defects. Four months later, she experienced a second episode of vision loss in the right eye and her inferior nasal visual fi eld was obviously lost. A third episode of vision loss in the right eye occurred in January 2011, 7 months after the fi rst attack. The remaining right infero-temporal visual fi eld was then lost. A second opinion was sought and concurred that it was probably NAION, although a rare variant with recurrences. No treatment was recommended. Her fourth visual attack was on her “good” left eye in June 2011, a year after the fi rst attack on her right eye. Her visual acuity of the left eye fell to 20/70 with superior and inferior arcuate defects. Her fi rst exam by a neuro-ophthalmology unit on June 7, 2011 showed visual acuities of 20/400 eccentrically in the right eye and 20/70 in the left eye. There was a defi nite right aff erent pupil defect. She had no proptosis, motility abnormality, severe biomicroscopic abnormality or retinopathy. There were small, visually insignifi cant lenticular changes appropriate for age.
Visual fi eld analysis of the right and left eyes performed using a Humphrey visual fi eld analyzer with program 30-2 revealed both superior temporal and inferonasal losses. Ocular coherence tomography of macular thickness showed thinning of the retinal nerve fi ber layers in the lowest percentile compared to her age-matched controls. Neurologic exam showed no defi cits except for the vision loss. She had no sign of a myelopathy. MRI of the brain did not demonstrate white matter lesions suggesting demyelinating disease and there was no orbital abnormality. Hematologic evaluation showed a low thyroid stimulating hormone, slightly elevated T3 and T4, normal complete blood count, normal chemistries, and a normal erythrocyte sedimentation rate.
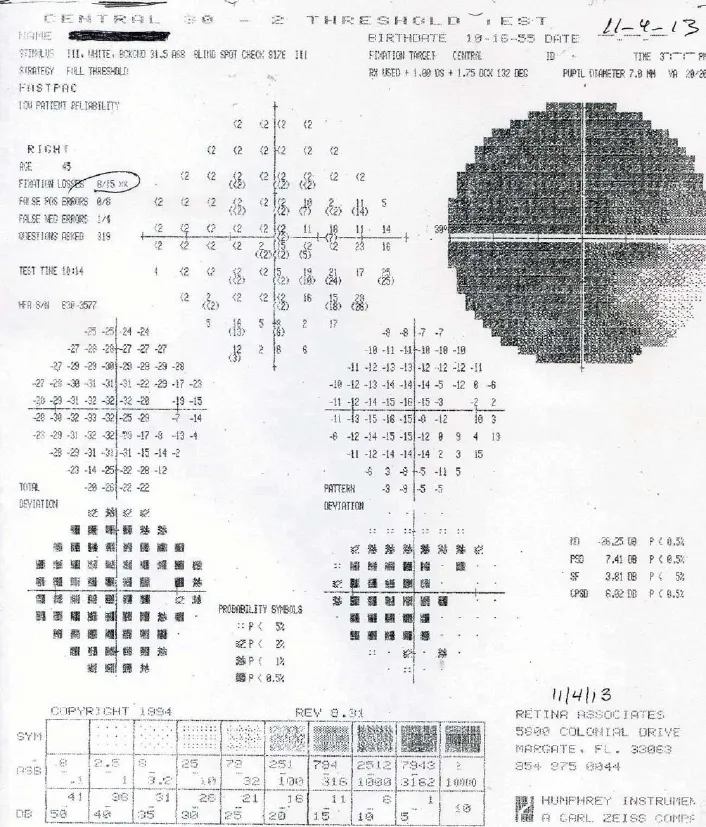
Figure 1 Preoperative Humphrey 30–2visual fi eld of the right eye on November 4, 2013.
