In vitro cholinesterase inhibitory and antioxidant effect of selected coniferous tree species
Fatma Sezer Senol, Ilkay Erdogan Orhan, Osman UstunDepartment of Pharmacognosy, Faculty of Pharmacy, Gazi University, 06330 Ankara, Turkey
In vitro cholinesterase inhibitory and antioxidant effect of selected coniferous tree species
Fatma Sezer Senol, Ilkay Erdogan Orhan*, Osman Ustun
Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, 06330 Ankara, Turkey
ARTICLE INFO
Article history:
Received 20 November 2014
Received in revised form 10 January2015
Accepted 20 February 2015
Available online 20 April 2015
Conifer
Cholinesterase inhibition
Antioxidant activity
Alzheimer's disease
Total phenol and flavonoid
Objective: To explore cholinesterase inhibitory and antioxidant effect of six coniferous trees (Abies bornmulleriana, Picea pungens, Juniperus communis, Cedrus libani, Taxus baccata, and Cupressus sempervirens var. horizantalis). Methods: Acetone (Ace), ethyl acetate (EtOAc), and ethanol (EtOH) extracts prepared from the needles and shoots of the six coniferous trees were screened for their acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity at 100 μg/mL. Antioxidant activity of the extracts was tested using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and N,N-dimethyl-p-phenylendiamine (DMPD) radical scavenging, metal-chelation capacity, ferric-(FRAP) and phosphomolibdenum-reducing antioxidant power (PRAP) assays. All of the assays were performed in ELISA microplate reader. Total phenol and flavonoid amounts in the extracts were determined spectrophotometrically. Results: Among thirty-six extracts in total, the shoot-Ace extract of Cupressus sempervirens var. horizantalis exerted the highest inhibition against AChE [(54.84±2.51)%], while the needle-Ace extract of Cedrus libani was the most effective in inhibiting BChE [(67.54±0.30)%]. The highest DPPH radical scavenging effect, FRAP and PRAP was observed in the shoot-Ace and EtOAc extracts from Taxus baccata, whereas all the extracts showed a variable degree of scavenging effect against DPMD radical. The shoot-EtOAc extract of Cedrus libani had the highest metalchelation capacity [(58.04±0.70)%]. The shoot extracts of Taxus baccata were determined to have the richest total phenol content, which may contribute to its marked antioxidant activity. Conclusions: The conifer species screened in this study may contain cholinesterase-inhibiting and antioxidant properties, which might be useful against Alzheimer's disease.
1. Introduction
Alzheimer's disease (AD) is an age-related progressive neurodegenerative disease and the most common form of dementia especially among the elder population in which irreversible neuronal loss and abnormal behavioral changes are evident in this disease[1]. Only a few hypotheses have been suggested for pathogenesis of AD, which are known as “cholinergic hypotheses” and “amyloid hypothesis”. In cholinergic hypothesis, shortage of acetylcholine (ACh) in the brain that is hydrolyzed by acetylcholinesterase (AChE) has been shown[2]. On the other hand, butyrylcholinesterase (BChE) that also inactivates ACh as well as butyrylcholine (BCh) is a new target in drug discovery for AD[3] and nowadays, cholinesterase inhibitors have become the most prescribed drug class in the treatment of this disease.
The conifers, majority of which are in tree form, are woody plants that bear cones. The coniferous trees are evergreen trees with long, needle- or scale-like leaves. On the other hand, plants have served as source of many drug molecules, nevertheless, an immense amount of plant species have been remained un-screened for their phytochemistry and pharmacological activities. Consequently, drug discovery from plants is a still attractive target for many researchers. During our ongoing pursuit on exploring new neuroprotective
agents of plant origin with cholinesterase inhibition and antioxidant effect, we previously investigated anticholinesterase effect of several coniferous genera including Juniperus species[4], Cupressus sempervirens (C. sempervirens)[5], Pinus species[6], and the lignans of Taxus baccata (T. baccata) L.[7]. In connection with our aforementioned studies, we have now designed the present study to search AChE and BChE inhibitory activity of the acetone (Ace), ethyl acetate (EtOAc), and ethanol (EtOH) extracts prepared from the needles and shoots of selected conifer species; Abies bornmulleriana (A. bornmulleriana) Matff. (AB) (fir), Picea pungens (P. pungens) Engelm. (PP) (blue spruce), and Cedrus libani (C. libani) A. Rich. (CL) (Lebanon cedar) from Pinaceae, C. sempervirens L. var. horizantalis (Mill.) Gord. (CS) (Mediterranean cypress) and Juniperus communis (J. communis) L. (JC) (juniper) from Cupressaceae, and T. baccata L. (TB) (European yew) from Taxaceae growing in Turkey. Since oxidative stress has been known to contribute to progression of AD[8], antioxidant activity of the extracts was also determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and N,N-dimethyl-p-phenylendiamine (DMPD) radical scavenging activity, metal-chelation capacity along with ferric-(FRAP) and phosphomolibdenum-reducing antioxidant power (PRAP) tests. Total phenol and flavonoid contents of the extracts were calculated spectrophotometrically.
