羧酸桥联的四核镧系簇合物的磁热效应和慢磁弛豫
刘遂军 崔雨 宋伟朝 王庆伦 卜显和*,
(1南开大学化学系,金属与分子基材料化学天津市重点实验室,天津化学化工协同创新中心,天津300071)
(2江西理工大学冶金与化学工程学院,赣州341000)
羧酸桥联的四核镧系簇合物的磁热效应和慢磁弛豫
刘遂军1,2崔雨1宋伟朝1王庆伦1卜显和*,1
(1南开大学化学系,金属与分子基材料化学天津市重点实验室,天津化学化工协同创新中心,天津300071)
(2江西理工大学冶金与化学工程学院,赣州341000)
在水热条件下,通过使用羧酸和螯合配体得到了一个系列的四核镧系簇合物,即[Ln4(mnba)12(tzp)2(H2O)2](Ln=Gd(1),Tb (2),Er(3);Hmnba=间硝基苯甲酸;tzp=2-(1H-1,2,4-三唑-3-基)吡啶))。这3个化合物是同构的,且具有线性的四核簇结构。磁性研究表明,化合物1和3中簇内镧系离子之间是弱铁磁耦合的,但化合物2中铽离子之间是弱的反铁磁相互作用和(或)铽离子激发的斯塔克能级的去布居。化合物1具有较大的磁热效应(-ΔSmmax=20.6 J·kg-1·K-1)。交流磁化率测试表明化合物3展现出频率和温度依赖的虚部信号,这是慢磁弛豫的典型特征,原因是铒离子的强各向异性和铁磁耦合的存在。
羧酸;镧系簇合物;磁热效应;慢磁弛豫
0 Introduction
The investigation of lanthanide(Ln)clusters has recently become an active field for their both fascinating structures and exceptional applications as molecular coolers and single-molecule magnets(SMMs)[1-6]. On one hand,GdⅢclusters could be regarded as candidate materials for magnetic refrigerators because of negligible magnetic anisotropy(D),large spin ground state(S)and low-lying excited spin states of GdⅢion and weak couplings between GdⅢions[7-11].Generally, the entropy change(-ΔSm)is employed to represent the magnetocaloric effect(MCE)of molecular magnetorefrigerants[12-13].The magnetic intensity(Mw/NGd)and magnetic interaction(θ)between GdⅢions are proposed to be main factors to affect MCE for the Gd-type magnetic refrigerants[14-16].To reduce Mw/NGdratio and |θ|value of GdⅢcomplexes,the utilization of light ligands to synthesize GdⅢclusters supply an effective tool.
On the other hand,Ln-based SMMs with large energy barriers have been a hot research topic for molecular magnets compared with 3d/3d-4f based SMMs[17-19].LnⅢclusters(especially for TbⅢ,DyⅢ,HoⅢand ErⅢtypes)have recently become favorable candidates to explore SMMs,since the S and D of LnⅢions could lead to an relatively large anisotropic energy barrier(Ueff)that prevents the reversal of the molecular magnetization[20-21].
Till now,most of Ln-clusters were constructed from Schiff-base and calix[4]arenes ligands with their chelating characteristic[22-25].The mixed-ligand strategy, especially the utilization of carboxylates and N-donor ligands,has been employed to construct discrete clusters and coordination polymers as a powerful synthetic approach,while the design and synthesis of discreteLn-clusterswithuniquestructuresand magnetic properties still remain a great challenge because of different affinities and coordination capabilities of the LnⅢions to O-donors and N-donors[26-27].
As an extension of our studies on the synthesis andmagneticinvestigationofLnⅢcomplexes[28-31],herein, we choose sterically hindered Hmnba(m-nitrobenzoic acid)andcornerligands2-(1H-1,2,4-triazol-3-yl) pyridine)(tzp)to construct low-dimensional structures (Scheme 1).Fortunately,a series of tetranuclear LnⅢclusters,namely[Ln4(mnba)12(tzp)2(H2O)2](Ln=Gd(1), Tb(2)and Er(3))were successfully synthesized.
