Monascus pilosus-fermented black soybean inhibits lipid accumulation in adipocytes and in high-fat diet-induced obese mice
Young-Sil Lee, Bong-Keun Choi, Hae Jin Lee, Dong-Ryung Lee, Jinhua Cheng, Won-Keun Lee, Seung Hwan Yang,*, Joo-Won Suh,,*
1Center for Nutraceutical and Pharmaceutical Materials, Myongji University, 116 Myongji-Ro, Cheoin-Gu, Yongin-Si, Gyeonggi-Do 449-728, Korea
2Interdisciplinary Program of Biomodulation, Myongji University, 116 Myongji-Ro, Cheoin-Gu, Yongin-Si, Gyeonggi-Do 449-728, Korea
3Division of Bioscience and Bioinformatics, College of Natural Science, Myongji University, 116 Myongji-Ro, Cheoin-Gu, Yongin-Si, Gyeonggi-Do 449-728, Korea
Monascus pilosus-fermented black soybean inhibits lipid accumulation in adipocytes and in high-fat diet-induced obese mice
Young-Sil Lee1#, Bong-Keun Choi1#, Hae Jin Lee2, Dong-Ryung Lee3, Jinhua Cheng1, Won-Keun Lee3, Seung Hwan Yang1,2*, Joo-Won Suh1,2,3*
1Center for Nutraceutical and Pharmaceutical Materials, Myongji University, 116 Myongji-Ro, Cheoin-Gu, Yongin-Si, Gyeonggi-Do 449-728, Korea
2Interdisciplinary Program of Biomodulation, Myongji University, 116 Myongji-Ro, Cheoin-Gu, Yongin-Si, Gyeonggi-Do 449-728, Korea
3Division of Bioscience and Bioinformatics, College of Natural Science, Myongji University, 116 Myongji-Ro, Cheoin-Gu, Yongin-Si, Gyeonggi-Do 449-728, Korea
ARTICLE INFO
Article history:
Received 15 January 2015
Received in revised form 20 February 2015
Accepted 15 March 2015
Available online 20 April 2015
Monascus pilosus
Black soybean
Adipocytes
High-fat diet-induced obese mice
Anti-obesity
Adipogenesis-related genes
Objective: To explore the anti-obesity effects and the mechanism of action of Monascus pilosus (M. pilosus)-fermented black soybean (MFBS) extracts (MFBSE) and MFBS powders (MFBSP) in adipocytes and high-fat diet (HFD)-induced obese mice, respectively. Methods: Black soybean was fermented with M. pilosus, and the main constituents in MFBS were analyzed by HPLC analysis. In vitro, MFBSE were examined for anti-adipogenic effects using Oil-Red O staining. In vivo, mice were fed a normal-fat diet (NFD) control, HFD control or HFD containing 1 g/kg MFBSP for 12 weeks, and then body weight gain and tissues weight measured. Real-time PCR and western blot assay were used to determine the mechanism of anti-adipogenic effects. Results: MFBSE inhibited lipid accumulation in 3T3-L1 adipocytes without exerting cell cytotoxicity. MFBSP treatment in HFD-fed mice significantly decreased the body weight gain compared with the HFD control mice. MFBSE and MFBSP treatment resulted in significantly lower mRNA levels of adipogenesis-related genes, such as peroxisome proliferator-activated receptor γ (PPARγ), fatty acid-binding protein 4 (FABP4), and fatty acid synthase (FAS), in adipocytes and in white adipose tissue (WAT) of HFD-induced obese mice. Conclusions: These results suggest that the anti-obesity effects of MFBS are elicited by regulating the expression of adipogenesis-related genes in adipocytes and WAT of HFD-induced obese mice.
