Expression and significance of netrin-1 and its receptor UNC5C in precocious puberty female rat hypothalamus
Yan-Chao Shang, Jie Zhang, Yan-Qiu Shang
Maternal and Children Health Hospital, Affiliated Hospital of Hebei United University, Tangshan, China
Expression and significance of netrin-1 and its receptor UNC5C in precocious puberty female rat hypothalamus
Yan-Chao Shang, Jie Zhang*, Yan-Qiu Shang
Maternal and Children Health Hospital, Affiliated Hospital of Hebei United University, Tangshan, China
ARTICLE INFO
Article history:
Received15 December 2014
Received in revised form 20 January 2015
Accepted 15 February 2015
Available online 20 March 2015
Netrin-1
Objective: To study expressions of netrin-1 and its receptor UNC5C in female precocious puberty rat hypothalamus, and explore its effect on precocious puberty process. Methods: Forty female one-week-old SD rats were randomly divided into four groups: experimental group A (precocious puberty early youth), experimental group B (precocious puberty medium youth), group A (normal pre-puberty), group B (normal early youth) with 10 rats in each group. Precocious puberty experimental rats were induced with Danazol and rats in control group were injected with saline. Uterus and ovaries were removed, specimens were weighed, uterus index and ovarian index were calculated, and amount of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were detected from the blood by ELISA. Real-time PCR was used to detect netrin-1 and its receptor UNC5C, as well as hypothalamic gonadotropinreleasing hormone (GnRH) mRNA expression in hypothalamus tissues; and then, a coimmunoprecipitation study of interactions between netrin-1 and its receptor UNC5C was carried out. Results: Relative target gene expression levels of control group A, control group B, experimental group A, and experimental group B (with β-actin as an internal control for normalization) were as follows: Netrin-1: 3.5±0.9, 5.4±0.7, 4.9±1.0, 5.3±0.3; UNC5C: 0.8± 0.04, 1.7±0.2, 1.82±0.23, 1.58±0.4; GnRH: 1.2±0.3, 2.7±0.3, 2.4±0.7, 3.2±0.4. Conclusions: LH and FSH concentrations, netrin-1 and its receptor expression are increased in precocious puberty animal models.
1. Introduction
Precocious puberty refers to endocrine system abnormalities which occurs in male children younger than nine years old and female children younger than eight years old. It results in abnormal secondary sexual characteristics development, and its incidence rate in China is nearly 1%. Central precocious puberty (CPP) is caused by elevated expression and secretion of gonadotropin-releasing hormone (GnRH) in advance, which activates gonadal axis function in the hypothalamus, then initiates gonad development and secretion of sex hormones, and causes genital development and emergence of secondary sexual characteristics[1].
Axon guidance factor is a class of secreted proteins that plays an important role in the correct formation of the nervous system. It has dual-orientation function as attracting or repelling function for axon guidance and cell migration. At present, axon guidance factors that have been identified include netrins, ephrin, slit, semaphorins family[2]. Netrins is an axon guidance factor that is necessary during mammalian nervous system development, which is mainly dependent on variously expressed UNC5 receptor homolog (UNC5H) or DCC growth cones, and can transmit various signals. The main caused for precocious puberty is premature activation of the hypothalamic-pituitary-gonadal axis (HPGA) function[3]; and abnormal expression of netrin-1 and its receptor UNC5C in the hypothalamus can cause dysfunction in this pathway. Therefore, we aimed to study the expressions of netrin-1 and its receptor UNC5C in the hypothalamus using female precocious puberty rat model, and explore the biological role that netrin-1 and its receptor UNC5Cmight play in this process.
2. Materials and methods
2.1. Experimental animals
Forty one-week-old specific pathogen free grade female SD rats, weighing (25±5) g, were purchased from Jun Nanjing Better Biotechnology Co. Ltd. Rats were randomly divided into four groups: experimental group A (precocious puberty at early stage), experimental group B (precocious puberty at medium stage), control group A (normal pre-puberty), control group B (normal puberty at early stage); and each group had 10 rats. During the experiment, rats were free to eat (maternal breastfeeding) and drink. The breeding room was well ventilated; and natural day and night lighting was provided in a room temperature maintained at 18-25 ℃.