2. Materials and methods
2.1. Plant samples
The needles and shoots of A. bornmulleriana (Bolu province), P. pungens, C. sempervirens L. var. horizantalis, T. baccata (Ankara province), C. libani (Burdur province), and J. communis (Antalya province) were collected from their corresponding places in Turkey during the year of 2010 and identified by one of us (O.U.). The voucher specimens are deposited at the Herbarium of Faculty of Pharmacy, Gazi University, Ankara, Turkey.
2.2. Preparation of the extracts
The air-dried and powdered needles and shoots of the plant samples were extracted by macerating 10 g of each plant part sequentially with 1 000 mL of Ace, EtOAc, and ethanol for 2 d. The filtrated organic phases of each plant part were evaporated until dryness in vacuo and preserved in the freeze dryer until the assays were performed.
2.3. Microtiter assays for enzyme inhibition
2.3.1. AChE and BChE inhibitory activity
AChE and BChE inhibitory activity of the extracts was determined by modified spectrophotometric method of Ellman et al[9]. Electric eel acetylcholinesterase (Type-VI-S, EC 3.1.1.7, Sigma) and horse serum butyrylcholinesterase (EC 3.1.1.8, Sigma) were used as the enzyme sources, while acetylthiocholine iodide and butyrylthiocholine chloride (Sigma, St. Louis, MO, USA) were employed as substrates of the reaction. 5,5´-Dithio-bis(2-nitrobenzoic)acid (DTNB, Sigma, St. Louis, MO, USA) was used for the measurement of the cholinesterase activity. All the other reagents and conditions were the same as described in our previous publication[10]. In brief, 140 μL of 0.1 mM sodium phosphate buffer (pH 8.0), 20 μL of 0.2 M DTNB, 20 μL of sample solutions and 20 μL of 0.2 M acetylcholinesterase/butyrylcholinesterase solution were added by multichannel automatic pipette (Gilson pipetman, France) in a 96-well microplate and incubated for 15 min at 25 ℃. The reaction was then initiated with the addition of 10 μ L of 0.2 M acetylthiocholine iodide/butyrylthiocholine chloride. The hydrolysis of acetylthiocholine iodide/butyrylthiocholine chloride was monitored by the formation of the yellow 5-thio-2-nitrobenzoate anion as a result of the reaction of DTNB with thiocholines, catalyzed by enzymes at a wavelength of 412 nm utilizing a 96-well microplate reader (VersaMax, Molecular Devices, USA). Galanthamine, the anticholinesterase alkaloid-type of drug isolated from the bulbs of snowdrop (Galanthus sp.), was purchased from Sigma (St. Louis, MO, USA) and was employed as reference.
2.3.2. Data processing for enzyme inhibition assays
The measurements and calculations were evaluated by using Softmax PRO 4.3.2.LS software. Percentage of inhibition of AChE/ BChE was determined by comparison of rates of reaction of test samples relative to blank sample (ethanol in phosphate buffer pH=8). Extent of the enzymatic reaction was calculated based on the following equation: E = (C-T)/C×100, where E is the activity of the enzyme. E value expresses the effect of the test sample or the positive control on acetylcholinesterase and butyrylcholinesterase enzyme activity articulated as the percentage of the remaining activity in the presence of test sample or positive control. C value is the absorbance of the control solvent (blank) in the presence of enzyme, where T is the absorbance of the tested sample (plant extract or positive control in the solvent) in the presence of enzyme. Data are expressed as average inhibition±standard error mean (SEM) and the results were taken from at least three independent experiments performed in triplicate.