1. Introduction
Obesity has become one of the most common metabolic disorders and is a serious health problem, since it is a risk factor for metabolic syndromes such as type 2 diabetes, hyperlipidemia, hypertension, and arteriosclerosis[1-3]. Therefore, prevention and treatment of obesity are important for achieving a healthy lifestyle. Obesity is characterized by increased fat accumulation in adipose tissue[3], which results from an imbalance between energy intake and expenditure. This imbalance leads to an increase in adipocyte number and size; adipogenesis, including structural changes; an increase in adipogenic/lipogenic gene expression; and accumulation of lipids stored as excess energy in the form of triglycerides (TG) [4-7]. Therefore, regulation of adipogenesis and TG accumulation is a potential therapeutic strategy to prevent and treat obesity and obesity-related diseases.
Monascus spp. have been consumed as the traditional edible fungi in Eastern Asia, including China and Korea. Monascus spp. are
known to produce several bioactive metabolites such as monacolins, and their metabolites and fermented products have been reported to be beneficial for obesity, regulation of lipid metabolism, and Alzheimer's disease in many previous in vitro and in vivo studies[8-12]. Black soybean (Glycine max L. Merrill) is a type of soybean with a black seed coat, which has been used as a traditional medicinal herb and health food for the prevention of several diseases and conditions such as diabetes and obesity[13-16]. In addition, black soybean has been fermented with filamentous fungi, including Aspergillus awamori, Aspergillus oryzae (A. oryzae), and Monascus pilosus (M. pilosus), and products of black soybean fermented with fungi have several useful biological effects, including antioxidant effects and reduced risk of atherosclerosis[17-19]. However, the effects of M. pilosus-fermented black soybean (MFBS) on obesity and the mechanisms involved in its anti-obesity effects are unknown. In this study, we examined the effects of MFBS extracts (MFBSE) and MFBS powders (MFBSP) on obesity and the underlying mechanisms in 3T3-L1 adipocytes and in high-fat diet (HFD)-induced obese mice.
2. Materials and methods
2.1. MFBSP and MFBSE preparation
Black soybean (Glycine max L. Merrill) purchased from National Agriculture Cooperative Federation (Gyeonggi-Do, Republic of Korea) was washed with water and was homogenized to a small particle size. The homogenates were mixed with 10% wheat bran of homogenate weight/solid media weight; the mixture was autoclaved at 121 ℃ for 90 min and then cooled under aseptic conditions at room temperature. Fermentation was performed following the method described by Lee et al[18]. The freeze-dried fermented sample (moisture content: <5%) was homogenized and was passed through 100 mesh sieve; the resulting material was used as a MFBSP. MFBSP (dry weight, 2 g) was extracted with 95% ethanol (20 mL) for 24 h and filtered. After filtration, the ethanol extracts were evaporated and dissolved in dimethyl sulfoxide (DMSO). The resulting material was used as MFBSE in experiments, and the final concentration of DMSO in the culture medium was adjusted to<0.1%.
2.2. 3T3-L1 adipocyte cell culture
Mouse 3T3-L1 pre-adipocytes obtained from Dr. Sang-Dal Rhee (Korea Research Institute of Chemical Technology (Daejeon, Korea) were cultured in Dulbecco's modified Eagle's medium (DMEM) (New York, NY, USA) supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin and 10 mg/mL streptomycin (Gibco BRL, Grand Island, NY, USA) at 37 ℃ under a humidified 5% CO2atmosphere. For differentiation of 3T3-L1 pre-adipocytes to mature adipocytes, postconfluent cells (defined as day 0) were cultured in differentiation medium containing 0.5 mM 3-isobutylmethylxanthine (Wako Pure Chemical Industries Ltd., Osaka, Japan), 5 μg/mL insulin (Sigma-Aldrich, St Louis, MO, USA), and 1 μM dexamethasone (Wako Pure Chemical Industries Ltd., Osaka, Japan) in DMEM supplemented with 10% FBS. After 3 d, the cell culture medium was changed to DMEM containing 5 μg/mL insulin and 10% FBS. Thereafter, the medium was replaced again with DMEM containing 10% FBS after 2 d for 4 d. To examine the effect of MFBSE on adipogenesis, the cells were treated with or without MFSBE during the adipogenic process.