Scheme 1Ligands used for the synthesis of 1~3
Magnetic analyses reveal that complex1 is weakly ferromagnetic coupled with-ΔSmmax=20.6 J·kg-1·K-1for ΔH=7 T at 2.0 K and complex 3 displays slow relaxation of the magnetization.Strong quantum tunnellingeffectexcludestheexistenceofslow magnetic relaxation for 2 although 2 kOe dc field was exerted.
1 Experimental
1.1Materials and instrumentation
All chemicals were of reagent grade and used as purchasedwithoutfurtherpurification.Elemental analysis(C,H and N)was performed on a Perkin-Elmer 240C analyzer(Perkin-Elmer,USA).The X-ray powder diffraction(PXRD)spectra were recorded on a Rigaku D/Max-2500 diffractometer at 60 kV,300 mA for a Cu-target tube and a graphite monochromator. Simulation of the PXRD spectra were carried out by the single-crystal data and diffraction-crystal module of the Mercury(Hg)program available free of charge via the Internet at http://www.iucr.org.IR spectra were measured in the range of 400~4 000 cm-1on a Tensor 27 OPUS FT-IR spectrometer using KBr pellets (Bruker,German).Magnetic data were measured by a Quantum Design MPMS-XL-7 SQUID magnetometer. Diamagnetic correctionswereestimatedbyusing Pascalconstantsandbackgroundcorrectionsby experimental measurement on sample holders.
1.2Preparation of 1~3
[Gd4(mnba)12(tzp)2(H2O)2](1):A mixture of Gd2O3(181 mg,0.5 mmol),Hmnba(334 mg,2 mmol)and tzp(66.6 mg,0.5 mmol)in 10 mL H2O was sealed in a Teflon-lined autoclave and heated to 160℃for 2days.Aftertheautoclavewascooledtoroom temperature in 12 h,Cubic colorless crystals were collected with 30%yield based on GdⅢ.Anal.Calcd. for C98H64O50N20Gd4(%):C,39.89;H,2.19;N,9.49. Found(%):C,39.78;H,2.78;N,9.35.IR(KBr,cm-1): 3579m,3504m,3161m,3087s,2 901m,2 765w,1580 s,1 483s,1 344s,1 263s,1 166m,1 080s,995w,912m, 831m,788s,725s,651m,576w,523w,416m.
[Tb4(mnba)12(tzp)2(H2O)2](2):The same procedure as that for 1 was used for this complex except that Gd2O3(181 mg,0.5 mmol)was replaced by Tb2O3(183 mg,0.5 mmol)and the holding time is 3 days.Block colorless crystals were collected with~40%yield based on TbⅢ.Anal.Calcd.for C98H64O50N20Tb4(%):C, 39.80;H,2.18;N,9.47.Found(%):C,40.13;H,2.89; N,9.60.IR(KBr,cm-1):3494w,3157w,3080m,2893 m,2767w,1598s,1517s,1415s,1350s,1266m,1082 m,995w,910w,790m,723s,649w.
[Er4(mnba)12(tzp)2(H2O)2](3):The same procedure as that for 1 was used for this complex except that Gd2O3(181 mg,0.5 mmol)was replaced by Er2O3(191 mg,0.5 mmol)and the holding time is 3 days.Block pink crystals were collected with~30%yield based on ErⅢ.Anal.Calcd.for C98H64O50N20Er4(%):C,39.36; H,2.16;N,9.37.Found(%):C,39.64;H,2.78;N, 9.43.IR(KBr,cm-1):3496w,3163w,3083m,2891w, 1 608s,1 525s,1 478s,1 410s,1 346s,1 267m,1 166w, 1 079m,1 001w,909w,830w,719s,650m,584w, 518w,413w.
1.3Crystallographic data and structure refinements
The single-crystal X-ray diffraction data of 1~3 were collected on a Rigaku SCX-mini diffractometer at 293(2)K with Mo Kα radiation(λ=0.071 073 nm) by ω scan mode.The program CrystalClear[32]was used for the integration of the diffraction profiles.The structures were solved by direct method using the SHELXS program of the SHELXTL package and refined by full-matrix least-squares methods with SHELXL[33].The non-hydrogen atoms were located in successive difference Fourier syntheses and refined with anisotropic thermal parameters on F2.All hydrogen atoms of ligands were generated theoretically at the specific atoms and refined isotropically with fixed thermal factors.The hydrogen atoms of water in 1~3 were added by the difference Fourier maps and refined with suitable constrains.A summary of the crystallographic data,data collection,and refinement parameters for 1~3 is provided in Table 1.
