基于四甲基取代六元瓜环的四核稀土镝簇合物的慢磁弛豫
陈文建 孔祥建 龙腊生 郑兰荪
(固体表面物理化学国家重点实验室,厦门大学化学化工学院化学系,厦门361005)
基于四甲基取代六元瓜环的四核稀土镝簇合物的慢磁弛豫
陈文建孔祥建*龙腊生*郑兰荪
(固体表面物理化学国家重点实验室,厦门大学化学化工学院化学系,厦门361005)
报道了2个基于四甲基取代六元瓜环的三明治型四核稀土簇合物,[Ln4(μ3-OH)4(μ2-OH)2(H2O)4(NO3)2(TMeQ[6])2]·(NO3)4·26H2O(Ln=Dy,1;Ln=Tb,2)。晶体结构分析显示2个簇合物包含2个四甲基取代瓜环夹心的四核稀土立方烷结构,[Ln4(μ3-OH)4]8+。磁性研究显示化合物1显示了慢磁弛豫行为。由于六元瓜环配体可以有效的传递能量给稀土铽离子,化合物2具有较好的发光性能。
簇合物;磁性;发光;六元瓜环
0 Introduction
Single-molecule magnets(SMMs)continue to be an inviting research field in recent decades,not only because of their intriguing properties,but also their potential applications in quantum computing[1],magnetic information storage[2],nanoelectrons[3],and molecular spintronics[4-5].Since the first SMM of[Mn12O12(AcO)16(H2O)4]appearance in the early 1990s[6],a large number of transition-metal polynuclear compounds with SMMs property have been reported.
It was found that high spin lanthanide ions are good candidates for constructing new SMMs,due to their large intrinsic magnetic anisotropy[8-11].However, because of the synthetic challenges and the difficulty in promoting magnetic interactions via connecting by bridging ligands,rational design and assembly of pure lanthanide based SMMs remain a challenge[9-11]. Investigations on the lanthanide cluster-based molecular magnetism suggest that selecting appropriate bridgingligand is crucial to the construction of pure lanthanide -based SMMs.So far,a number of organic ligands, such as aminoacids[11-12],o-vanillin[8b],Schiff bases[13], carboxylates[14],β-diketones[15],and calixarenes[9,16]have been used to construct lanthanide SMMs,among which Schiff base based on o-vanillin[8b,10b,17]has been most studied.
Cucurbit[n]urils(Q[n]s,Fig.1a)and their alkylsubstituted derivatives have proved to be an excellent class of both ligands and organic building blocks due to the two opening portals of these macrocycles with its unique cavity rimmed with π-rich dipole carbonyl groups[18-21].Although a large number of Q[n]s supported transition metal-containing[22]and lanthanide-containing coordination compounds have been reported[23],Q[n]s supported polynuclear lanthanide SMMs are rare[24]. Herein we report a TMeQ[6](Fig.1b)supported Dy4cluster complex,formulated as[Dy4(μ3-OH)4(μ2-OH)2(H2O)4(NO3)2(TMeQ[6])2]·(NO3)4·26H2O(1),which features a disordered[Dy4(μ3-OH)4]8+cubane cluster coresandwichedbytwoTMeQ[6]macrocycles. Alternating current susceptibility measurements reveal that the compound 1 exhibits slow relaxation of magnetization.To the best of our knowledge,this is the first example of a TMeQ[6]supported lanthanide cluster with slow relaxation of magnetization.We also obtain its Tb3+analogue[Tb4(μ3-OH)4(μ2-OH)2(H2O)4(NO3)2(TMeQ [6])2](NO3)4·26H2O(2),which exhibitsinteresting luminescent property.

Fig.1 Molecular structure of Q[n]s(a)and TMeQ[6](b)
1 Experimental
1.1Materials and methods
All reagents were of commercial origin with 99% purity and were used as received.TMeQ[6]was prepared by procedures reported elsewhere[19e].The C, H and N microanalyses were carried out with a CE instruments EA 1110 elemental analyzer.TGA curve was obtained on a SDT Q600 thermal analyzer. Magnetic susceptibility was measured by a Quantum Design MPMS superconducting quantum interference device(SQUID).