2.3. Oil-Red O staining
After differentiation, cells were fixed with 4% formaldehyde solution in phosphate-buffered saline (PBS) for 1 h and then were washed three times with distilled water. The cells were stained with Oil-Red O (0.5% in 60% isopropanol) for 1 h and were washed three times with distilled water. Stained cells were photographed under an optical microscope. Stained lipid droplets were extracted with isopropanol, and the absorbance of the extracted lipids was measured at 520 nm using a microplate reader (Tecan, Mannedorf, Switzerland).
2.4. Cell viability assay
Cells were treated with various concentrations (0, 25, 50, and 100 μM) of MFBSE in the DMEM containing 10% FBS for 48 h. Filtered MTT reagent (5 mg/mL) was added to cells at a final concentration of 0.5 mg MTT/mL, and cells were incubated further for 4 h at 37 ℃. Violet formazan crystals were dissolved in DMSO, and the absorbance was measured at 570 nm using a microplate reader. Cell viability (%) was calculated by comparing the absorbance of the treated samples with the absorbance of the control (untreated samples).
2.5. Animal experiments
Five-week-old male C57BL/6J mice were purchased from Orient Bio (Sung-Nam, South Korea). The mice were housed under temperature-[(23±3) ℃] and humidity-controlled conditions with a 12-h light/dark cycle and were given free access to water and food throughout the experiment. After acclimatization with a standard rodent normal-fat diet (NFD) (CRF-1, Charles River, Japan) for 1 week, the mice were fed either the NFD (NFD control) or HFD (60% Kcal Fat, Cat #101556; Research Diets, NJ, USA) for 4 weeks to induce obesity. Based on their body weights, HFD-fed mice were divided into two experimental groups (n=5/group) and treated without (HFD control) or with 1 g/kg M. pilosus-fermented black soybean powder (MFBSP). MFBSP was mixed with distilled water and administered by oral gavage once daily for 12 weeks
while continuing the HFD. The control NFD and HFD groups were administered distilled water alone. Body weight and food intake were measured twice per week throughout the study. The experimental design was approved by the Ethics Committee of the Wonkwang University (Iksan, Jeonbuk, Republic of Korea), and the mice were maintained in accordance with their guidelines.
2.6. Collection of liver and white adipose tissue (WAT)
At the end of the 12-week treatment period, the mice were sacrificed, and the liver and WAT were excised immediately, rinsed, weighed, frozen in liquid nitrogen, and stored at -80 ℃ until analysis.
2.7. Total RNA isolation and gene expression analysis
Total RNA was isolated from 3T3-L1 adipocytes using the SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer's protocol. Total RNA (1 μg) was reversetranscribed to cDNA by using a reverse transcription system (a3500; Promega, Madison, WI, USA) according to the manufacturer's protocol. The mRNA expression levels of adipogenesis-related genes were evaluated by real-time PCR using gene-specific primers and SYBR Green Master PCR Kit (Takara, Shiga, Japan), according to the manufacturer's instructions, with the real-time PCR iQTM5 system (Bio-Rad Laboratories, Hercules, CA, USA). The primers were as follows: PPARγ, 5'-TCACAAGAGGTGACCCAATG-3' and 5'-CCATCCTTCACAAGCATGAA-3'; C/EBP α, 5'-GTGTGCACGTCTATGCTAAACCA-3'and 5'-G C C G T TA G T G A A G A G T C T C A G T T T G-3'; F A B P 4, 5'-C C A AT G A G C A A G T G G C A A G A-3'and 5'-GATGCCAGGCTCCAGGATAG-3'; FAS, 5'-T G G T G G-G T T T G G T G A AT T G T C-3' a n d 5'-G C T T G T C C T G C T C TA AC T G G A AG T-3'; a n dβ -actin, 5'-TGTCCACCTTCCAGCAGATGT-3' and 5'-AGCTCAGTAACAGTCCGCCTAGA-3'.