2.4. Antioxidant activity assays
2.4.1. DPPH radical scavenging assay
The hydrogen atom or electron donation capacity of the corresponding extracts was computed from the bleaching property of the purple-colored methanol solution of 2,2-diphenyl-1-
picrylhydrazyl (DPPH). The stable DPPH radical scavenging activity of the extracts was determined by the method of Blois[11]. The samples (2 700 μL) dissolved in ethanol (75%) were mixed with 300 μL of DPPH solution (1.5×10-4M). Remaining DPPH amount was measured at 520 nm using a Unico 4802 UV-visible double beam spectrophotometer (Dayton, NJ, USA). The results were compared to that of gallic acid employed as the reference.
2.4.2. DMPD radical scavenging assay
Principal of the assay is based on reduction of the purple-colored radical DMPD+(N,N-dimethyl-p-phenylendiamine)[12]. According to the method, a reagent comprising of 100 mM DMPD, 0.1 M acetate buffer (pH=5.25), and 0.05 M ferric chloride solution, which led to formation of DMPD radical, was freshly prepared and the reagent was equilibrated to an absorbance of 0.900±0.100 at 505 nm. Then, the reagent (1 000 μL) was mixed up with 50 μL of the extract dilutions dissolved in ethanol (75%) and absorbance was taken at 505 nm using a Unico 4802 UV-visible double beam spectrophotometer (USA). Quercetin was employed as the reference and the experiments were done in triplicate.
2.4.3. Fe2+-ferrozine test system for metal-chelation
The metal-chelating effect of the extracts by Fe2+-ferrozine test system was estimated in consistent with Chua et al's method[13]. Accordingly, 740 μL of ethanol and 200 μL of the samples dissolved in ethanol (75%) were incubated with 2 mM FeCl2solution. The reaction was initiated by the addition of 40 μL of 5 mM ferrozine solution into the mixture, shaken vigorously, and left standing at ambient temperature for 10 min. The absorbance of the reaction mixture was measured at 562 nm. The ratio of inhibition of ferrozine-Fe2+complex formation was calculated as given in 4.6. and ethylenediaminetetraacetic acid (EDTA) was employed as the reference in this assay.
2.4.5. Ferric-reducing antioxidant power (FRAP) assay
The FRAP of the extracts and reference was tested using the assay of Oyaizu[14] based on the chemical reaction of Fe(Ⅲ) => Fe(Ⅱ). Different concentrations of the extracts dissolved in ethanol (75%) were added into 2500 μL of phosphate buffer (pH 6.6) and 2 500 μL of potassium ferricyanide [K3Fe(CN)6] (1%, w/v). Later, the mixture was incubated at 50 ℃ for 20 min and then 2 500 μL of trichloroacetic acid (10%) was added. After the mixture was shaken vigorously, this solution was mixed with 2 500 μL of distilled water and FeCl3(100 μL, 0.1%, w/v). After 30 min incubation, absorbance was read at 700 nm using a Unico 4802 UV-visible double beam spectrophotometer (Dayton, NJ, USA). Analyses were achieved in triplicate. Chlorogenic acid was the reference in this assay.
2.4.6. Phosphomolibdenum-reducing antioxidant power (PRAP) assay
In order to perform PRAP assays on the extracts, each dilution was mixed with 10% phosphomolybdic acid solution in ethanol (w/v)[15]. The solution was subsequently subjected to incubation at 80 ℃ for 30 min and the absorbance was read at 600 nm using a Unico 4802 UV-visible double beam spectrophotometer (USA) and compared to that of quercetin as the reference.
2.4.7. Data processing for antioxidant activity assays
Inhibition of DPPH, DMPD, super oxide, and nitric oxide radicals and metal-chelation capacity was calculated as given below and the results were expressed as percent inhibition (I%):
I%=[(Ablank-Asample)/Ablank]×100, where Ablank is the absorbance of the control reaction (containing all reagents except the test sample), and Asampleis the absorbance of the extracts. Analyses were run in triplicate and the results were expressed as average values with SEM.
For FRAP and PRAP assays, the analyses were also achieved in triplicate and increased absorbance of the reaction meant increased reducing power in both assays.