CCDC:978830,1;978831,2;978832,3.

Table 1Crystal data and structure refinements for 1~3

Continued Table 1
2 Results and discussion
2.1Synthesis
The mix-ligand strategy has been employed to constructthelinearLn-clusterssuccessfully.As effective terminal co-ligands,tzp plays a key role in theformationofdiscretelanthanideclusters. Compared with lanthanide salts,the use of Ln2O3provides not only a slow-release LnⅢion source but also a pH regulator of the reactions.
2.2Description of crystal structures
Single crystal X-ray analysis reveals that 1~3 are isostructural,thus only the structure of 1 is described in detail.Complex 1 crystallizes in the triclinic space group P1 with the asymmetric unit including two GdⅢions,six mnba ligands,one tzp ligand and one coordinated H2O.Gd1 is located in a seven coordinated environment constructed with seven O atoms from one water molecule and six carboxylates.Gd2 center exhibits an eight-coordinated environment with two N atoms of one tzp ligand and six carboxylate O atoms from five mnba ligands.Gd1 and Gd2 are connected by four carboxylate groups with syn,syn-μ2-η1∶η1to form a linear Gd4cluster(Fig.1).
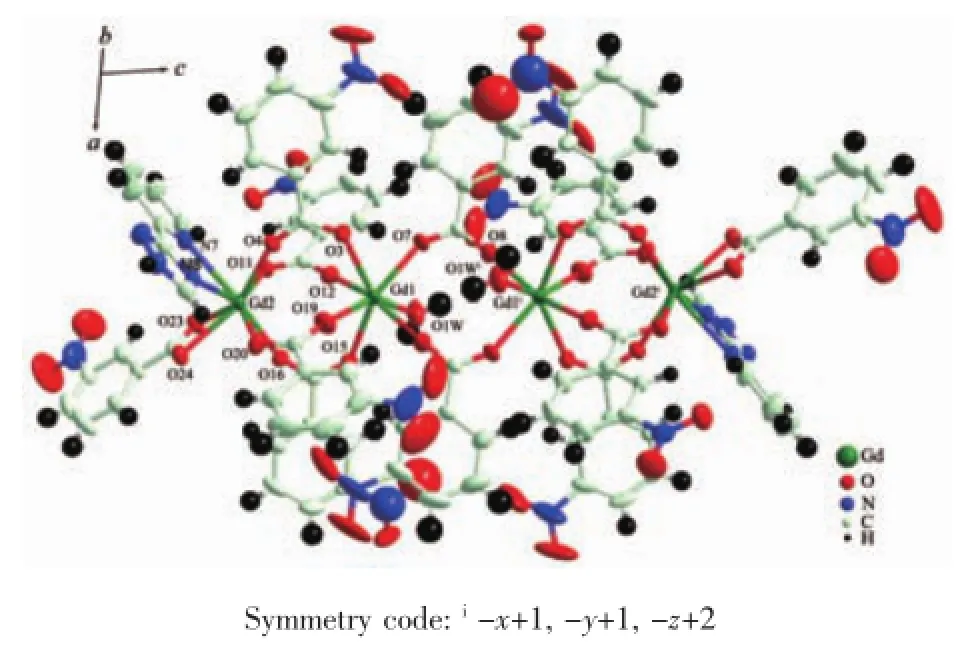
Fig.1 View of the molecular structure showing 30% probability thermal ellipsoids of 1
2.3Magnetic studies
The magnetic properties of 1~3 were studied by solid state magnetic susceptibility measurements in 2.0~300 K range at 1 kOe dc field and the isothermal field-dependent magnetizations M(H)at fields up to 70 kOe at 2.0 K.Before the magnetic measurements of 1~3,their crushed crystalline samples were used to measure X-ray powder diffraction(PXRD,Fig.S1,SI) to confirm their phase purities.