1.2Synthesis
1.2.1[Dy4(μ3-OH)4(μ2-OH)2(H2O)4(NO3)2(TMeQ[6])2] (NO3)4·26H2O(1)
TMeQ[6](0.138 g,0.125 mmol),Dy(NO3)3·5H2O (0.484 g,1.00 mmol)and 1H-[3-(4-pyridyl)pyrazole]-acetic acid(0.104 g,0.50 mmol)were dissolved in 40.0 mL of water while stirring at 70℃.The mixture was heated to 100℃and refluxed for 2 h.The filtrate was left to stand at room temperature in an open beaker(50 mL).After six days,colorless crystals of 1 were obtained and collected in a yield of 36%on the basis of TMeQ[6].Anal.Calcd.for 1(%):C,25.48;H, 4.12;N,20.06.Found(%):C,25.58;H,4.22;N, 20.12.
1.2.2[Tb4(μ3-OH)4(μ2-OH)2(H2O)4(NO3)2(TMeQ[6])2] (NO3)4·26H2O(2)
This compound was prepared using the same procedure as described above for the synthesis of its Dy cognate,but using 0.481 g Tb(NO3)3·5H2O(1.00 mmol)instead of Dy(NO3)3·5H2O.Colorless crystals of 2 were obtained after a week and collected in a yield of 40%on the basis of TMeQ[6].Anal.Calcd.for 2 (%):C,25.58;H,4.13;N,20.14.Found(%):C,25.66; H,4.20;N,20.22.
1.2.3Single-Crystal X-ray structure determination
Data collections were performed on a Bruker Apex-2000diffractometerusinggraphite monochromated Mo Kα radiation(λ=0.071 073 nm)at 173 K.Absorption corrections were applied using the ultiscan program SADABS[25].The structures were solved by indirect methods(SHELXTL Version 5.10)[25]. Non-hydrogen atoms were refined anisotropically by full-matrix least-squares method on F2.The hydrogen atoms of the organic ligand were generated geometrically(C-H,0.096 nm).Because of severe disorder, 21 water molecules and 3NO3-in the unit cell have been taken into account by the SQUEEZE.Details of the crystal parameters,data collection conditions and refinement parameters for compounds 1 and 2 aresummarized in Table 1.
CCDC:929607,1;929608,2.

Table 1Crystal Data and Structure Refinement Details for Compounds 1 and 2
2 Results and discussion
2.1Synthesis
The reaction of TMeQ[6],Ln(NO3)3,and 1H-[3-(4-pyridyl)pyrazole]-acetic acid(1∶8∶4)in distilled water produces 1(Ln=Dy)or 2(Ln=Tb)in good yields. Originally,we intent to synthesize rare-earth-metal coordination polymers that conatin 1H-[3-(4-pyridyl) pyrazole]acetic acid ligand and TMeQ[6].However, the experimental results show that 1H-[3-(4-pyridyl) pyrazole]-acetic acid is not coordinated with rare earth ions in complexes 1 and 2.The ligand of 1H-[3-(4-pyridyl)pyrazole]-aceticacidisnecessaryforthe reactions,although it is not incorporated into the structures of 1 and 2.Absence of 1H-[3-(4-pyridyl) pyrazole]-acetic acid would result in the formation of an one-dimensional chain comprising TMeQ[6]molecules and[Ln(H2O)8]3+with a 1∶1 ratio through hydrogen bonding(Fig.S1).The ligand may play the role of controlling the hydrolysis of the rare earth ions to limitthedegreeofaggregationofthehydroxo intermediates.