2.2. Drug reagents and equipment
Danazol was purchased from Jiangsu Lian Huan Pharmaceutical Co. Ltd; rat luteinizing hormone (LH) ELISA kit was purchased from C-X Biotechnology Co. Ltd; follicle stimulating hormone (FSH) rapid test kit was purchased from Chemtronbio Co., Ltd.; tissue RNA extraction kit (RNeasy Plus Mini Kit) was purchased from QIAGEN; reverse transcription kit (D61101A) was purchased from TaKaRa; Real-time PCR fluorescence quantification kit (SsoAdvanced SYBR Green Super mix) was purchased from Bio-Rad; immunoprecipitation kit (SeizePrimary) was purchased from Fisher Biosciences; Netrin-1 and UNC5C antibodies were purchased from Santa Cruz Biotechnology; β-actin monoclonal antibody was Wuhan Boster Biological Company; Horseradish peroxidase (HRP) labeled secondary antibody was from Wuhan Boster Biological Technology Co., Ltd.; ECL color kit was purchased from Millpore; PVDF membrane (Polyvinylidene fluoride) was purchased from Millpore; and Skim milk was purchased from Oxoid. Ultraviolet spectrophotometer UV-1750 from Shimadzu; precision balances from Sartorius; quantitative fluorescence PCR detection system, CFX96 Touch from Bio-Rad.
2.3. Methods
2.3.1. Precocious puberty rat model and animal groups
After two days of adaptive feeding, rats were randomly divided into four groups: experimental group A (precocious puberty at early stage), experimental group B (precocious puberty at medium stage), control group A (normal pre-puberty), control group B (normal at early stage); and each group had 10 rats. Each rat in experimental groups A and B were subcutaneously injected with 300 μg of danazol to establish the precocious puberty model. Each rat in control groups A and B were subcutaneously injected with equal volumes of normal saline. When rats were 20-days-old, vaginal openings were checked and vaginal opening time (VO) was recorded. These were recorded on the 20th day if the vaginal was open at the first inspection[4].
Rats in experimental group A were sacrificed after the first estrus cycle of vaginal opening; and control group A was also sacrificed at the same time. Then, rats in control group B were sacrificed at the first estrus cycle after puberty and rats in experimental group B were sacrificed at the second estrus cycle. Uterus and ovary specimens were taken from all rats; and uterus index and ovary index (organ wet weight/body mass) were calculated. Hypothalamic tissues were carefully cut, removed and stored in liquid nitrogen.
2.3.2. ELISA assay
Specific procedures for serum LH and FSH quantitative analysis by ELISA assay. Standard wells, test sample wells, and blank wells were set up, respectively. Seven standard wells were set and different standard concentrations of test samples were added; samples were incubated for 2 h at room temperature. Then, 100 μL of detecting solution (biotinylated antibody) was added into each hole and incubated for one hour at room temperature. Liquid from the wells were then discarded and each well was immersed three times with 350 μL of washing liquid for two minutes; 100 μL of HRP labeled secondary antibody was added in each well and incubated for 30 min at room temperature; and 90 μL of TMB substrate was added in each well and incubated at 37 ℃ for 25 min. Reaction was terminated before blue gradients obviously appeared in 3-4 standard wells and 50 μL of 2M H2SO4was added. OD values of each well were immediately measured at 450 nm with a microplate reader. Data were expressed as mean±standard deviation (SD) meter. The two groups were compared by student-t test. P<0.05 was considered as significant difference.
2.3.3. Real-time RCR
Rat hypothalamic tissues were grinded from liquid nitrogen and total RNA was extracted by RNA extraction kit. Rnase-free water was used to dissolve RNA precipitation and an ultraviolet spectrophotometer was used to measure RNA concentration. RT cDNA was processed by TaKaRa Reverse transcription kit; Realtime PCR was used to detect mRNA expression levels of netrin-1, UNC5C and GnRH. NCBI database was queried for netrin-1, UNC5C and GnRH mRNA sequences; primers were designed by Real-time PCR; and all primers were synthesized by Invitrogen Corporation. Specific sequences are shown in Table 1. Target gene relative expression levels were calculated by double Ct value method: target gene relative expression level=2-ΔΔCt, ΔCt=Ct-Ct(β -actin); ΔΔCt= ΔCt-ΔCt(contro1)[5].