2.8. Statistical analysis of data
Data obtained from in vitro enzyme inhibition and antioxidant experiments were expressed as the mean±SEM. Statistical differences between the reference and the sample groups were evaluated by ANOVA (one way). Dunnett's multiple comparison tests were used as post hoc tests. P<0.05 was considered to be significant.
2.9. Determination of total phenol and flavonoid contents in the extracts
Phenolic content of the extracts was determined in accordance with Folin-Ciocalteau's method[16]. In brief, a number of dilutions of gallic acid dissolved in ethanol (75%) were obtained to prepare a calibration curve. The extracts and gallic acid dilutions were mixed with 750 μL of Folin-Ciocalteau's reagent and 600 μL of sodium carbonate in test tubes. The tubes were then vortexed and incubated at 40 ℃ for 30 min. Afterward, absorption was measured at 760 nm at a Unico 4802 UV-visible double beam spectrophotometer (USA). Total flavonoid content of the extracts was calculated by aluminum chloride colorimetric method[17]. To sum up, a number of dilutions of quercetin dissolved in ethanol (75%) were obtained to prepare a calibration curve. Then, the extracts and quercetin dilutions were mixed with 95% ethanol, aluminum chloride reagent, 100 μL of sodium acetate as well as distilled water. Following incubation for 30 minutes at room temperature, absorbance of the reaction mixtures was measured at wavelength of 415 nm with a Unico 4802 UV-
visible double beam spectrophotometer (USA). The total phenol and flavonoid contents of the extracts were expressed as gallic acid and quercetin equivalents (mg/g extract), respectively.
3. Results