2.8. Protein extraction and Western blot analysis
To prepare whole cell lysates, adipocytes were treated with various concentrations of MFBSE. After the treatment, cells were washed with ice-cold PBS and harvested in a lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF). The lysates were centrifuged at 12 000 rpm for 20 min, and the supernatants were collected. Total protein concentration was determined by Bio-Rad protein assay (Bradford, USA) using BSA as a standard. Aliquots (30 μg protein) were separated by 10% SDS-PAGE gel electrophoresis and transferred to polyvinylidene difluoride membranes (Amersham, Cleveland, OH, USA). The membranes were blocked with 5% (w/ v) non-fat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) and were incubated with anti-mouse actin and FABP antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or with anti-rabbit PPARγ and CEBP/α antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:1 000 v/v) in 5% BSA in TBS-T. Following 16 h incubation at 4 ℃, the membranes were washed with TBS-T and were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary antibody (Bio-Rad Laboratories, Hercules, CA, USA) in 5% nonfat dry milk in TBS-T. After washing, the immunocomplexes were visualized by enhanced chemiluminescence reagents and detected by chemiluminometer (CLINX Science Instruments Co. Ltd., Shanghai, China). The expression levels of PPARγ, C/EBP α, and FABP relative to those in the control group were determined.
2.9. High-performance Liquid Chromatography (HPLC)
The main constituents in MFBS were analyzed by HPLC analysis. Chromatographic analysis of MFBSE was performed on an Agilent 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA). The column was a reversed-phase column, with dimensions of 4.6 mm×250 mm. (Waters, Milford, MA, USA). The four isoflavones (daidzein, daidzin, genistein, and genistin) were analyzed as follows: the column temperature was maintained at 40 ℃; the solvent was a mixture of (A) water: methanol: acetic acid (88:10:2, v/v/v) and (B) methanol: acetic acid (98:2, v/v) and was delivered at 1 mL/min in a gradient mode (10%-40% B in 21 min, 40% B for 11 min, 40%-60% B in 3 min); and the isoflavones were detected by UV-Vis spectroscopy at 260 nm. Monacolin K was analyzed as follows: the column temperature was maintained at 40 ℃; the mobile phase was (A) 0.2% phosphoric acid and (B) acetonitrile and was delivered at 1 ml/min in a gradient mode (35%-75% B in 20 min, 75%-100% B in 1 min, 100% B for 14 min, 100%-35% B in 1 min, 35% B for 14 min); and Monacolin K was detected by UV-Vis spectroscopy at 237 nm.
2.10. Statistical analysis
Data are expressed as means±SEM. The differences among treatment groups were analyzed by one-way ANOVA, followed by Duncan test, using Origin 7 Software (MicroCal Software, Northampton, MA, USA). Values of P<0.05 were considered to indicate statistical significance.
3. Results
3.1. HPLC analysis of MFBS
Monacolin K and four isoflavones (daidzein, daidzin, genistein, and genistin) from MFBS were analyzed by HPLC. To compare the relative contents of these constituents, monacolin K and isoflavones
were measured from the 75% ethanol extracts of M. piloususfermented and unfermented black soybean. Although monacolin K was not detected in unfermented black soybean extracts, MFBSE significantly produced monacolin K (Figure 1A). Additionally, isoflavone aglycones, such as daidzein and genistein, were markedly induced by fermentation of MFBSE (Figure 1B). The ratios of isoflavone aglycones daidzein and genistein were increased approximately 2.9- and 4.4-fold, respectively, based on the peak areas.
3.2. Effects of MFBSE on lipid accumulation and cell viability in 3T3-L1 adipocytes
To determine the effect of MFBSE on lipid accumulation during differentiation, 3T3-L1 preadipocytes were induced to differentiate into adipocytes with MFBSE. Lipid accumulation was measured by Oil-Red O staining. MFBSE inhibited lipid accumulation in 3T3-L1 adipocytes in a dose-dependent manner (Figure 2A). Treatment with 50 and 100 μg/mL of MFBSE significantly reduced the lipid content by 25% (P<0.05) and 45% (P<0.001), respectively, compared with control. We performed the MTT assay to investigate the effect of MFBSE on cell viability of 3T3-L1 preadipocytes. MFBSE did not show any cytotoxicity after 48 h incubation at the tested concentrations (Figure 2B). These results demonstrated that MFBSE inhibits lipid accumulation.