Further testing for autoimmune disorders included a neuromyelitis optica antibody measuring 1.398 (below the 3.0 cut-off for “positive”), a positive thyroid stimulating immunoglobulin (313, n < 130) for Graves’ disease, a positive microsomal antibody at 380 (n < 35) for auto-immune thyroid disorders, a slightly elevated thyroglobulin antibody at 50 (n< 29), a positive anti-Saccharomyces Cerevisiae Ab 32 (ASCA IgA, n < 20) for gluten-sensitive enteropathy. Important negatives were antinuclear antibodies, rapid plasma reagin, angiotensin converting enzyme, lysozyme, toxoplasmosis, Lyme’s Ab, herpes simplex virus 1 and 2 Ab, B12 and anti-phospholipid antibodies. The patient deleted gluten from her diet.
Blood sent to an Oregon laboratory revealed anti-optic nerve antibodies to both 35 and 70 kDa proteins. Multiple bands are common in auto-immune disorders, but are non-specifi c.
An endocrinologist treated her with methimazole and synthroid and she became euthyroid.
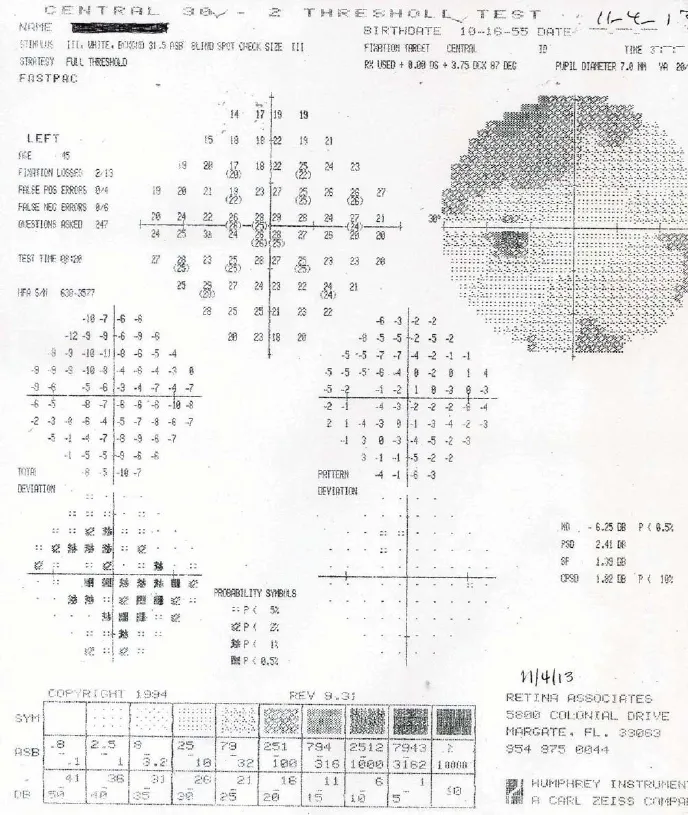
Figure 2 Preoperative Humphrey 30–2visual fi eld of the left eye on November 4, 2013.
The patient received a steroid pulse therapy after the second episode of vision loss in the right eye and the visual acuity in the left eye improved from 20/70 to 20/20. There was no change in the visual acuity in the right eye. Daily mycophenylate (1,500 mg) began but did not prevent two more attacks of vision loss in the left eye to 20/70 (NMO Ab 1.496 on August 8, 2011) and 20/80 (NMO Ab 1.9 on 3/12). Her neuromyelitis optica antibody titers never exceeded 3.0 and were not rechecked until August 14, 2011. After the third steroid pulse therapy, her visual acuities were between 20/350–20/400 in the right eye and 20/70 in the left eye, and her general health remained stable from March, 2012, until her SCOTS treatment in November, 2013. Postoperative visual fi eld analysis was performed using the Humphrey Field Analyzer 30–2program (Carl Zeiss Meditech Inc., Oberkochen, Germany) at the SCOTS center in Florida, USA on November 4, 2013. Results showed a mean deviation (MD) of –26.25 in the right eye and –6.25 in the left eye. Humphrey 30–2visual fi eld data of the right (Figure 1) and left eyes (Figure 2).
