基于1,3-二(2-苯并咪唑基)苯的双核钴及钴配位聚合物的晶体结构及磁性
刘法谦 赵杰 张东 段小群 王磊 邓月义*, 李伟华*,
基于1,3-二(2-苯并咪唑基)苯的双核钴及钴配位聚合物的晶体结构及磁性
刘法谦*,1赵杰2张东3段小群3王磊1邓月义*,3李伟华*,2
(1青岛科技大学高性能聚合物及成型技术教育部工程研究中心,青岛266042)
(2中国科学院海洋研究所,青岛266071)
(3桂林医学院药学院,桂林541004)
在不同的条件下,合成了2个基于刚性配体MBimB的钴配合物,即Co2(MBimB)2Cl4·2H2O·EDA(1)和[Co(MBimB)Cl2]n(2) (MBimB=1,3-二(2-苯并咪唑基)苯,EDA=乙二胺),并解析了其单晶结构。在配合物1中,2个MBimB配体采用顺式构象桥接2个钴原子,形成双核金属杂环结构。在配合物2中,MBimB配体采用反式配位方式,桥接2个邻近的钴原子,形成一维链状结构。磁性研究表明:随温度降低,配合物1和2均先表现为铁磁耦合,继而表现为反铁磁耦合。同配合物2相比,配合物1中Co…Co原子距离更短,且存在更强的π共轭效应,因此,配合物1中存在更为强烈的铁磁耦合及旋轨耦合作用。
1,3-二(2-苯并咪唑基)苯;配位聚合物;双核钴配合物;磁性
0 Introduction
In recent years,metal complexes based on bis(2-benzimidazole)ligands have received much attention. They exhibited diverse biological properties,including excellent antibacterial activities[1-2]and liver disease control[3],which are of interest in the application fields of proton sponges[4],ion exchange membrane[5-6], catalysts for polymerization[7-9],and agents for electron transfer system[10].As a result of extensive research of molecule-based magnets,magnetic properties of complexes based on bis(2-benzimidazolyl)have been reported[11-13].Owning to their capacity of mediating sufficiently strong magnetic interactions between metal ions[14-16],bis(2-benzimidazole)compounds can be used as bridging ligand to construct the complexes with bulk magnetic ordering.
The bis(2-benzimidazole)ligand can be flexible or rigid.A literature survey showed that lots ofcoordination complexes have been synthesized using flexible bis(2-benzimdazolyl)alkanes as ligands.As a result of the long flexible alkane chain,diverse structures which included mononuclear[17-19],binuclear[20-23]and multinuclear[17,24]complexes have been reported.Compared to flexible bis(2-benzimidazole)ligands,studies on complexesbased on the rigid ligandsare limited[12,25-26]. In our previous studies,we have prepared some complexes based on the rigid ligand OBimB(OBimB =1,2-bis(2-benzimidazolyl)benzene)[27-28].Herein,we reportthe synthesis,structure characterization,thermal and magnetic properties of two cobalt(Ⅱ)complexes Co2(MBimB)2Cl4·2H2O·EDA(1)and[Co(MBimB)Cl2]n(2)using rigid MBimB as ligand(MBimB=1,3-bis(2-benzimidazolyl)benzene,EDA=ethylenediamine).
1 Experimental
1.1 Materials and instruments
All the chemicals were purchased commercially and used without further purification.Elemental analyses(C,H and N)were carried out on a Perkin-Elmer 1400C analyzer.IR spectra were obtained on a Nicolet 170SX spectrometer.TG curves are obtained on a TGA/DSC 2 thermogravimetric analyzer(Mettler-Toledo AG)in nitrogen up to 900℃.Magnetic susceptibility data were obtained from 2 to 300 K at a magnetic field of 1 000 Oe on a Quantum Design MPMS-7 SQUID magnetometer.1H NMR spectrum of MBimB was obtained on a Bruker DOX 300 instrument.