2.2Description of crystal structures
Complexes 1 and 2 are isomorphous,so only the structure of 1 is described in detail.X-ray Singlecrystal structure analysis reveals that complex1 crystallizes in triclinic,P1 space group.As shown in Fig.2,complex 1 possesses a sandwich structure of [Dy4(μ3-OH)4(μ2-OH)2(H2O)4(NO3)2(TMeQ[6])2]4+unit,4 nitrate anions and 26 guest water molecules.It shouldbe noted that all dysprosium ions and μ3-OH atoms have an occupancy factor of 50%,owing to positional disorder,similar to that reported disordered Ln cluster cores[16a-b].
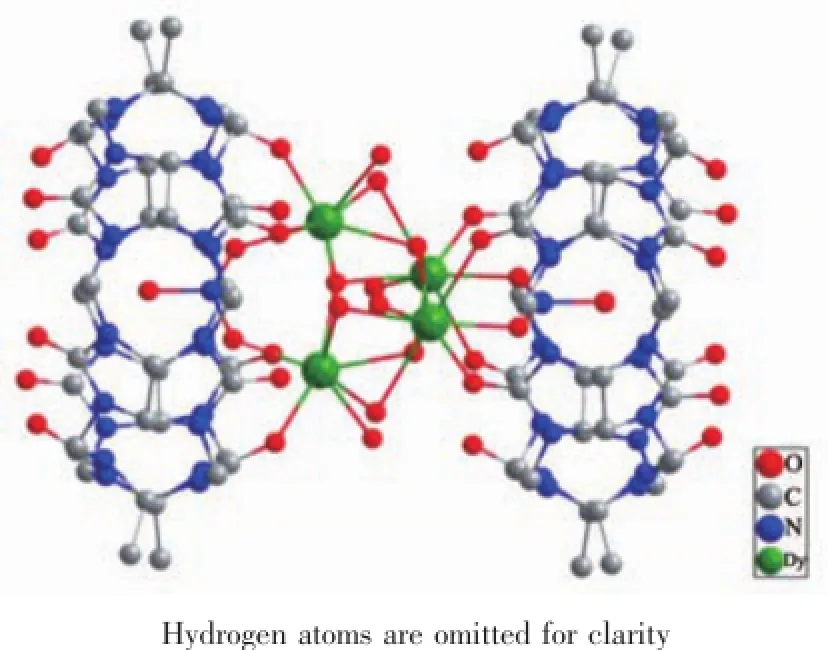
Fig.2 Crystal structure of[Dy4(μ3-OH)4(μ2-OH)2(H2O)4(NO3)2(TMeQ[6])2]4+units in 1
The cationic cluster of 1 has a distorted cubaneshaped core of[Dy4(μ3-OH)4]8+.Four Dy atoms form a nearly perfect tetrahedron.Two opposite edges of the tetrahedron are further bridged by two μ2-OH anions, while another two opposite edges are bridged by two NO3-anions(Fig.3a).This[Dy4(μ3-OH)4]8+cubane cluster core is sandwiched by two TMeQ[6]macrocycles displaying an antiparallel orientation.As shown in Fig.3b,each of Dy ion locates in the center of a square antiprism geometry and is octa-coordinated with contributions from three μ3-OH,one μ2-OH anion, two O atoms from a TMeQ[6]ligand,one O atom from nitrate anion,and one terminal aqua ligands.The bond lengths of Dy-O rang from 0.195 4(12)to 0.271 8(9)nm (Table S1),comparable to those in the reported complexes containing the same[Dy4(μ3-OH)4]8+core[10-11,17b]. The Dy-Ohydroxy-Dy angles in the[Dy4(μ3-OH)4]8+cubane of 1 are in the range of 99.8(5)°~113.6(7)°obviously larger than 99°[10c].
Complex 2 is isomorphic to 1.The bond lengths of Tb-O range from 0.210 0(5)to 0.269 0(4)nm and the angles of Tb-Ohydroxy-Tb range from 99.7(2)°to 113.5(4)°(Table S2),comparable to those in reported Tb complex[24b,26].