Fig.2 Plots of χMT vs T for 1~3
Complex 1 contains isotropic GdⅢ(f7)with a ground state8S7/2,and the first excited state6P7/2is very high in energy,while complexes 2 and 3 include other anisotropic LnⅢions.Generally,the magnetism of lanthanide(Ln)clusters is very difficult to explain because of the exchange-coupling and large orbital contributions as well as the crystal field perturbation[34-35].The magnetic properties in the form of χMT vs T plots of 1~3 are shown in Fig.2.The room-temperature χMT products estimated as 31.45(1),46.39(2)and 47.96 (3)emu·mol-1·K are in relative good agreement with the presence of four lanthanide metal ions:four GdⅢions(S=7/2,L=0,J=7/2,g=2,C=7.88 emu·mol-1·K) for 1,four TbⅢions(S=3,L=3,J=6,g=3/2,C=11.82 emu·mol-1·K)for 2 and four ErⅢions(S=3/2,L=6,J=15/2,g=6/5,C=11.48 emu·mol-1·K)for 3.For 1,as the temperature decreases,the χMT value stays nearly constant in the high temperature range with a value of 30.86 emu·mol-1·K at 14 K.Upon further cooling the temperature to 2.0 K,χMT abruptly increases to a maximum value(32.31 emu·mol-1·K,indicating the ferromagnetic(F)interaction between GdⅢions in the Gd4cluster.For 2,up lowering of the temperature to 2.0 K,χMT value stays nearly constant at high temperatures,and then decreases sharply to a minimum value(28.62 emu·mol-1·K),which indicates weak antiferromagnetic(AF)interaction in the Tb4cluster and/or depopulation of the TbⅢexcited Stark sublevels.The Stark sub-levels of the anisotropic TbⅢions may be progressively thermally depopulated leading to a decrease of the χMT value.For 3,as the temperature decreases,the value ofχMT slowly decreases down to a minimum value of 39.96 emu·mol-1·K at 4.5 K.On cooling the temperature to 2 K,χMT abruptly increases to the maximum value(41.17 emu·mol-1·K), indicating ferromagnetic coupling between ErⅢions in the Er4cluster.