1.2 Preparation of the complexes 1 and 2
1.2.1 Synthesis of MBimB
MBimB was synthesized by the condensation between m-phthalic acid and o-diaminobenzene using a method reported before[29](Scheme 1).Typically, about4.7 g o-diaminobenzene(44 mmol)and 3.3 g mphthalic acid(20 mmol)were mixed and thoroughly ground in a mortar.Then,the uniform mixed powder was transferred to a flask.About40 mL polyphosphoric acid was added to the flask and the resulting mixture was heated to 230℃for 4 h with continuous stirring. After that,the reaction solution was poured into copious amounts of ice water with strong stirring. Finally,the resulting raw solid was stirred in a saturated NaHCO3solution for12 h(Yield:3.3 g,39%). Yellow single crystals suitable for X-ray diffraction were obtained by recrystallization from methanol. Anal.Calcd.(%)for C20H14N4:C 77.40,H 4.55,N 18.05;Found(%):C 77.68,H 4.35,N 17.97.IR(KBr, cm-1):3 058(w,νAr-H),1 534(w),1 439(s,νC=C,C=N), 1 278(w),1 008(w),742(s).1H NMR(DMSO-d6):δ 12.95(2H,s,NH),7.3~7.8(12H,m,Ar).
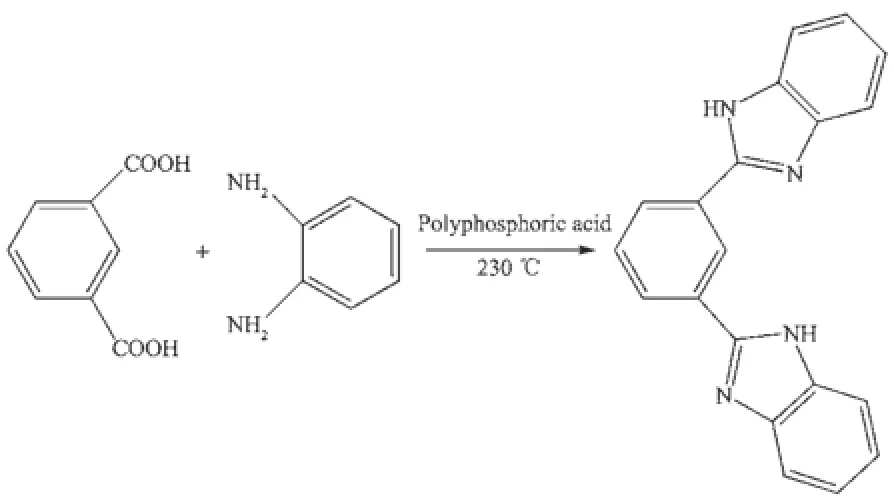
Scheme 1 Schematic view of the synthesis of MBimB
1.2.2 Preparation of Co2(MBimB)2Cl4·2H2O·EDA(1)
CoCl2·6H2O(0.048 g,0.2 mmol)and MBimB (0.03 g,0.1 mmol)were added to the mixed solution ofethanol(10 mL)and acetonitrile(10 mL).Then,two drops of ethylenediamine was added to improve the solubility.The resulting solution was refluxed for 30min with continuous stirring.Finally,the blue filtrate was kept at room temperature for about2 days to give blue crystals(45%yield based on Co).Anal.Calcd.(%) for C42H40Cl4Co2N10O2:C 51.7,H 4.1,N 14.3;Found(%): C 52.1,H 4.1,N 14.1.IR(KBr,cm-1):3 423(br,νN-H), 1 631(w,νC=C,C=N),1 572(w),1 442(m,νC=C,C=N),1 394 (s),1 285(m),1 227(w),891(w),745(m),677(w).
1.2.3 Preparation of[Co(MBimB)Cl2]n(2)
A mixture of MBimB(0.03 g,0.1 mmol),CoCl2· 6H2O(0.048 g,0.2 mmol),NaOH(0.004 g,0.1 mmol), ethanol(6 mL),and benzene(10 mL)was heated in a 20 mL Teflon autoclave at 110℃for 3 days and then cooled to ambient temperature at a rate of 5℃·h-1. The dark blue block crystals were separated by filtration.Anal.Calcd.(%)for C20H14Cl2CoN4:C 54.6,H 3.2,N 12.7;Found(%):C 54.2,H 3.4,N 12.9.IR (KBr,cm-1):3 431(br,νN-H),1 626(w,νC=C,C=N),1 590(w), 1 437(m,νC=C,C=N),1 289(m),1 027(w),768(m),647(w).