Fig.3 (a)Structure of the[Dy4(μ3-OH)4(μ2-OH)2(NO3)2]4+core unit of 1;(b)the coordination geometry of Dy ion in 1
2.3TG analysis
The thermogravimetric(TG)curve of the two complexes under N2atmosphere are shown in Fig.4.1 exhibitsthefirstweightlossof12%inthe temperature range from 24 to 125℃,corresponding to the weight loss of 26 lattice water molecules in 1 (Calculated weight loss 12%),and the second weight loss of 2%in the temperature range from 125 to 335℃corresponding to the loss of four coordination water molecules in 1(Calculated weight loss 2%),and then themetal-organiccomplexstartstodecompose accompanying loss of organic ligands.The TGA curve of complex 2 is similar to that of complex 1,the first and second weight loss of complex 2 are 12%(Calcd. 12%)and 2%(Calcd.2%),corresponding to the weight loss of 26 lattice water molecules and 4 coordination water molecules,respectively.Thesimulatedand experimental PXRD patterns for complex 1 and 2 areare almost identical as indicated in Fig.S10 and Fig. S11.

Fig.4 Thermogram of 1 and 2 showing TGA at the heating rate of 10℃·min-1
2.4Magnetic properties
The temperature dependence of direct-current (dc)magnetic susceptibility of crushed crystalline sample of 1 and 2 were carried out in an applied magnetic field of 1 000 Oe in the temperature range of 2~300 K.As shown in Fig.5,the observed χMT value of 1 is 55.78 cm3·mol-1·K at 300 K,close to the expected value of 56.68 cm3·mol-1·K for four uncoupled Dy3+ions(S=5/2,L=5,6H15/2,g=4/3).The χMT gradually decreases until 50 K and then quickly decreases to a minimum of 36.35 cm3·mol-1·K at 2 K, which is lower than four times the χMT value of an isolated mononuclear Dy complex at 2 K,suggesting antiferromagnetic coupling between Dy3+ion.Thus the decreaseinχMTwithdecreasingtemperatureis probably ascribed to a combination of the antiferromagnetic interaction between the Dy3+ions and the thermal depopulation of excited Stark sublevels[27].The data in the range of 30~300 K can be fitted to the Curie-Weiss law,yielding C=63.69 cm3·mol-1·K and θ=-5.01 K for 1.

Fig.5 Plots of temperature dependence of χMT vs T and χM-1vs T for 1
The field dependence of magnetization of 1 is shown in Fig.6.The magnetization at 2 K increases rapidly below 1.5 T,and then slowly and linearly increases without complete saturation up to 7 T.The maximum value for M is 23.08μBat 7 T,which is slightly larger than the calculated value for four uncorrelated Dy3+magnetic moments(4×5.23μB)[10]. Indeed,the values are lower than the expected saturation value of 40μB(10μBfor each Dy3+ion for J= 15/2 and g=4/3)[10c-10d],which suggests the presence of a significant anisotropy and low-lying excited states, consistent with the observed nonsuperposition M vs H/ T plots at different magnetic fields(Fig.6)[9].
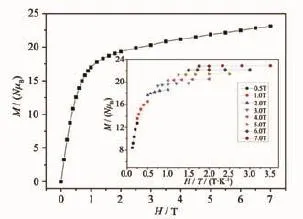
Fig.6 M vs H/T plots for 1 measured in different fields below 7 T
To probe the dynamics of magnetization for 1,the temperature dependence of ac magnetic susceptibility under Hdc=0 Oe and Hac=3 Oe was characterized at the indicated frequencies(1~1500 Hz).As shown in Fig.7,complex1displaysanobviousfrequency dependent out-of-phase signal,indicating the slow relaxation of the magnetization.However,the energy barriercannotbederivedbyfittingthepeak temperatures to an Arrhenius type expression due to the absence of maxima of out-of-phase susceptibility signals above 2.0 K(Fig.S5).However,the Eaand τ0valuescanbeobtainedfromfittingtheac susceptibility data by adopting Debye model and using the relationship ln(χ″/χ′=lnτ0+Ea/(KBT),if it is assumed thatthereisonlyonecharacteristicrelaxationprocess[28].The obtained Ea=35.4 K,τ0=1.5×10-5s(Fig. 8)are in agreement with the observed values for some other Dy4SMMs.For 1,the slow magnetic relaxation may result from a coupled system involving the four Dy (III)and the magnetic exchange coupling,although the interactions are expected to be very weak.