Cholinesterase inhibitory activity of the extracts was tested against AChE and BChE at 100 μg/mL using ELISA microplate reader. According to the results we obtained; occurrence of the most remarkable inhibition against AChE was observed in the shoot-Ace extract of C. sempervirens var. horizantalis [(54.84±2.51)%), followed by the needle-Ace extract of P. pungens (45.09±3.54%) and the shoot-EtOH extract of C. libani [(44.07±0.20)%] (Table 1). Among the tested extracts, the highest BChE inhibition rates (over 60% at 100 μg/mL) were caused by the shoot-Ace extract of C. libani [(67.54±0.30)%] along with the needle- [(64.29± 1.00)%) and shoot-Ace [(60.77±0.56)%] extracts of C. sempervirens var. horizantalis. Our findings indicated that majority of the extracts exerted higher inhibition towards BChE than AChE (Table 1).
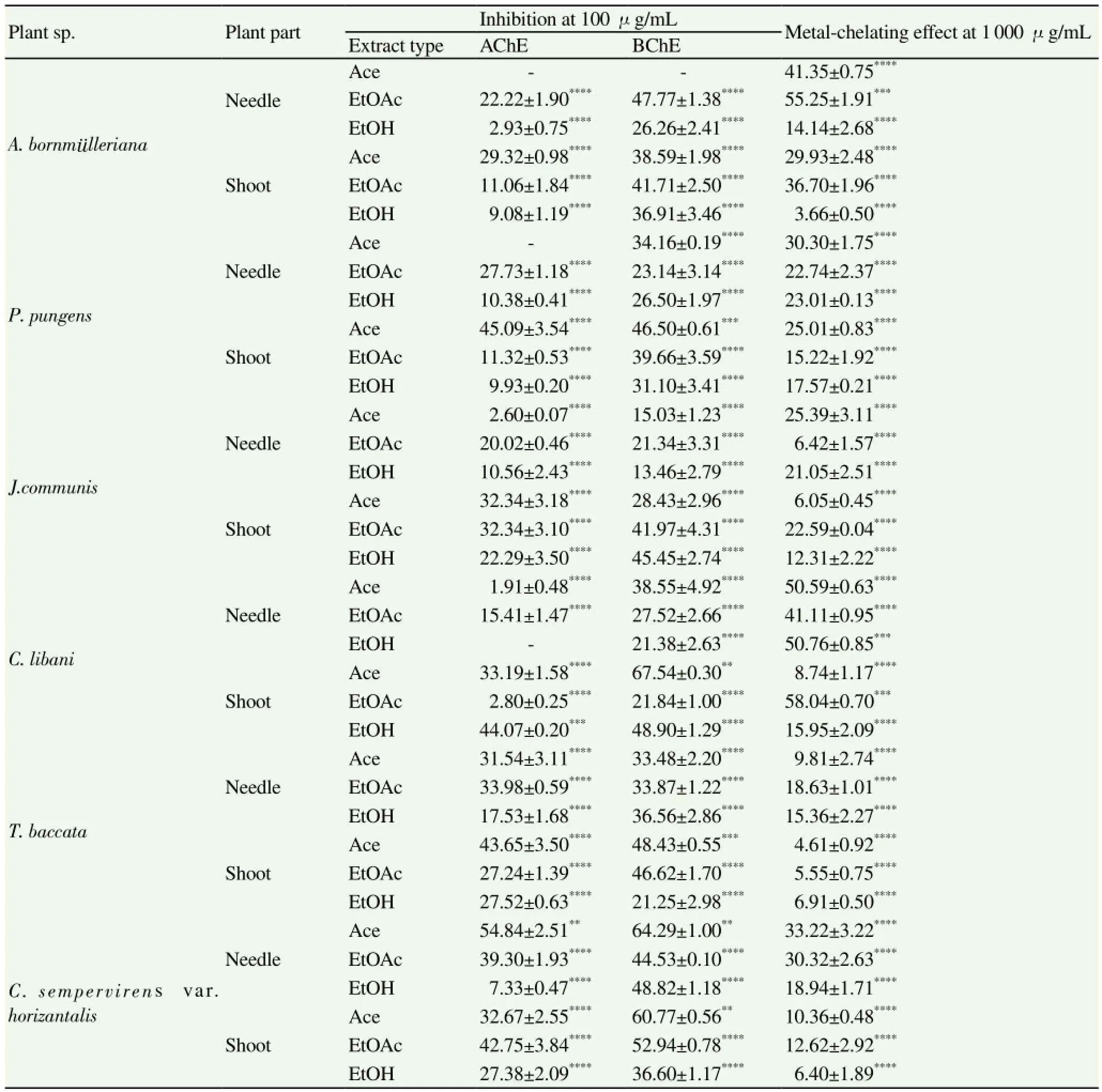
Table 1 Inhibitory effect of the extracts against AChE and BChE and their metal-chelating effect.
DPPH and DMPD radical scavenging, metal-chelation, FRAP, and PRAP assays were performed to determine in vitro antioxidant activities of the extracts. Table 2 represents the percentages of radical scavenging activity of the extracts. Accordingly, occurrence of the highest DPPH scavenging activity, all of which ranged between (10.93±2.77)% and (91.57±0.76)%, was observed in the Ace [(91.57 ±0.76)%] and EtOAc [(87.95±2.51)%] extracts from the shoots of T. baccata. In consistent with these data, the three extracts obtained from the shoots of T. baccata had the most marked scavenging effect towards DMPD radical (Table 2) and also the most remarkable FRAP and PRAP values (Figure 1). In the PRAP assay, the shoot-Ace extracts from C. libani and T. baccata showed a comparable effect to that of the reference (quercetin).
The richest extracts in terms of total phenol contents were revealed to be the shoot-Ace [(248.17±2.69) μg/g extract], EtOAc (231.07 ±5.61) μg/g extract), and EtOH [(200.66±4.21) μg/g extract] extracts of T. baccata (Table 1). However, total flavonoid content was the most abundant in the EtOAc extracts of C. libani shoots and needles [(66.85±4.42) and (64.73±5.57) μg/g extract, respectively].