1.3 X-ray crystallographic study
Diffraction measurements were made with a Bruker SMART 1000 CCD area detector X-ray single crystal diffractometer.The structure was solved via the SHELXS 97 program[30]and refined with SHELXL 97[31]by full-matrix leastsquares on F2.H atomsbonded to the C and N atoms were positioned geometrically (C-H 0.095 or 0.099 nm,and N-H 0.088 nm)and allowed to ride on their parent atoms.H atoms attached to O atoms were located in a difference map and were refined with bonds restraints(O-H 0.085(3) nm,H…H 0.137(2)nm)and with Uiso(H)=0.1. Additionaldetails ofthe data collection and refinement for 1 and 2 are given in Table 1.Selected bond lengths and bond angles are listed in Table 2.
CCDC:993989,1;993990,2.

Table 1 Crystals and structures refinement data for 1 and 2

Table 2 Selected bond lengths(nm)and bond angles(°)for 1 and 2
2 Results and discussion
2.1 Synthesis
The ligand MBimB was synthesized by the condensation of o-diaminobenzene and m-phthalic acid.Polyphosphoric acid was used here as both reaction solution and acid catalyst[32-33].Complex 1 with binuclear structure was obtained by the reaction of CoCl2·6H2O and MBimB with the molar ratio of 2∶1 in a mixture of ethanol and acetonitrile.However, coordination polymer structure in 2 was formed with the same metal-ligand molar ratio under solvothermal reaction condition with the presence of benzene.This result indicates that the assemblies of metals and ligands are influenced by the reaction condition and the nature of solvate.Two reasons may explain the structural difference.First,the polymer structure of 2 formed under high temperature and high pressure has a lower energy compared to the binuclear structure of 1.Second,under the high temperature and pressure, benzene may acts as a templating regent and prevents the construction ofbinuclear complex sterically.
2.2 Description of the Structures
An ORTEP view of 1 with the atomic numbering scheme is shown in Fig.1,showing 50%probability displacement ellipsoids.Single-crystal X-ray analysis reveals that 1 has a binuclear structure.Two distorted tetrahedral Cobalt(Ⅱ),each bonded by two benzimidazole nitrogens and two chlorine anions,are bridged by two MBimB ligands to form familiar centrosymmetric,metallaycyclic arrangement.The bidental MBimB ligand adopts a“cis”conformation with two coordinated nitrogens at the same direction.The Co-N distances are almost equal(Co1-N4 0.204 9(3)nm,Co1-N6 0.202 6(3)nm),which are slightly longer than those of complex[Co2(BTBI)Br4]·4DMF(BTBI=1,2,4,5-tetrakis (benzimidazo1-2-yl)benzene,Co-N 0.201 6(6)and 0.200 4(7)nm)[34].The angles at cobalt range from 102.0(11)°to 124.58(8)°.In the metallaycyclic framework,the distance between two Cobalt(Ⅱ)atoms is 0.727 5(6)nm and the parallel central benzene rings are separated by 0.521 9(6)nm.The dihedralangles of the benzimidazolyl ring planes with the central phenyl ring plane are 40.85°and 14.52°,respectively.

Fig.1 Structure of complex 1,showing 50%probability displacement ellipsoids and the atomic numbering scheme
The structure of 2 is shown in Fig.2.Each MBim B as bidental ligand links two Co(Ⅱ)atoms into 1D zigzag chain.The MBimB ligand affords two coordination sites and adopts a“trans”conformation with two coordinated nitrogens at the oppositedirection,resulting in coordination polymer conformation.The stereochemistry at each cobalt center is distorted tetrahedral,with angles at cobalt ranging from 103.86(8)°to 120.62(8)°and are similar to those of1.The bond lengths of Co-N(Co-N3 0.203 7(3)nm, Co-N1 0.205 1(3)nm)are much closed to those in 1. The distance between two adjacent Cobalt atoms in the 1D chain is 0.785 9 nm.Benzimidazole rings are twisted by 49.01°and 29.83°relative to the central benzene ring,which are bigger than those of 1.