Fig.7 Temperature dependence of the out-of-phase ac susceptibilities at the indicated frequencies for 1 under zero dc field
The χMT value of 2 is 45.66 cm3·mol-1·K at 300 K (Fig.S4),which is slightly lower than the expected value of 47.28 cm3·mol-1·K for four uncoupled Tb3+ions(S=3, L=3,7F6,g=3/2).Similar to 1,a steady decrease of the χMT values of 2 is observed with deceasing temperature down to 50 K,and then decrease dramatically to 33.05 cm3·mol-1·K at 2 K.The data from 30 to 300 K are fitted to the Curie-Weiss law,leading to C=44.52 cm3· mol-1·K and θ=-5.80 K.As shown in Fig.S6,the magnetization increases rapidly below 1.5 T at 2 K,and then slowly and linearly increases to 18.91μBat 7 T. Notably,the ac susceptibility results of 2 show that no frequency dependent out-of-phase signal is observed in the region of 2~10 K(Fig.S7).Although Dy3+and Tb3+ions have large spin and high anisotropy,only the Dy4cluster exhibits slow paramagnetic relaxation,which may be ascribed to the spin parity effect[26a,29].

Fig.8 Plots of natural logarithm of χ″/χ′vs 1/T for 1
2.5Luminescent properties
The solid-state luminescence of complex 2 at room temperature is shown in Fig.9.Complex 2 exhibits intense photoluminescence upon excitation at 375 nm. The emission spectrum of 2 can be ascribed to the characteristic5D4→7FJtransitions(J=6,5,4,3).The most intense peak with its maximum at 546 nm is attributed to the5D4→7F5transition.Besides this main emission line,the second intense peak at 492 nm(J=6), and much less intense two peaks at 588 nm(J=4)and 622 nm(J=3),respectively,are also observed.This means that TMeQ[6]ligand exhibits efficient energy transfer to Tb3+ion.When excited at 265 nm in the solid state at room temperature,compound 1 displays a wide luminescence spectrum with emission maximum at 390 nm(Fig.S8),whichcanbeassignedtoligand fluorescence(Fig.S9).Compared with the emission of the free ligand at 378 nm,the red shift in 1 may be ascribed to the increase of ligand conformational rigidity.

Fig.9 Emission spectrum of 2 under 375 nm excitation in the solid state at room temperature
3 Conclusions
In summary,two TMeQ[6]-supported lanthanide sandwich complexes containing a cubane-like[Ln4(μ3-OH)4]8+cluster core were prepared and characterized. MagneticstudiesrevealthatDy4exhibitsslowmagnetic relaxation behavior.While the Tb3+analogue of[Ln4(μ3-OH)4]8+cluster core displays interesting luminescent property.The present work not only affords the first example of TMeQ[6]supported lanthanide hydroxide cluster with slow magnetic relaxation behavior,but also provides a new synthetic approach to prepare new lanthanide SMMs based on Cucurbit[n] urils.