3.3. Effects of MFBSE on the expression levels of adipogenic transcription factor and related genes
To examine the possible mechanism by which adipogenesis is inhibited, we analyzed the effects of MFBSE on the expression
of adipogenic transcription factors and their target genes by using quantitative real-time PCR. PPARγ mRNA expression levels were decreased by 44% and 62% in 50 and 100 μg/mL MFBSE groups, respectively, compared with the control group (P<0.05; Figure 3A). C/EBP α mRNA expression levels were decreased by 44% in the 100 μg/mL MFBSE group compared with the control group (P<0.05; Figure 3B). FABP4 and FAS mRNA expression levels were reduced significantly in a dose-dependent manner in MFBSE-treated groups (FABP4: P<0.05 and P<0.01; FAS: P<0.05 and P<0.01; at 50 and 100 μg/mL, respectively; Figure 3C and D).
3.4. Effects of MFBSE on the expression levels of adipogenesis-related proteins
We confirmed whether MFBSE affects the protein expression of adipogenesis-related proteins. PPARγ expression levels were reduced in a dose-dependent manner in MFBSE-treated groups and were reduced significantly in the 100 μg/mL MFBSE group (Figure 4A and B). C/EBPα and FABP4 protein expression levels were reduced significantly in both the 50 and 100 μg/mL MFBSE groups (Figure 4A, C and D). These results demonstrated that MFBSE inhibits lipid accumulation through the downregulation of adipogenic transcription factors and their targets.
3.5. Effects of MFBSP on body weight gain, food intake, and organ weight
The body weight gain, food intake, and organ weight are shown in Table 1. Body weight gain in the HFD control group was higher than the body weight gain in the NFD control group (P<0.005). Body weight gain in the MFBSP group was significantly lower than that in the HFD control group (P<0.05). Food intake was not significantly different among all groups. WAT and liver weights in the MFBSP group tented to be lower, but not significantly lower than in the HFD group. These results suggest that the MFBSP reduces the body weight gain without affecting food intake.
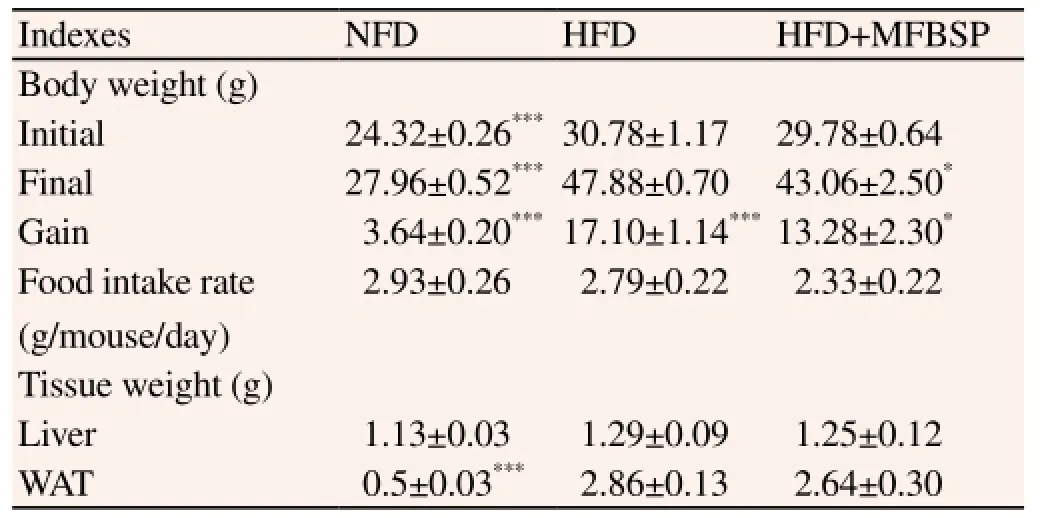
Table 1 Effects of MFBSP on body weight, food intake and tissues weight in HFD-induced obese mice for 12 weeks.