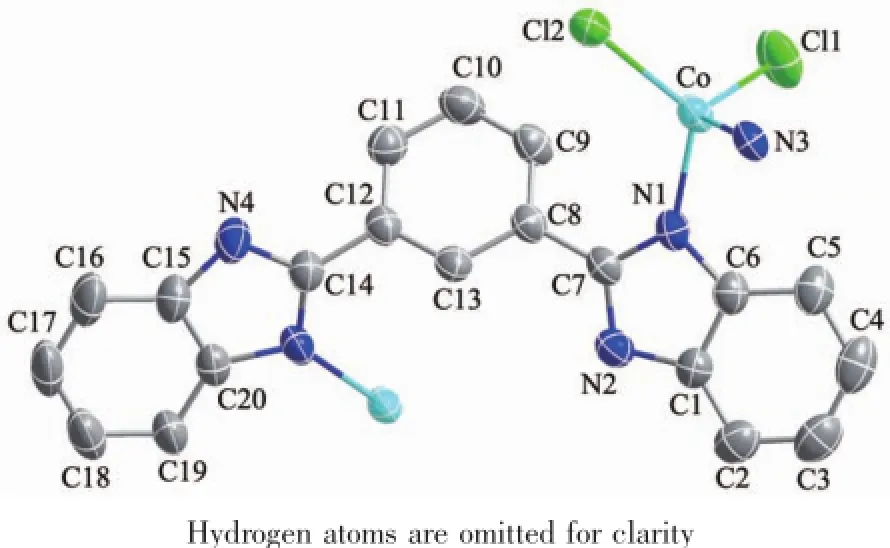
Fig.2 Structure of complex 2,showing 50%probability displacement ellipsoids and the atomic numbering scheme
In complex 1,the strong classical hydrogen bonds,constructed by NH groups of MBimB ligand, water molecules and NH2groups of ethylenediamine, link the Co2(MBimB)2Cl4units into 2D layered structure along the ac plane(Fig.3).There is also weakπ…πstacking interactions among the imidazole rings of the MBimB ligands(the distance of the centroids of the two imidazole rings is ca.0.388 5 nm).For complex 2,the chlorine anion is hydrogenbonded to the amine NH groups attached to the benzimdazole(N2i-Cl2,0.321 9(4)nm;Symmetry code:i1-x,-1/2+y,1/2-z),which further stabilize the 1D chain structure(Fig.4).
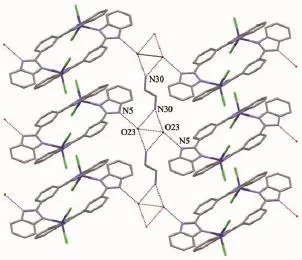
Fig.3 3D layered structure formed by N-H…O and O-H…O classical hydrogen bonds in 1
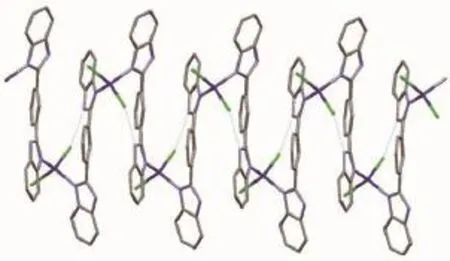
Fig.4 N-H…Cl classical hydrogen bonds in complex 2

Fig.5 TG curves of title complexes 1 and 2
2.3 Thermal decomposition properties
The thermal decomposition curves of complexes 1 and 2 in nitrogen are shown in Fig.5.As shown in Fig.5a,the first stage of weight loss of complex 1 corresponds to the release of water molecules and EDA molecules in the crystal lattice(Found 9.72%, Calcd.9.84%).The second stage of weight loss from ca.285℃to ca.650℃is ascribed to the gradualloss of Cl atoms and the decomposition of MBimB ligands(Found 78.02%,Calcd.78.08%).The residue is calculated to be Co(Found 12.26%,Calcd.12.07%). The thermal decomposition curve of complex 2 is shown in Fig.5b.Complex 2 is stable in nitrogen until the temperature reaches up to ca.275℃.After that, the gradual loss of Cl atoms and the decomposition of MBimB ligands were observed,which is similar to that of complex 1.The final residue is Co(Found 14.01%;Calcd.13.39%).