Supportinginformationisavailableathttp://www.wjhxxb.cn
References:
[1]Troiani F,Affronte M.Chem.Soc.Rev.,2011,40:3119-3129
[2]Rogez G,Donnio B,Terazzi E,et al.Adv.Mater.,2009,21: 4323-4333
[3](a)Sessoli R,Gatteschi D,Caneschi A,et al.Nature,1993, 365:141-143
(b)Benelli C,Gatteschi D.Chem.Rev.,2002,102:2369-2387
(c)Bagai R,Christou G.Chem.Soc.Rev.,2009,38:1011-1026
(d)Kostakis G E,Akoab A M,Powell A K.Chem.Soc.Rev., 2010,39:2238-2271
[4](a)Coronado E,Day P.Chem.Rev.,2004,104:5419-5448 (b)Sanvito S.Chem.Soc.Rev.,2011,40:3336-3355
[5]Sokol J J,Hee A G,Long J R.J.Am.Chem.Soc.,2002, 124:7656-7657
[6]Sessoli R,Tsai H L,Schake A R,et al.J.Am.Chem.Soc., 1993,115:1804-1816
[7]Murrie M.Chem.Soc.Rev.,2010,39:1986-1995
[8](a)Woodruff D N,Winpenny R E P,Layfield R A.Chem. Rev.,2013,113:5110-5148
(b)Hewitt I J.Tang J K,Madhu N T,et al.Angew.Chem. Int.Ed.,2010,49:6352-6356
(c)Guo Y N,Xu G F,Gamez P,et al.J.Am.Chem.Soc., 2010,132:8538-8539
[9]Bi Y F,Wang X T,Liao W P,et al.Inorg.Chem.,2009,48: 11743-11747
[10](a)Abbas G,Lan Y H,Kostakis G E,et al.Inorg.Chem., 2010,49:8067-8072
(b)Lin P H,Burchell T J,Ungur L,et al.Angew.Chem. Int.Ed.,2009,48:9489-9492
(c)Ke H S,Gamez P,Zhao L,et al.Inorg.Chem.,2010,49: 7549-7557
(d)Tang J K,Hewitt I,Madhu N T,et al.Angew.Chem.Int. Ed.,2006,45:1729-1733
[11]Kong X J,Wu Y L,Long L S,et al.J.Am.Chem.Soc., 2009,131:6918-6919
[12]Wang R,Selby H D,Liu H,et al.Inorg.Chem.,2002,41: 278-286
[13]Deacon G B,Feng T,Hockless D C R,et al.Chem.Commun., 1997:341-342
[14]Peng J B,Kong X J,Zhang Q C,et al.J.Am.Chem.Soc., 2014,136:17938-17941
[15]Bürgstein M R,Gamer M T,Roesky P W,et al.J.Am. Chem.Soc.,2004,126:5213-5218
[16]Liu C M,Zhang D Q,Hao X,et al.Cryst.Growth Des., 2012,12:2948-2954
[17](a)Hewitt I J,Lan Y,Anson C E,et al.Chem.Commun., 2009:6765-6767
(b)Gao Y,Xu G F,Zhao L,et al.Inorg.Chem.,2010,48: 11495-11497
(c)Habib F,Lin P O,Long J,et al.J.Am.Chem.Soc., 2011,133:8830-8833
[18](a)Behrend R,Meyer E,Rusche F.Justus Liebigs Ann. Chem.,1905,339:1-37
(b)Freeman W A,Mock W L,Shih N Y.J.Am.Chem.Soc., 1981,103:7367-7368
(d)Day A I,Blanch R J,Arnold A,et al.Angew.Chem., Int.Ed.,2002,41:275-277
[19](a)Flinn A,Hough G C,Stoddart J F,et al.Angew.Chem. Int.Ed.,1992,31:1475-1477
(b)Zhao J Z,Kim H J,Oh J,et al.Angew.Chem.,Int.Ed., 2001,40:4233-4235
(c)Jon S Y,Selvapalam N,Oh D H.et al.J.Am.Chem. Soc.,2003,125:10186-10187
(d)Huang W H,Zavalij P Y,Isaacs L.Angew.Chem.Int. Ed.,2007,119:7569-7571
(e)Zhao Y J,Xue S F,Zhu Q J,et al.Chin.Sci.Bull., 2004,49:1111-1116
[20]Chen W J,Yu D H,Xiao X,et al.Inorg.Chem.,2011,50: 6956-6964
[21](a)Hernandez-Molina R,Sokolov M N,Sykes A G.Acc. Chem.Res.,2001,34:223-230