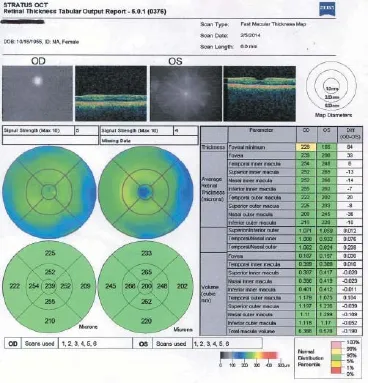
Figure 3 Fast macular thickness maps taken on February 5, 2014. OD: Right eye; OS: left eye.
The patient was assigned to SCOTS Arm 3 with a vitrectomy and intra-optic nerve injection of BMSCs in the right eye. Her left eye received Arm 2 with retrobulbar, subtenon and intravitreal injection of BMSCs. She also received an intravenous stem cell infusion. The surgery was performed under general anesthesia without complications and with no cessation of her mycophenylate on November 5, 2013. On December 9, 2013 (1 month after SCOTS treatment), she reported “brighter and clearer vision” and was able to safely drive the hour and a half to her check-up. Her visual acuities on that day had improved to 20/300 in the right eye and 20/15 in the left eye.
At 3 months after SCOTS treatment, the macular thickness had improved to 6.388 in the right eye and 6.578 in the left eye.
On February 5, 2014, 3 months after SCOTS treatment, her fast retinal nerve fi ber layer thickness had improved to 39.02 on average in the right eye and 83.02 in the left eye (Figure 3). Optic nerve thickness measured at the same date is shown in Figure 4.
On May, 2014, 6 months after SCOTS treatment, her best corrected visual acuity remained 20/150 in the right eye and 20/15 in the left eye. In August, 2014, 9 months after SCOTS treatment, the patient reported that she subjectively felt healthier than she had felt since 2010. She had tapered her mycophenylate to 500 mg per day, required no steroids since surgery, and was on stable amounts of methimazole (5 mg), evista, dehydroepiandrosterone (DHEA), and vitamin E. Her blood tests demonstrated a thyroid stimulating immunoglobulin down to 88 from the 313 in November 6, 2013, microsomal Ab down to 149 from 380, and ASCA IgA 0 and NMO antibody 0.0. She still had a measurable anti-optic nerve antibody to the 35 kDa protein, and no Ab to the 70 kDa protein.
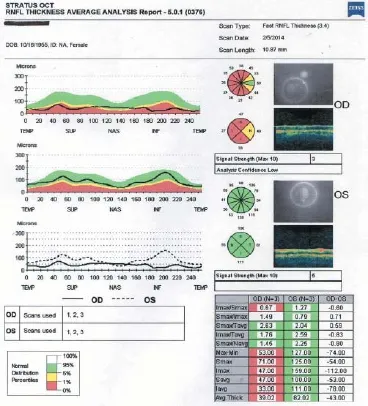
Figure 4 Fast retinal nerve fi ber layer optic nerve thickness maps taken on February 5, 2014.
On November 12, 2014, i.e., 12 months after SCOTS treatment, the patient had a Snellen acuity of 20/300 in the right eye through a now-denser posterior subcapsular cataract and 20/15 in the left eye through a trivial cataract. Her Early Treatment of Diabetic Retinopathy Study (ETDRS) distance score was 15 in the right eye and 50 in the left eye. There was still an aff erent pupillary defect in the right eye. Biomicroscopy showed a posterior subcapsular cataract in the right eye and a lesser one in the left eye with an intraocular pressure of 9 in the right eye and 11 in the left eye.
At 12 months after SCOTS treatment, the visual fields for the right eye averaged –20.52 dB with a temporal island of intact vision sliding toward fi xation (Figure 5) and that for the left eye averaged –0.56 dB with a residual inferonasal spotty defect (Figure 6). The visual field improved at 12 months in both eyes despite the right macula measured thinner than it had at the 3 and 6 month visits (Figure 7).