2.4 Magnetic properties
Magnetic susceptibility measurements were performed on crushed single crystals of 1 and 2 with an applied field of 1 000 Oe from 5 to 300 K.Roomtemperature magnetic moments for complexes 1 and 2 (1:μeff=5.19μB;2:μeff=4.22μB)are all in excess of the spin-only value of the cobalt(Ⅱ)ion(3.87μB),which indicated that spin-spin coupling in these systems is likely to be weak or nonexistent.As T decreases,the values ofχmT for 1 and 2 increase(Fig.6),suggesting the existence of ferromagnetic coupling,which may be associated with a compensation of ferromagnetism by the combined effects of spin-orbit coupling of the cobalt(Ⅱ)ions[35-36].Then,χm T begins to drop off,which is a signal of antiferromagnetic coupling.In 1,the temperature dependence of the reciprocal susceptibility(χm-1)in the range of110~300 K follows the Curie-Weiss law with a Weiss constantθof 55.58 K,thus indicating a strong ferro-magnetic interaction and/or spin-orbital coupling between the cobalt(Ⅱ) ions.Reciprocal susceptibility versus temperature plot for 2 in the range of 50~300 K is also linear and the Weiss constantθis 5.86 K,which is far less than that of 1.The decrease can be ascribed to the longer distance of Co…Co and weaker intervening aromatic π-system in 2 than those of1.
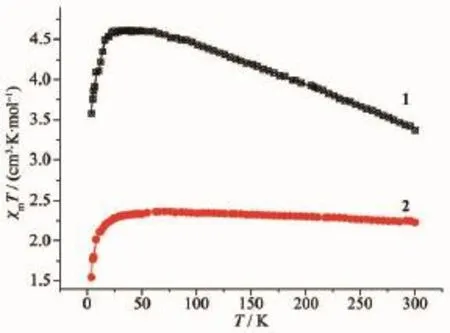
Fig.6 Temperature dependence of magnetic susceptibility of 1 and 2
3 Conclusions
Two cobalt(Ⅱ)complexes,Co2(MBim B)2Cl4· 2H2O·EDA(1)and[Co(MBimB)Cl2]n(2),have been synthesized under different reaction conditions and structurally characterized by X-ray diffraction analysis. Complex 1 exhibits a binuclear metallaycyclic framework,while complex 2 adopts a coordination polymer conformation.The magnetic studies reveal that both complexes exhibit ferromagnetic coupling firstly and then antiferromagnetic coupling as T decreases. Compared to complex 2,complex 1 shows stronger ferromagnetic interaction and/or spin-orbital coupling between adjacent cobalt(Ⅱ)ions.
[1]Poeta M D,Schell W A,Dykska C C,et al.Antimicrob. Agents Chemother.,1998,42:2495-2502
[2]Salunke N M,Revankar V K,Mahale V B.Transition Met. Chem.,1994,19:53-56
[3]Tidwell R R,Jones S K,Naiman N A.Antimicrob.Agents Chemother.,1993,37:1713-1716
[4]Stibrany R T,Schugar H J,Potenza J A,etal.Acta Crystallogr. Sect.E,2002,58:o1142-o1144
[5]Hoorn H J,de Joode P,Driessen W L,et al.React.Funct. Polym.,1995,27:223-235
[6]Hoorn H J,de Joode P,Dijkstra D J,et al.J.Mater.Chem., 1997,7:1747-1754
[7]Stibrany R T,Schulz D N,Kacker S,et al.Macromolecules, 2003,36:8584-8586
[8]Patil A O,Zushma S,Stibrany R T,et al.J.Polym.Sci.Part A:Polym.Chem.,2003,41:2095-2106
[9]Stibrany R T,Kacker S.US Patent,6479425.2002-11-12
[10]Xie B,Elder T,Wilson L J,et al.Inorg.Chem.,1999,38:12-19
[11]Galan-Mascaros J R,Dunbar K R,et al.Angew.Chem.Int. Ed.,2003,42:2289-2293
[12]Stibrany R T,Lobanov M V,Schugar H J,et al.Inorg. Chem.,2004,43:1472-1480
[13]Isele K,Broughton V,Matthews C J,et al.J.Chem.Soc., Dalton Trans.,2002:3899-3905
[14]Wang X Y,Wang Z M,Gao S.Chem.Commun.,2008:281-294
[15]Murrie M.Chem.Soc.Rev.,2010,39:1986-1995
[16]Woodruff D N,Winpenny R E P,Layfield R A.Chem.Rev., 2013,113(7):5110-5148
[17]Albada G A,van Smeets W J J,Veldman N,et al.Inorg. Chim.Acta,1999,290:105-112