3.6. Effects of MFBSP on adipogenesis-related genes expression
We analyzed the effects of MFBSP on the mRNA levels of adipogenesis-related genes in WAT. In WAT, PPARγ mRNA expression levels tended to be reduced, but this reduction was not
significant (P=0.052; Figure 5A). aP2 and FAS mRNA expression levels were markedly reduced by 78% and 46%, respectively, in the MFBSP group compared with the HFD control group (P<0.05; Figure 5B and C). These results suggest that MFBS downregulates the mRNA expression of adipogenesis-related genes in WAT.
4. Discussion
Obesity is a risk factor for metabolic syndromes including hyperlipidemia, type 2 diabetes, and cardiovascular disease[20,21]. Therefore, many studies have focused on prevention and treatment of obesity for achieving a healthy life. There are many drugs for obesity prevention, and treatments include reduction of nutrient absorption and regulation of lipid mobilization and utilization. Owing to the adverse side effects associated with many anti-obesity drugs, more recent trials have focused on screening for natural sources that have been reported to reduce body weight and that generally have minimal side effects[22]. This study is the first to investigate the in vitro and in vivo effects of M. pilosus-fermented black soybean (MFBS) on antiobesity and the mechanisms involved using 3T3-L1 adipocytes and HFD-induced obese mice.
Monascus sp. have been used as traditional edible fungi, natural food colorant and preservative, and traditional medicine for improving blood circulation. Monacolin K, a secondary metabolite of Monascus sp., is a known inhibitor of HMG-CoA reductase in cholesterol metabolism[8,9]. Additionally, black soybean and its products fermented with various filamentous fungi have been used as foods for promotion of health[19]. In this study, we found that the content of monacolin K, daidzein, and genistein was higher in MFBS than in unfermented black soybean. This finding suggests that the endogenous glucoside form of isoflavone in black soybean is converted into aglycones by M. pilosus-fermentation, in agreement with the increase in content of isoflavone aglycones and decrease of glucosides observed upon soaking soybeans in water and processing[12]. It has been reported that aglycones have the ability to lower fat accumulation and plasma lipids levels in vitro and in vivo[9,13,14]. We found that MFBS extracts showed less lipid accumulation in adipocytes than M. pilosus extracts and black soybean extracts (data not shown). These results suggest that MFBS containing monacolin K, daidzein, and genistein might regulate lipid accumulation more efficiently than non-fermented black soybean, and the interaction between these bioactive components could play important roles in the regulation of obesity. Thus, MFBS was employed in additional experiments.
The results from lipid staining by Oil-Red O in adipocytes show that MFBSE suppresses adipogenesis without cell cytotoxicity. In addition, our in vivo study demonstrated that MFBSP significantly reduced the body weight gain of mice, although it showed a tendency to lower WAT weight. These results suggest that MFBS exerts anti-obesity effects in adipocytes and in HFD-induced obese mice. Previous studies have also confirmed that Monascus-fermented products have anti-obesity and hypolipidemia effects in adipocytes and in HFD-induced obese animals[11,23,24].