(b)Fedin V P.J.Coord.Chem.,2004,30:151-152
(c)Hernandez-Molina R,Sokolov M N,Clausen M,et al. Inorg.Chem.,2006,45:10567-10575
(d)Gushchin A L,Ooi B,Harris P,et al.Inorg.Chem., 2009,48:3832-3839
[22](a)Lü J,Lin J X,Cao M N,et al.Coord.Chem.Rev.,2013, 257:1334-1357
(b)Ni X L,Xue S F,Tao Z.Coord.Chem.Rev.,2015,287:
89-113
[23](a)Tripolskaya A A,Mainicheva E A,Mitkina T V,et al. Russ.J.Coord.Chem.,2005,31:768-774
(b)Thuery P.Inorg.Chem.,2009,48:4497-4513
(c)Thuery P.Inorg.Chem.,2010,49:9078-9085
(d)Thuery P.Inorg.Chem.,2011,50:10558-10560
(e)Kushwaha S,Rao S A,Sudhakar P P.Inorg.Chem., 2012,51:267-273
(f)Liang L L,Ni X L,Zhao Y,et al.Inorg.Chem.,2013, 52:1909-1915
(g)Liang L L,Zhao Y,Tao Z,et al.CrystEngComm,2013, 15:3943-3950
(h)Liu J X,Hu Y F,Lin R L,et al.CrystEngComm,2012, 14:6983-6989
[24](a)Gerasko O A,Mainicheva E A,Naumova M I,et al. Inorg.Chem.,2008,47:8869-8880
(b)Gerasko O A,Mainicheva E A,Naumova M I,et al. Eur.J.Inorg.Chem.,2008,3:416-424
[25]SHELXTL Program Package,Version 6.10,Bruker AXS, Inc.,Madison,WI,2000.
[26](a)Yan P F,Lin P H,Habib F.et al.Inorg.Chem.,2011, 50:7059-7065
(b)Jami A K,Baskar V,Sanudo E C,et al.Inorg.Chem., 2013,52:2432-2438
[27]Langley S K,Moubaraki B,Forsyth C M,et al.Dalton Trans.,2010,39:1705-1708
[28]Bartolomé J,Filoti G,Kuncser V,et al.Phys.Rev.B,2009, 80:014430
[29](a)Wernsdorfer W,Bhaduri S,Boskovic C,et al.Phys.Rev. B,2002,65:180403
(b)Wernsdorfer W,Chakov N E,Christou G,et al.Phys. Rev.Lett.,2005,95:037203
Slow Magnetic Relaxation in Sandwich-Type Tetranuclear Dysprosium Complex with TMeQ[6](TMeQ[6]=α,α,δ,δ-Tetramethylcucurbit[6]uril)
CHEN Wen-JianKONG Xiang-Jian*LONG La-Sheng*ZHENG Lan-Sun
(State Key Laboratory of Physical Chemistry of Solid Surface and Department of Chemistry,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen,Fujian 361005,China)
Two TMeQ[6]-supported sandwich tetranuclear complexes,[Ln4(μ3-OH)4(μ2-OH)2(H2O)4(NO3)2(TMeQ[6])2] (NO3)4·26H2O(Ln=Dy,1;Ln=Tb,2),have been prepared and characterized.Crystal structural analysis reveals that both complexes contain a cubane-like[Ln4(μ3-OH)4]8+cluster core sandwiched between two TMeQ[6]macrocycles.Magnetic investigations indicate that complex 1 displays slow magnetization relaxation.Complex 2 exhibits intense photoluminescence owing to the efficient energy transfer from TMeQ[6]ligand to Tb3+ion.CCDC: 929607,1;929608,2.
cluster;magnetism;photoluminescence;curcurbit[6]
O614.342
A
1001-4861(2015)09-1867-08
10.11862/CJIC.2015.226
2015-06-01。收修改稿日期:2015-07-07。
国家自然科学基金(nos.21422106,21371144,21431005)资助项目。
*通讯联系人。E-mail:xjkong@xmu.edu.cn;lslong@xmu.edu.cn;会员登记号:S06N455S1203(陈文建);S06N3944M1007(龙腊生)。