[18]Albada G A,van Smeets WJ J,Spek A L,etal.Inorg.Chim. Acta,2000,299:35-40
[19]Bernardinelli G,Kübel-Pollak A,Rüttimann S,et al.Chimia, 1992,46:155-162
[20]Bernardinelli G,Kübel-Pollak A,Rüttimann S,et al.Zeit. Krist.,1993,203:132-136
[21]Albada G A,van Lakin M T,Veldman N,etal.Inorg.Chem., 1995,34:4910-4917
[22]Bau R,Drabnis M H.Inorg.Chim.Acta,1997,259:27-50
[23]Chen C L,Tan H Y,Yao J H,et al.Inorg.Chem.,2005,44: 8510-8520
[24]Guo X F,Li Z F,Yue C,et al.Polyhedron,2010,29:384-390
[25]Stibrany R T,Potenza J A.Acta Crystallogr.Sect.C,2008, 64:m213-m216
[26]Carina R F,Williams A F,Bernardine G.Inorg.Chem.,2001, 40:1826-1832
[27]Deng Y Y,Liu F Q,Jin Y L.Transition Met.Chem.,2012, 37:309-314
[28]Deng Y Y,Wu H Z,Jin Y L,et al.J.Chem.Crystallogr., 2012,42:739-745
[29]DENG Yue-Yi(邓月义),WU Hong-Zhi(吴鸿志),JIN Yan-Ling(金燕玲),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28:2463-2467
[30]Sheldrick G M.Acta Crystallogr.Sect.A,1992,46:467-469
[31]Sheldrick G M.SHELXL-97,Program for X-ray Crystal Structure Refinement,University of Göttingen,Germany, 1997.
[32]Vyas P C,Oza C K,Goyal A K.Chem.Ind.,1980:287-288
[33]Hein D W,Alheim R J,Leavitt J J.J.Am.Chem.Soc., 1957,79:427-429
[34]Tandon S S,Thompson L K,Bridson J N,et al.Inorg.Chem., 1994,33:54-61
[35]Abragam A,Bleaney B.Electron Paramagnetic Resonance of Transition Ions.New York:Dover Publications,1970.
[36]Kahn O.Molecular Magnetism.Weinheim:VCH,1993:38-42
Binuclear Cobalt(Ⅱ)Complex and Cobalt(Ⅱ)Coordination Polymer of Bidentate Ligand 1,3-Bis(2-benzimidazolyl)benzene: Crystal Structures and Magnetic Properties
LIU Fa-Qian*,1ZHAO Jie2ZHANG Dong3DUAN Xiao-Quan3WANG Lei1DENG Yue-Yi*,3LI Wei-Hua*,2
(1Engineering Research Center of High Performance Polymer and Molding Technology,
Ministry of Education,Qingdao University of Science and Technology,Qingdao,Shandong 266042,China)
(2Institute of Oceanology,Chinese Academy of Sciences,Qingdao,Shandong 266071,China)
(3College of Pharmacy,Guilin Medical University,Guilin,Guangxi 541004,China)
Two cobalt(Ⅱ)complexes,Co2(MBimB)2Cl4·2H2O·EDA(1)(MBimB=1,3-bis(2-benzimidazolyl)benzene, EDA=ethylenediamine)and[Co(MBimB)Cl2]n(2),have been synthesized based on the rigid ligand MBimB and characterized by single crystal X-ray diffraction.In complex 1,two MBimB molecules adopt“cis”conformation and bridge two cobalt(Ⅱ)ions to form a binuclear metallaycyclic framework.In complex 2,MBimB ligand adopts“trans”conformation and bridges two adjacent cobalt(Ⅱ)ions into 1D zigzag chain.Magnetic susceptibility of 1 and 2 showed ferromagnetic coupling firstly and then antiferromagnetic coupling as the temperature decreases. Compared to complex 2,stronger ferromagnetic interaction and/or spin-orbital coupling between the cobalt(Ⅱ)ions in 1 can be ascribed to the shorter distance of Co…Co and enhanced intervening aromaticπ-system between the metalcenters.CCDC:993989,1;993990,2.
1,3-bis(2-benzimidazolyl)benzene;coordination polymer;binuclear cobalt(Ⅱ)complex;magnetic properties
O614.81+2
A
1001-4861(2015)07-1402-07
10.11862/CJIC.2015.166
2015-02-28。收修改稿日期:2015-04-02。
国家自然科学基金(No.21371105,51372125,51179182),青岛市科技发展计划(No.14-2-4-41-jch,13-1-4-209-jch)资助项目。
*通讯联系人。E-mail:faqianliu@yahoo.com,dengyyqust@hotmail.com,1685680@163.com