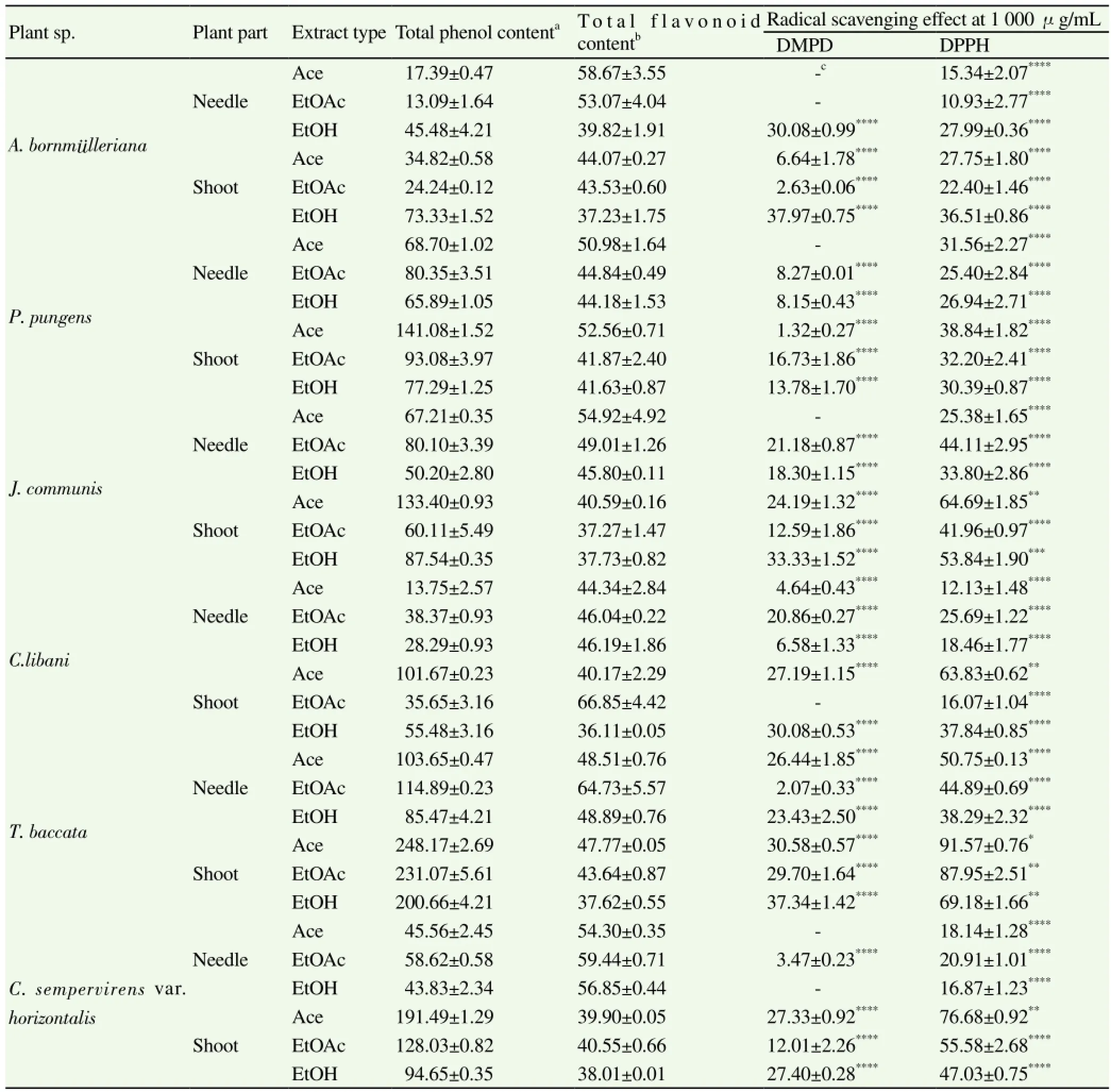
Table 2 Total phenol and flavonoid contents and inhibitory effect of the extracts against DMPD and DPPH radicals
4. Discussion
Cholinesterase inhibition is an important drug treatment strategy against AD and, therefore, an extensive research is also being conducted on plant sources in order to find new inhibitors. For this purpose, our data revealed that the conifer trees screened herein may contain some potential components which could serve as inhibitors against cholinesterase enzyme family. In our previous work, the lignans (lariciresinol, taxiresinol, 3'-demethylisolariciresinol-9'-hydroxyisopropylether, isolariciresinol, and 3-demethylisolariciresinol) isolated from the bark of T. baccata (European yew), a conifer native to Europe, Turkey, northwest Africa, northern Iran, and southwest Asia[18], was tested in the same manner all of which were found to display a moderate level inhibition only against BChE[7]. Although presence of lignans, flavonoids, steroids, and some sugar derivatives have been reported in T. baccata[18], those lignans isolated from the bark of this tree that may possibly present in the shoots and needles could contribute to the BChE inhibitory of the needle and shoot extracts of this plant in the current study. On the other hand, since the isolated lignans from the bark of T. baccata were previously shown by us to be quite effective in the antioxidant assays[7], the high antioxidant activity of T. baccata extracts could be mostly attributed to the lignan derivatives.