At 1 year after SCOTS treatment, her optical coherence tomography macular retinal thickness data showed that manular thickness was 5.973 in the right eye (in the lowest 5thpercentile compared to her age-matched controls) (Figure 7) and 6.905 in the left eye (in the normal 5–95thpercentile for her age).
At 1 year after SCOTS treatment, her circular peripapillary fast retinal nerve fi ber layer thickness was 40.56 in the right eye (1stpercentile), with the nasal quarter of the nerve now averaging in the normal thickness for her age, and 67.64 in the left eye (still the lowest fi rst percentile), with the temporal half of the nerve back in the normal retinal nerve fi ber layer thickness (Figure 8).

Figure 5 Humphrey visual fi eld of the right eye on November 12, 2014 (1 year after the Stem Cell Ophthalmology Treatment Study treatment).
Discussion
In this case report, a relapsing auto-immune optic neuropathy caused progressive bilateral visual loss, which started in June 2010 and lasted till fall 2013 with visual acuities of 20/350–20/400 in the right eye and 20/70 in the left eye.
The patient chose to participate in the SCOTS study and underwent a right eye vitrectomy with intra-optic nerve injection of BMSCs in the right eye and retrobulbar, subtenon and intravitreal injection of BMSCs in the left eye.
After SCOTS treatment, her central acuity was 20/150 for the right eye at 3 months after SCOTS treatment, remained this level for a 6-month period, and then declined to 20/300 at 12 months after SCOTS treatment at which time a PSC cataract had formed in the vitrectomized eye.
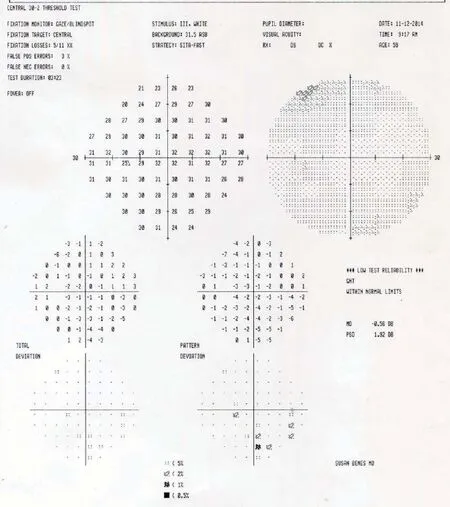
Figure 6 Humphrey Visual Field of the left eye on November 12, 2014 (1 year after the Stem Cell Ophthalmology Treatment Study treatment).
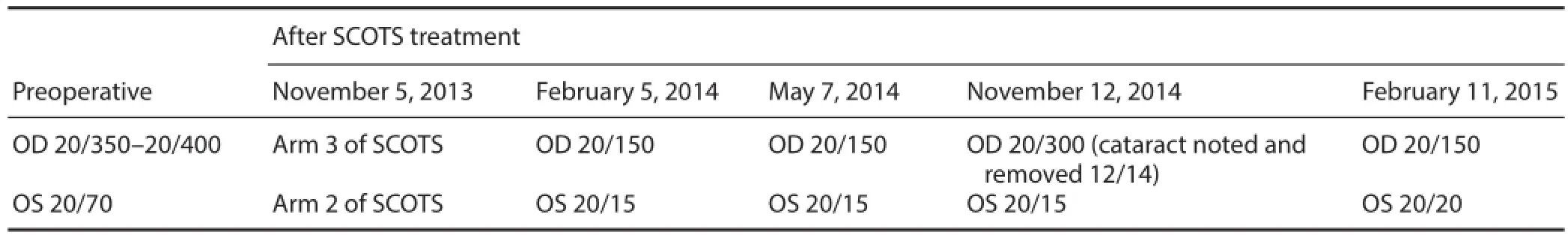
Table 1 Summary of best corrected visual acuities
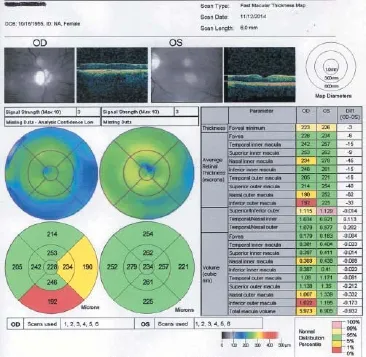
Figure 7 Macular thickness maps taken on November 12, 2014 (1 year after the Stem Cell Ophthalmology Treatment Study treatment).