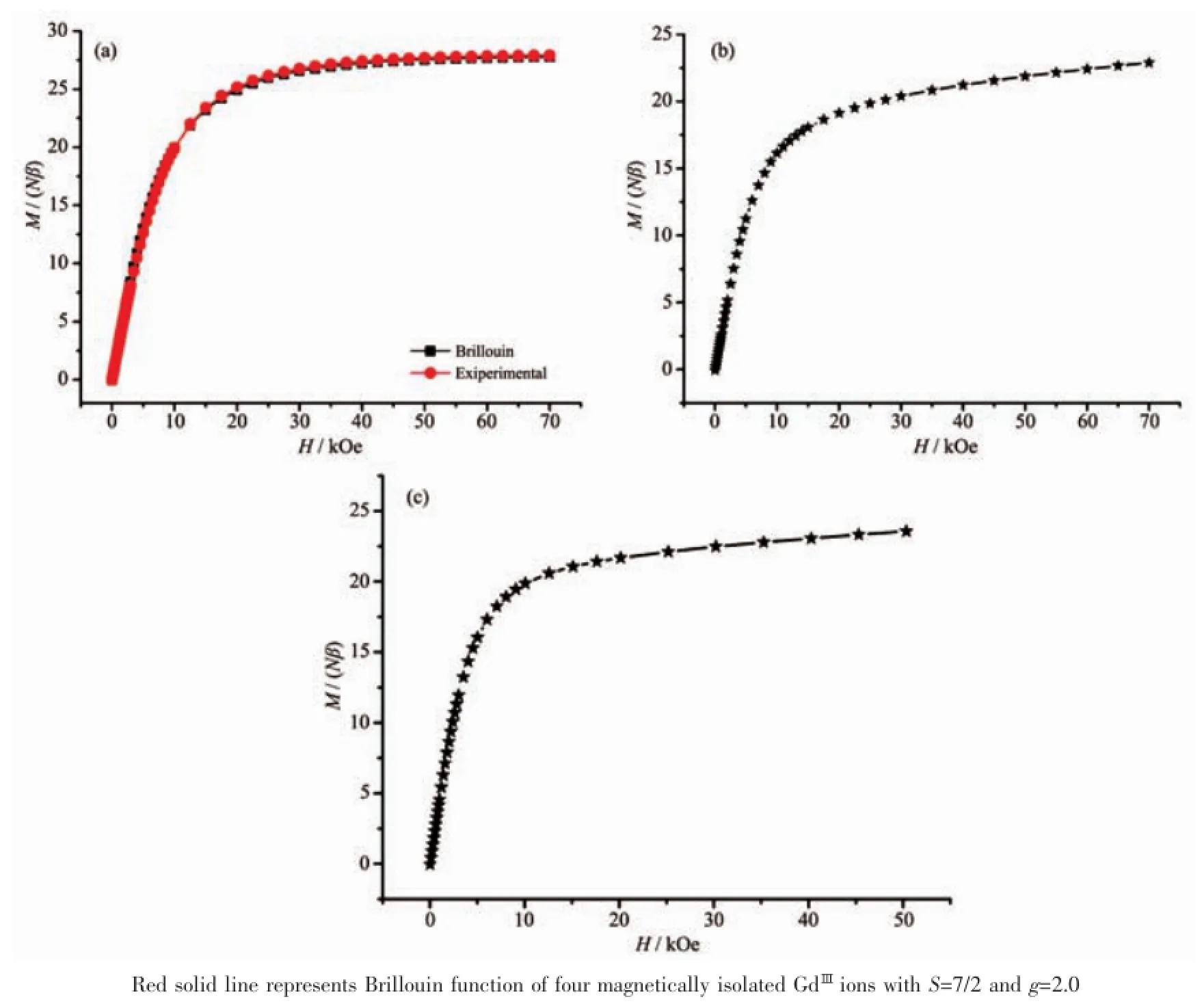
Fig.3 M vs H curves of 1(a),2(b)and 3(c)at 2.0 K
The magnetizations slowly increase and tend to a value of 27.77Nβ at 70 kOe,and the experimental magnetization plot of 1 is nearly consistent with the red line that presents the Brillouin function for four magnetically uncoupled GdⅢions with S=7/2 and g= 2.0,which further confirms the weak F behavior for 1 as similar literatures[3-4](Fig.3a).The field dependences of the magnetizations at 2.0 K for 2 and 3 show rapid increases of the magnetizations at low fields,reaching about 16.13Nβ and 19.87Nβ at 10 kOe,and linear increases at high fields without achieving a complete saturation at 70 or 50 kOe(22.90Nβ for 2 and 23.57 Nβ for 3,Fig.3b and 3c),which could be explained by the fact that the depopulation of the Stark levels of the LnⅢ2S+1LJground state under the ligand-field perturbation produces a much smaller effective spin.For 2 and 3,the M vs H/T(Fig.4)data at 2~4 K shows non-superposition plots and a rapid increase of the magnetization at low fields without any sign of saturation at 50 kOe.The reason is most likely because of anisotropy and important crystal-field effect at the TbⅢor ErⅢions,which eliminates the degeneracy of the7F6and4I15/2ground states.Reduced magnetization curves do not superimpose,further indicating the presence of a significant magnetic anisotropy and/or low lying excited states[15].
Therecentlydevelopednon-criticalscaling theory could be used to study the F/AF behaviors of 2 and 3 based on the sum of two exponential functions (as shown in Eq.1)[36-38].