In one of our earlier studies[4], we screened AChE and BChE inhibitory and antioxidant effects of the aqueous and ethanol extracts of the leaves, ripe fruits, and unripe fruits of five Juniperus species including J. communis subsp. nana using the same methods as used herein and those extracts also exhibited similar inhibitory profiles to the current ones studied in this work, which can be considered to be mild or moderate. Among the extracts of J. communis screened herein, its shoot-EtOH extract exerted the highest BChE activity [(45.45±2.74)%], while the shoot-Ace and EtOAc extracts of this plant were the most active in inhibiting AChE [(32.34±3.18)% and (32.34±3.10)%, respectively]. In our previous study, umbelliferone was detected in the leaf-EtOH extract of J. communis subsp. nana, which could also possibly exist in the shoot extracts. Consequently, this compound may be speculated to donate to BChE inhibitory action of the shoot-EtOH extract as it was formerly shown by us to possess BChE-inhibiting property[19]. In accordance with our data, J. communis fruit extracts from Turkey were reported to have a low cholinesterase inhibitory effect[20].
We have recently reported about cholinesterase inhibitory and antioxidant activities of several extracts of the cones and leaves (needles) of C. sempervirens var. horizantalis and var. pyramidalis[5], where they exerted a mild to moderate cholinesterase inhibition below 40%. However, the needle and shoot extracts of the same plant had better anticholinesterase activity in this study that inhibited BChE up to (64.29±1.00)%. A number of flavonoids (quercetin, rutin, cupressuflavone, amenoflavone, quercitrin, and myricitrin) have been identified in C. sempervirens[21] and notable antioxidant activity of the shoot extracts of this plant in DPPH radical scavenging activity, FRAP, and PRAP assays could be attributed to presence of these compounds.
Our literature survey has shown that there has been no report on anticholinesterase activity of C. libani, A. bornmulleriana, and P. pungens up to date. However, piperidine alkaloids found in C. libani, A. bornmulleriana, and P. pungens[22,23] could be associated with their cholinesterase inhibitory effect as many piperidine alkaloids were revealed to have potent inhibitory properties against cholinesterases[24,25]. These species also contain some flavonoid derivatives which make a contribution to their antioxidant activity to some extent[26].
In the current study, we have evaluated possible in vitro neuroprotective effect of the needles and shoots of A. bornmulleriana, P. pungens, J. communis, C. libani, T. baccata, and C. sempervirens var. horizantalis through their cholinesterase inhibitory and antioxidant properties. The extracts displayed a variable level of inhibition towards both cholinesterases, some of which possessed a marked AChE and BChE-inhibiting effects over 50%. The shoot extracts of T. baccata appeared to be highly effective in most of the antioxidant assays performed. To the best of our knowledge, we herein disclose the first study on anticholinesterase effects of P. pungens, A. bornmulleriana, and C. libani as well as their antioxidant actions by the assays performed. Our results suggest that the conifers screened may contain some beneficial compounds with cholinesterase inhibitory and antioxidant properties and, therefore, these species deserve a further evaluation, which is progress in our
laboratory.
Conflict of interest statement
We declared that we have no conflict of interest.
Acknowledgements
F.S. Senol would like to extend her genuine gratitude to the Scientific and Technological Research Council of Turkey (TUBITAK) for the scholarship provided her for Ph.D. program.
[1] Orhan G, Orhan I, Sener B. Recent developments in natural and synthetic drug research for Alzheimer's Disease. Lett Drug Design Disc 2006; 3: 268-274.
[2] Terry Jr AV, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 2003; 306: 821-827.
[3] Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. PNAS 2005; 102: 17213-17218.
[4] Orhan N, Orhan IE, Ergun F. Insights into cholinesterase inhibitory and antioxidant activities of five Juniperus species. Food Chem Toxicol 2011; 49: 2305-2312.
[5] Tumen I, Senol FS, Orhan IE. Evaluation of possible in vitro neurobiological effects of two varieties of Cupressus sempervirens through their antioxidant and enzyme inhibitory actions. Turk J Biochem 2012; 37: 283-289.
[6] Ustun O, Senol FS, Kurkcuoglu M, Orhan IE, Kartal M, Baser KHC. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oil of Turkish Pinus species and pycnogenol. Ind Crops Prods 2012; 38: 115-123.