Obesity is characterized by increased fat accumulation, which is associated with an increase in the cell number and/or cell size of adipocytes differentiated from preadipocytes[3,4,6,7]. Adipogenesis is the process by which undifferentiated preadipocytes are converted to differentiated adipocytes, which is closely associated with the etiologies of obesity[25]. The entire adipogenic process is accompanied by sequential expression and activation of transcription factors, such as C/EBPα, PPARγ, and ADD/SREBP-1[26,27]. Among them, PPARγ, a ligand-activated transcription factor that is expressed during the early to middle stages of adipogenesis, regulates adipogenesis through modulating the expression of its target genes, including fatty aP2 and FAS, which are responsible for TG synthesis and accumulation during adipocyte differentiation[28,29]. C/EBP α is expressed in the late stage of adipogenesis and stimulates the differentiation of preadipcoytes in cooperation with PPARγ. In this study, MFBSE reduced the mRNA and protein expression levels of adipogenesis-related genes such as PPARγ, C/EBPα, FABP4, and FAS. Furthermore, mRNA expression levels of these genes were reduced in WAT of HFD-induced obese mice, although the WAT weights tended to be reduced in MFBSP-treated group. These results suggest that reduction of expression of adipogenesis-related genes by MFBSE and MFBSP might explain the inhibition of differentiation and lipid accumulation of adipocytes and WAT of HFD-induced obese mice.
In conclusion, our results show that MFBS prevents the development of obesity in adipocytes and in HFD-induced obese mice by regulating the mRNA expression of genes associated with adipogenesis. These results suggest that MFBS can potentially be
used as an anti-obesity therapy.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Björntorp P. Obesity. Lancet 1997; 350(9075): 423-426.
[2] Kopelman PG. Obesity as a medical problem. Nature 2000; 404(6778): 635-643.
[3] Spieglman BM, Filer JS. Obesity and the regulation of energy balance. Cell 2001; 104(4): 531-543.
[4] Prins JB, O'Rahilly S. Regulation of adipose cell number in man. Clin Sci 1997; 92(1): 3-11.
[5] Devlin MJ, Yanovski SZ, Wilson GT. Obesity: what mental health professionals need to know. Am J Psychiatry 2000; 157(6): 854-866.
[6] Fujioka K. Management of obesity as a chronic disease: nonpharmacologic, pharmacologic, and surgical options. Obes Res 2002; 10(Suppl): 116S-123S.
[7] Choi H, Eo H, Park K. A water-soluble extract from Cucurbita moschata shows anti-obesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. Biochem Biophys Res Commun 2007; 359(3): 419-425.
[8] Endo A, Hasumi K, Negishi S. Monacolins J. New inhibitors of cholesterol biosynthesis produced by Monascus reber. J Antibiot (Tokyo) 1985; 38(3): 420-422.
[9] Bey L, Maigret P, Laouenan H, Hamilton MT. Induction of lipoprotein lipase gene expression in 3T3-L1 preadipocytes by atorvastain, a cholesterol-and triglyceride-lowering drug. Pharmacology 2002; 66(1): 51-56.
[10] Chen WP, Ho BY, Lee CL, Lee CH, Pan TM. Red mold rice prevents the development of obesity, dyslipidemia and hyperinsulinemia induced by high-fat diet. Int J Obes (London) 2008; 32(11): 1694-1704.
[11] Jeon T, Hwang SG, Hirai S, Matsui T, Yono H, Kwada T, et al. Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci 2004; 75(26): 3195-3203.
[12] Lee CL, Wang JJ, Pand TM. Red mold rice extract represses amyloid beta peptide-induced neurotoxicity via potent synergism of anti-inflammatory and antioxidative effects. Appl Micobiol Biotechnol 2008; 79(5): 829-841.
[13] Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr 2002; 76(6): 1191-1201.
[14] Davis J, Higginbotham A, O'Connor T, Moustaid-Moussa N, Tebbe A, Kim YC, et al. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann Nutr Metab 2007; 51(1): 42-52.
[15] Kanamoto Y, Yamashita Y, Nanba F, Yoshida T, Tsuda T, Fukuda I, et al. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J Agric Food Chem 2012; 59(16): 8985-8993.
[16] Kurimoto Y, Shibayama Y, Inoue S, Soga M, Takikawa M, Ito C, et al. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. J Agric Food Chem 2013; 61(23): 5558-5564.
[17] Lee IH, Chou CC. Distribution profiles of isoflavone isomers in black bean kojis prepared with various filamentous fungi. J Agric Food Chem 2006; 54(4): 1309-1314.