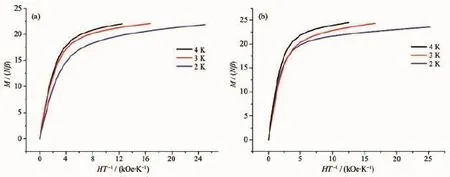
Fig.4 Curves of M vs H/T for 2(a)and 3(b)

In Eq.1,A+B is the high-temperature extrapolated Curie constant andE1andE2denotethe magnitude of the intracluster magnetic interaction. The first term in Eq.1 represents an F/AF contribution to the moment that is dominant at low temperatures, whereas the second term reflects the crystal-field effect because the interionic interactions between the internal 4f electrons are usually very weak.The best fit of the experimental data gives that A+B=47.22 emu ·mol-1·K,E1=-0.61 K and E2=-20.94 K for 2 and A+ B=48.54 emu·mol-1·K,E1=0.08 K and E2=-18.12 K for 3(Fig.2).The small values of E1of 2 and 3 further indicate very weak magnetic interactions between the TbⅢ/ErⅢions,which is in good agreement with the prediction that the Ln-Ln interaction is expected to be very weak,due to the shielding of the f-orbitals and the consequent poor overlap with the bridging ligand orbitals[12].
To characterize the low-temperature behaviors of 1,the temperature dependencies of field-cooled(FC) andzero-field-cooled(ZFC)magnetizationwere measured under a field of 50 Oe upon warming from 2.0 K(Fig.5).The FC curve coincides with the ZFC curve and the magnetizations increase monotonically with the decrease of temperature,and no maximum is observed.These results indicate that 1 does not exhibit magnetic ordering above 2.0 K.

Fig.5 FC/ZFC curves at 2~30 K for 1
Considering the weak magnetic couplings between the GdⅢions and potential application of GdⅢcomplexes for magnetic refrigeration,we investigated the magnetocaloric properties of 1.We used the magnetic entropy change(ΔSm)to evaluate MCE,which could be calculatedbytheMaxwellequation(ΔSm(T)ΔH=∫[∂M(T,H) /∂T]HdH)[39-41].According to the equation,we could obtain the-ΔSmfrom the experimental magnetization data(Fig.6a),and the curves of-ΔSmare depicted in Fig. 6b.The obtained-ΔSmmaxgives the value of 20.6 J·kg-1·K-1(the theoretical-ΔSmmaxis 23.4 J·kg-1·K-1calculated with-ΔSmmax=4Rln(2S+1),R is the gas constant) for a field change of 7 T at 2.0 K.If-ΔSmmaxis given per unit of volume,it is equivalent to 36.96 mJ·cm-3· K-1.Although various discrete GdⅢclusters have been constructed,theirmagnetocaloricpropertieshave rarely been reported.Previous literatures report only twelve GdⅢclusters with significant MCE(-ΔSmmax>20 J·kg-1·K-1),as shown in Table 2.

Fig.6 (a)M vs H curves of 1 at T=2~10 K and H=2.5~70 kOe;(b)Experimental-ΔSmobtained from magnetization data of 1 at different fields and temperatures

Table 2Comparison of-ΔSmmax(larger than 20.0 J·kg-1·K-1)among 1 and GdⅢclusters associated with potential molecule-based magnetic coolers*
To investigate possible SMM behaviors of 2 and 3,alternating current(ac)susceptibility measurements were carried out in the temperature range of 15~2.0 K under Hdc=0 Oe and Hac=3.5 Oe for variable frequencies (from 1 488 to 10 Hz).Unfortunately,although all the in-phasecurves(χ′)are almost consistent without peaks, there is no frequency dependent out-of-phase signal even up to 997 Hz(Fig.S2a and S2c).In order to weaken the quantum tunneling effect,2 kOe dc field were applied to further study the dynamic properties. The ac signal of 2 is still poor and slow magnetic relaxation is not observed(Fig.S2b),while there is weak frequency dependent out-of-phase signal for 3(Fig.S2d).Then 5 kOe dc field was exerted and attempted to obtain better ac signals.Therefore,the peaks can be observed obviously both in χM′and χM″curves(see Fig.7),which suggested the existence of slow magnetic relaxation behavior in 3.As aforementioned,strong anisotropy of ErⅢions and weak ferromagnetic interaction presumably lead to the fieldinduced slow magnetic relaxation behavior in 3.