Figure 8 Fast retinal nerve fi ber layer thickness analysis report taken on November 12, 2014 (1 year after the Stem Cell Ophthalmology Treatment Study treatment).
This posterior subcapsular cataract was removed. An intraocular lens was inserted at 13 months after SCOTS treatment and vision returned to 20/150 at 15 months after SCOTS treatment. The visual acuity of the left eye was 20/15 within the fi rst 3 weeks after SCOTS treatment and remained stable throughout the 1st year. At 15 months after SCOTS treatment, her visual acuity was 20/20 in the left eye. Both eyes continued to have visual fi eld improvements at 1 year after SCOTS treatment. At 3 and 6 months after SCOTS treatment, both macular thickness maps and fast retinal nerve fiber layer thickness improved. Despite the thinner measurements of the macular thickness and fast retinal nerve fi ber layer thickness in the right eye at 9 and 12 months after SCOTS treatment, the visual fi eld function continued to improve in both eyes. Based on the scans, the decreases in macular thickness and fast retinal nerve fi ber layer thickness in the right eye mainly occurred in the nasal and inferior regions, corresponding to the superior temporal fi eld. A summary of acuities is shown in Table 1.
At 1 year after SCOTS treatment, the impaired visual fi eld of the right eye remained primarily in the temporal part. Kok et al. (2013) reported a linear relationship between the optical coherence tomography-eff ective optical density of cataracts and an underestimation of retinal nerve fi ber layer thickness. Given the development of a moderately dense cataract in the right eye in conjunction with an improved visual field, it is plausible that a degree of the measured decrease in retinal nerve fi ber layer thickness is an artifact, according to Mwanza et al. (2011). The patient also reduced her mycophenylate dose and required no additional steroid pulses. Immune modulation as described by Karantalis and Hare (2015) may play a role in this later response. In a review article by Pula and MacDonald (2012), optic neuritis can be classifi ed as typical (as in multiple sclerosis) or atypical (not associated with multiple sclerosis and which may improve after steroid treatment). In atypical optic neuritis, autoimmune optic neuritis is associated with connective tissue diseases such as systemic lupus erythematosus, vasculitis, sarcoidosis, and neuromyelitis optica. Treatment for typical or demyelinating optic neuritis may include intravenous steroids. As shown in the Optic Nerve Treatment Trial, steroids may hasten the visual recovery but do not change the overall outcomes of visual acuity after 6 months of treatment.
The benefi ts of steroids for the overall demyelinating process remain unclear. Therapeutic plasma exchange may be used to potentially help remove pathogenic immunoglobulins or complement from plasma; immunoadsorption is sometimes used similar to therapeutic plasma exchange but with absorption of immunoglobulins. Other interventions explored have included the use of statins, heat killed Mycobacterium w, and Fingolimod (used for the prevention of multiple sclerosis relapses).
There are few studies regarding recurrent autoimmune optic neuropathy to which we might compare SCOTS. In a study by Stiebel-Kalish et al. (2010), patients with recurrent infl ammatory optic neuropathy treated with intravenous immunoglobulins acquired stable vision and in most cases, steroid avoidance was achieved but there was no visual improvement. Treatments for atypical optic neuritis also primarily involve steroids and mainly focus on associated systemic diseases, if identifi ed. Systemic causes can include vasculitis such as microscopic polyarteritis, Wegener’s granulomatosis, Henoch-Schönlein purpura; collagen vascular diseases include systemic lupus erythematosus, neurosarcoidosis, and Sjögren’s syndrome.