[18] Lee SI, Kim SD, Lee YK, Kim MJ, Lee IA, Choi JK, et al. Dietary effects of black bean fermented by Monascus pilosus on body weight gain, serum lipid profiles and activities of hepatic antioxidative enzymes in mice fed high fat diets. Korean J Nutr 2013; 46(1): 5-14.
[19] Huang CC, Huang WC, Hou CW, Chi YW, Huang HY. Effect of black soybean koji extract on glucose utilization and adipocyte differentiation in 3T3-L1 cells. Int J Mol Sci 2014; 15(5): 8280-8292.
[20] Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation 2003; 107(10): 1448-1453.
[21] Wing RR, Jeffery RW. Effect of modest weight loss on changes in cardiovascular risk factors: are there differences between men and women or between weight loss and maintenance? Int J Obes Relat Metab Disord 1995; 19 (1): 67-73.
[22] Kishino E, Ito T, Fujita K, Kiuchi Y. A mixture of the Salacia reticulata (Kotala himbutu) aqueous extract and cyclodextrin reduces the accumulation of visceral fat mass in mice and rats with high-fat dietinduced obesity. J Nutr 2006; 136(2): 433-439.
[23] Pyo YH, Seong KS. Hypolipidemic effects of Monascus-fermented soybean extracts in rats fed a high-fat and -cholesterol diet. J Agric Food Chem 2009; 57(18): 8617-8622.
[24] Wang LC, Lung TY, Kung YH, Wang JJ, Tsai TY, Wei BL, et al. Enhanced anti-obesity activities of red mold dioscorea when fermented using deep ocean water as the culture water. Mar Drugs 2013; 11(10): 3902-3925.
[25] Spiegelman BM, Choy L, Hotiamisligil GS, Graves RA, Tontonoz P. Regualtion of adipocyte gene expression in differenctiation and syndromes of obesity/diabetes. J Biol Chem 1993; 268(10): 6823-6826.
[26] Ntambi JM, Kim YC. Adipocytes differentiation and gene expression. J Nutr 2000; 130(8): 3122S-3126S.
[27] Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 2000; 16: 145-171.
[28] Ha DH, Trung TH, Phoung TT, Yim N, Chen QC, Bae KH. The selected flavonol glycoside derived from Sophorae Flos improves glucose uptake and inhibits adipocyte differentiation via activation AMPK in 3T3-L1 cells. Bioorg Med Chem Lett 2010; 20(20): 6076-6081.
[29] Kim KJ, Lee OH, Lee BY. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci 2010; 86(21-22): 791-797.
ment heading
10.1016/S1995-7645(14)60330-8
#These authors contributed equally to this work.
*Corresponding author: Seung Hwan Yang and Joo-Won Suh, Center for Nutraceutical and Pharmaceutical Materials, Myongji University, 116 Myongji-Ro, Cheoin-Gu, Yongin-Si, Gyeonggi-Do 449-728, Korea.
Tel: +82-31-330-6190
Fax: +82-32-321-7361
E-mail: ymichigan@mju.ac.kr, jwsuh@mju.ac.kr
Foundation project: This work was carried out with the support of the "Cooperative Research Program for Agriculture Science & Technology Development (Project No.PJ009582)" of the Rural Development Administration, Republic of Korea.
 Asian Pacific Journal of Tropical Medicine2015年4期
Asian Pacific Journal of Tropical Medicine2015年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- A brief review on biomarkers and proteomic approach for malaria research
- Trigonelline protects the cardiocyte from hydrogen peroxide induced apoptosis in H9c2 cells
- In vitro cholinesterase inhibitory and antioxidant effect of selected coniferous tree species
- Antiprotozoal assessment and phenolic acid profiling of five Fumaria (fumitory) species
- Profile and geographical distribution of reported cutaneous leishmaniasis cases in Northwestern Saudi Arabia, from 2010 to 2013
- Change of MicroRNA-134, CREB and p-CREB expression in epileptic rat