[7] Kucukboyaci N, Orhan I, Sener B, Nawaz SA, Choudhary MI. Assessment of enzyme inhibitory and antioxidant activities of lignans from Taxus baccata L. Z Naturforsch 2010; 65: 187-194.
[8] Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 2010; 69: 155-167.
[9] Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88-95.
[10] Orhan I, Senol FS, Kartal M, Dvorska M, Zemlicka M, Smejkal K, et al. Cholinesterase inhibitory effects of the extracts and compounds of Maclura pomifera (Rafin.) Schneider. Food Chem Toxicol 2009; 47: 1747-1751.
[11] Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181: 1199-1200.
[12] Schlesier K, Harvat M, Bohm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Rad Res 2002; 36: 177-187.
[13] Chua MT, Tung YT, Chang ST. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophleum. Biores Technol 2008; 99: 1918-1925.
[14] Oyaizu M. Studies on products of browning reactions-antioxidative activities of products of browning reaction prepared from glucosamine. Jap J Nutr 1986; 44: 307-315.
[15] Falcioni G, Fedeli D, Tiano L, Calzuola I, Mancinelli L, Marsili V, et al Antioxidant activity of wheat sprouts extract in vitro: Inhibition of DNA oxidative damage. J Food Sci 2002; 67: 2918-2922.
[16] Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolibdic-phosphotungtic acid reagents. Am J Enol Viticult 1965; 16: 144-158.
[17] Woisky R, Salatino A. Analysis of propolis: some parameters and procedures for chemical quality control. J Apicol Res 1998; 37: 99-105.
[18] Kucukboyaci N, Sener B. Biological activities of lignans from Taxus baccata L. growing in Turkey. J Med Plants Res 2010; 4: 1136-1140.
[19] Orhan I, Tosun F, Sener B. Coumarin, anthroquinone and stilbene derivatives with anticholinesterase activity. Z Naturforsch 2008; 63: 366-370.
[20] Ozturk M, Tumen I, Ugur A, Aydogmus-Ozturk F, Topcu G. Evaluation of fruit extracts of six Turkish Juniperus species for their antioxidant, anticholinesterase and antimicrobial activities. J Sci Food Agric 2011; 91: 867-876.
[21] Harborne JB. The flavonoids - Advances in research. London: Chapman and Hall; 1993, p. 460.
[22] Todd FG, Stermitz FR, Blokhin AV. Piperidine alkaloid content of Picea pungens (Colorado blue spruce). Phytochemistry 1995; 40: 401.
[23] Stermitz FR, Kamm CD, Tawara JN. Piperidine alkaloids of spruce (Picea) and fir (Abies) species. Biochem System Ecol 2000; 28: 177-181.
[24] Viegas Jr C, Bolzani VS, Pimentel LSB, Castro NG, Cabral RF, Costa RS, et al. New selective acetylcholinesterase inhibitors designed from natural piperidine alkaloids. Bioorg Med Chem 2005; 13: 4184-4190.
[25] Castro NG, Costa RS, Pimentel LSB, Danuello A, Romeiro NC, Viegas Jr C, et al. CNS-selective noncompetitive cholinesterase inhibitors derived from the natural piperidine alkaloid (-)-spectaline. Eur J Pharmacol 2008; 580: 339-349.
[26] Slimestad R. Flavonoids in buds and young needles of Picea, Pinus and Abies. Biochem System Ecol 2003; 31: 1247-1255.
ment heading
10.1016/S1995-7645(14)60329-1
*Corresponding author: Ilkay Erdogan Orhan, Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, 06330 Ankara, Turkey.
Tel: +90 312 2023186
Fax: +90 312 2235018.
E-mail: iorhan@gazi.edu.tr.
 Asian Pacific Journal of Tropical Medicine2015年4期
Asian Pacific Journal of Tropical Medicine2015年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- A brief review on biomarkers and proteomic approach for malaria research
- Trigonelline protects the cardiocyte from hydrogen peroxide induced apoptosis in H9c2 cells
- Monascus pilosus-fermented black soybean inhibits lipid accumulation in adipocytes and in high-fat diet-induced obese mice
- Antiprotozoal assessment and phenolic acid profiling of five Fumaria (fumitory) species
- Profile and geographical distribution of reported cutaneous leishmaniasis cases in Northwestern Saudi Arabia, from 2010 to 2013
- Change of MicroRNA-134, CREB and p-CREB expression in epileptic rat