Fig.7 Temperature dependence of the ac χMat different frequencies for 3 with Hdc=5 kOe
3 Conclusions
A type of linear tetranuclear lanthanide clusters (1~3)constructed fromthemonocarboxylate and terminal co-ligand has been synthesized in hydrothermal reactions.Magnetic investigation indicates that 1~3 are weakly coupled with 1 displaying large MCE with-ΔSmmax=20.6 J·kg-1·K-1and 3 exhibiting slow magnetic relaxation behavior for the strong anisotropy and ferromagnetic contribution.Complex 2 does not show slow magnetic relaxation behavior because of weak antiferromagneticinteractionandstrongquantum tunneling effect,although 2 kOe dc field was exerted.
References:
[1]Zheng Y Z,Zhou G J,Zheng Z,et al.Chem.Soc.Rev.,2014, 43:1462-1475
[2]Sharples J W,Collison D.Polyhedron,2013,54:91-103
[3]Woodruff D N,Winpenny R E P,Layfield R A.Chem.Rev., 2013,113:5110-5148
[4]Liu J L,Chen Y C,Guo,F S,et al.Coord.Chem.Rev., 2014,281:26-49
[5]Rinehart J D,Long J R.Chem.Sci.,2011,2:2078-2085
[6]Habib F,Murugesu M.Chem.Soc.Rev.,2013,42:3278-3288
[7]Wang P,Shannigrahi S,Yakovlev N L,et al.Chem.Asian J.,2013,8:2943-2946
[8]Liu S J,Zhao J P,Tao J,et al.Inorg.Chem.,2013,52:9163-9165
[9]Sharples J W,Zheng Y Z,Tuna F,et al.Chem.Commun., 2011,47:7650-7652
[10]Chang L X,Xiong G,Wang L,et al.Chem.Commun.,2013, 49:1055-1057
[11]Guo F S,Chen Y C,Mao L L,et al.Chem.Eur.J.,2013, 19:14876-14885
[12]Guo F S,Leng J D,Liu J L,et al.Inorg.Chem.,2012,51: 405-413
[13]Evangelisti M,Roubeau O,Palacios E,et al.Angew.Chem. Int.Ed.,2011,50:6606-6609
[14]Liu S J,Xie C C,Jia J M,et al.Chem.Asian J.,2014,9: 1116-1122
[15]Lorusso G,Sharples J W,Palacios E,et al.Adv.Mater., 2013,25:4653-4656
[16]Han S D,Miao X H,Liu S J,et al.Inorg.Chem.Front., 2014,1:549-552
[17]Blagg R J,Muryn C A,McInnes E J L,et al.Angew.Chem. Int.Ed.,2011,50:6530-6533
[18]Gao F,Cui L,Liu W,et al.Inorg.Chem.,2013,52:11164-11172
[19]Guo Y N,Xu G F,Wernsdorfer W,et al.J.Am.Chem. Soc.,2011,133:11948-11951
[20]Jiang S D,Liu S S,Zhou L N,et al.Inorg.Chem.,2012,51: 3079-3087
[21]Jiang S D,Wang B W,Sun H L,et al.J.Am.Chem.Soc., 2011,133:4730-4733
[22]Rell N M,Anwar M U,Drover M W,et al.Inorg.Chem., 2013,52:6731-6742
[23]Anwar M U,Thompson L K,Dawe L N,et al.Chem. Commun.,2012,48:4576-4578
[24]Liu C M,Zhang D Q,Hao X,et al.Cryst.Growth Des., 2012,12:2948-2954
[25]Xu X,Zhao L,Xu G F,et al.Dalton.Trans.,2011,40:6440-6444
[26]Liu S J,Zhao J P,Song W C,et al.Inorg.Chem.,2013,52: 2103-2109
[27]Sessoli R,Powell A K.Coord.Chem.Rev.,2009,253:2328-2341
[28]Jia J M,Liu S J,Cui Y,et al.Cryst.Growth Des.,2013,13: 4631-4634
[29]Liu S J,Zeng Y F,Xue L,et al.Inorg.Chem.Front.,2014, 1:200-206
[30]Han S D,Miao X H,Liu S J,et al.Chem.Asian J.,2014,9:3116-3120
[31]Miao X H,Han S D,Liu S J,et al.Chin.Chem.Lett.,2014, 25:829-834
[32]Rigaku.CrystalClear,Process-AutoRigakuAmericas Corporation,The Woodlands,Texas,1998.