Neuromyelitis optica or Devic’s disease is an infl ammatory disease of the central nervous system producing optic neuritis
and myelitis. According to Collongues et al. (2010), there is a female predominance; the median age of onset is the mid to late 30s with more than 80% of patients having a relapsing course with progressive disability (Collongues et al., 2010). Neuromyelitis optica tends to be worse than multiple sclerosis for both visual loss and physical disability. Testing for neuromyelitis optica-IgG is now routine. This identifi es an antibody to the plasma membrane channel aquaporin-4 which is responsible for water movement. Aquaporin-4 is present in the astrocytes which form the blood-brain barrier and neuromyelitis optica-IgG is associated with 77–91% of sensitivity and 94–100% of specifi city for neuromyelitis optica, as concluded by Wingerchuk et al. (2006) and Takahashi et al. (2007). As noted by Lennon et al. (2004), neuromyelitis optica may be present without transverse myelitis. In this patient, neuromyelitis optica antibodies were only weakly present, and given the other antibodies identifi ed, a diagnosis of relapsing autoimmune optic neuritis was made.
This 54 year old female patient had signifi cant improvements in visual acuity, visual field and ocular coherence tomography following treatment with autologous BMSCs as given in the protocols developed for the SCOTS. While this is a single patient and results may not be reproducible, autologous BMSCs may prove a valuable addition to treatment of relapsing autoimmune optic neuritis as well as other optic neuropathies, demonstrating an immunologic component.
Author contributions: JNW and SL designed the study. JNW performed the research. All authors collected and interpreted the data, wrote the paper, and approved the final version of the paper.
Confl icts of interest: None declared.
Collongues N, Marignier R, Zéphir H, Papeix C, Blanc F, Ritleng C, Tchikviladzé M, Outteryck O, Vukusic S, Fleury M, Fontaine B, Brassat D, Clanet M, Milh M, Pelletier J, Audoin B, Ruet A, Lebrun-Frenay C, Thouvenot E, Camu W, et al. (2010) Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology 74:736-742.
Karantalis V, Hare JM (2015) Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 116:1413-1430.
Kok PH, van den Berg TJ, van Dijk HW, Stehouwer M, van der Meulen IJ, Mourits MP, Verbraak FD (2013) The relationship between the optical density of cataract and its infl uence on retinal nerve fi bre layer thickness measured with spectral domain optical coherence tomography. Acta Ophthalmol 91:418-424.
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106-2112.
Mwanza JC, Bhorade AM, Sekhon N, McSoley JJ, Yoo SH, Feuer WJ, Budenz DL (2011) Effect of cataract and its removal on signal strength and peripapillary retinal nerve fi ber layer optical coherence tomography measurements. J Glaucoma 20:37-43.
Pula JH, MacDonald CJ (2012) Current options for the treatment of optic neuritis. Clin Ophthalmol 6:1211-1223.
Stiebel-Kalish H, Hammel N, van Everdingen J, Huna-Baron R, Lee AG (2010) Intravenous immunoglobulin in recurrent-relapsing infl ammatory optic neuropathy. Can J Ophthalmol 45:71-75.
Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, Watanabe S, Shiga Y, Kanaoka C, Fujimori J, Sato S, Itoyama Y (2007) Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 130:1235-1243.
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66:1485-1489.
Copyedited by Lin W, Xie Z, Yue JP, Li CH, Song LP, Zhao M
*Correspondence to:
Steven Levy, M.D.,
stevenlevy@mdstemcells.com.
orcid:
0000-0002-9313-3448 (Steven Levy)
10.4103/1673-5374.165525
http://www.nrronline.org/
Accepted: 2015-08-02
- 中国神经再生研究(英文版)的其它文章
- Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy
- Elastic modulus aff ects the growth and diff erentiation of neural stem cells
- Optimal concentration and time window for proliferation and diff erentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours
- Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells
- Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury
- Transplantation of erythropoietin gene-modifi ed neural stem cells improves the repair of injured spinal cord