[33]Sheldrick G M.SHELXL97,Program for Crystal Structure Refinement,University of Göttingen,Göttingen,Germany, 1997.
[34]Wu M F,Wang M S,Guo S P,et al.Cryst.Growth Des., 2011,11:372-381
[35]Kahn M L,Sutter J P,Golhen S,et al.J.Am.Chem.Soc., 2000,122:3413-3421
[36]Zheng Y Z,Lan Y H,Wernsdorfer W,et al.Chem.Eur.J., 2009,15:12566-12570
[37]Souletie J,Rabu P,Drillon M.Phys.Rev.B,2005,72:214427 [38]Drillon M,Panissod P,Rabu P,et al.Phys.Rev.B,2002, 65:104404
[39]Zheng Y Z,Evangelisti M,Winpenny R E P.Angew.Chem. Int.Ed.,2011,50:3692-3695
[40]Zheng Y Z,Pineda E M,Helliwell M,et al.Chem.Eur.J., 2012,18:4161-4165
[41]FAN Shao-Yong(范少勇),HUANG Ke-Di(黄科棣),TANG Qing-Mei(唐清美),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30:1167-1173
[42]Peng J B,Kong X J,Zhang Q C,et al.J.Am.Chem.Soc., 2014,136:17938-17941
[43]Adhikary A,Jena H S,Biswas S,et al.Chem.Asian J., 2014,9:1083-1090
[44]Adhikary A,Sheikh J A,Biswas S,et al.Dalton Trans., 2014,43:9334-9343
[45]Sheikh J A,Adhikary A,Konar S.New J.Chem.,2014,38: 3006-3014
Carboxylate-Bridged Tetranuclear Lanthanide Clusters: Magnetocaloric Effect and Slow Magnetic Relaxation
LIU Sui-Jun1,2CUI Yu1SONG Wei-Chao1WANG Qing-Lun1BU Xian-He*,1
(1Department of Chemistry,TKL of Metal-and Molecule-Based Material Chemistry and Collaborative Innovation Center of Chemical Science and Engineering(Tianjin),Nankai University,Tianjin 300071,China)
(2School of Metallurgical and Chemical Engineering,Jiangxi University of Science and Technology,Ganzhou,Jiangxi 341000,China)
By using carboxylate and chelating ligands,a family of tetranuclear lanthanide clusters,namely [Ln4(mnba)12(tzp)2(H2O)2](Ln=Gd(1),Tb(2)and Er(3),Hmnba=m-nitrobenzoic acid,tzp=2-(1H-1,2,4-triazol-3-yl) pyridine),has been obtained under hydrothermal conditions.The three complexes exhibit linear tetranuclear clusters bridged by carboxylates with syn,syn-μ2-η1∶η1mode.Magnetic investigation indicates weak ferromagnetic interaction between adjacent GdⅢor ErⅢions of the Ln4cluster in 1 and 3,while weak intra-molecular antiferromagnetic interaction between TbⅢions and/or depopulation of the TbⅢexcited Stark sub-levels in 2. Complex 1 exhibits a significant magnetocaloric effect with-ΔSmmax=20.6 J·kg-1·K-1and ac susceptibility measurements reveal frequency-and temperature-dependent out-of-phase signal under 5 kOe dc field in 3,being typical slow magnetic relaxation behavior due to strong anisotropy of ErⅢand ferromagnetic coupling.CCDC: 978830,1;978831,2;978832,3.
carboxylate;lanthanide clusters;magnetocaloric effect;slow magnetic relaxation
O614.33+9;O614.341;O614.344
A
1001-4861(2015)09-1894-09
10.11862/CJIC.2015.240
2015-06-08。收修改稿日期:2015-07-22。
国家自然科学基金(No.21290171),江西省科技厅青年自然科学基金(No.20151BAB213003)资助项目。
*通讯联系人。E-mail:buxh@nankai.edu.cn