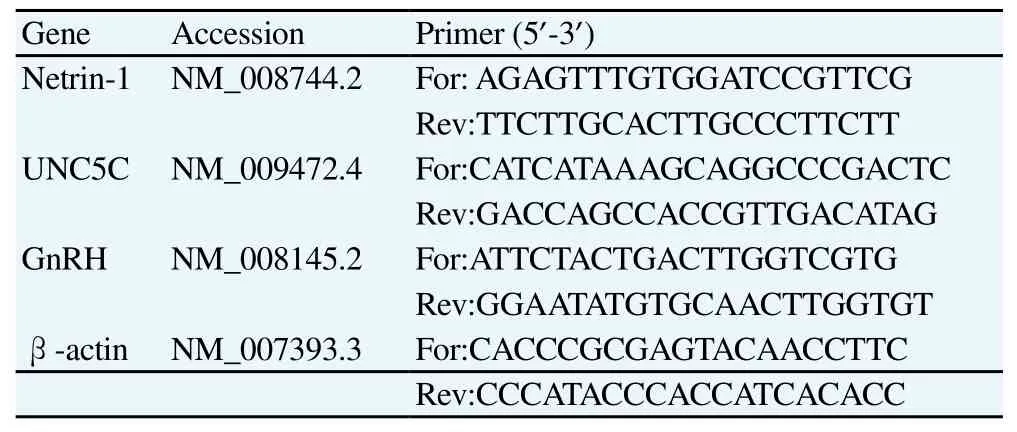
Table 1 Primer used in Real-time PCR.
2.3.4. Western blot
Tissue samples were grinded from liquid nitrogen and lysed using RIPA. At the same time, a protease inhibitor cocktail was added, mixed by pipetting. After the samples were placed on ice for 30 min, cells were lysed by ultrasonic waves. Appropriate short frequency shocks were emitted on ice using ultrasound probes. The lysed mixture was placed at 4 ℃ and centrifuged at 13 000 rpm/min for 20 min; protein concentrations in the new supernatant were determined using a protein assay kit.
After SDS-PAGE electrophoresis completion, the gel was immersed into transfer buffer equilibrium for 10 min. Transfer “sandwich” was assembled, transfer buffer was added and the electrode was plugged at 100 V for 45-60 min. After the film was transferred, PVDF membrane was rinsed with TBS for 10-15 min. The film was placed in a TBS/T blocking buffer containing 5% (w/v) skimmed milk powder, shaken for 1 h at room temperature, appropriate dilution of primary antibody [containing 1% (w/v) skimmed milk powder in TBS/T dilution] was added, incubated at room temperature for 2 h, and the membrane was rinsed with TBST three times every 5-10 min. The film was incubated in a TBST diluted secondary antibody (1:10 000, HRP labeled) containing 0.05% (w/v) nonfat dry milk at room temperature for 1 h, the film was rinsed with TBS/T three times every 5-10 min, and exposed for scanning.
2.3.5. Co-immunoprecipitation
According to manufacturer's instructions, netrin-1 monoclonal antibody was cross-linked to AminoLink® Plus support material, washed with PBS three times, and the supernatant was removed by centrifugation. Tissue samples were lysed using RIPA lysis buffer, added to the cross-linked antibody complex, then incubated in a 4 ℃shaker for 4 h to enable netrin-1 and its interacting protein to bind on the antibody complex. After incubation was completed, it was washed with PBS again three times to remove unbound proteins and the supernatant was removed by centrifugation. After SDS-PAGE, Western blot assay was applied to measure the concentration of eluted protein from the eluted buffer precipitated mixture.
2.4. Statistical analysis
SPSS 11.5 statistical software was applied to the experimental data, and results were expressed as mean±SD. Multiple groups of data were analyzed using ANOVA (one-way ANOVA) and t-test was used to compare two groups. P<0.05 was considered as statistically significant difference.
3. Results
3.1. Vaginal opening time, first estrus cycle, ovarian index and uterus index of rats in each group
Compared with control group, vaginal opening and first estrus cycle time of experimental groups were significantly decreased (P<0.05). And uterus index of experimental groups were significantly increased (P<0.05). There was no significant difference in ovarian index (Table 2).
3.2. FSH and LH in serum of rats in each group by ELISA assay
LH and FSH levels were significantly increased in experimental groups compared with control group A (P<0.05) (Table 3).
3.3. Real-time PCR detection of netrin-1, UNC5C, GnRH mRNA expressions in hypothalamic tissues
Netrin-1, GnRH and UNC5C levels in control group B and experimental groups were significantly increased compared with that of control group A (P<0.05) (Figure 1).
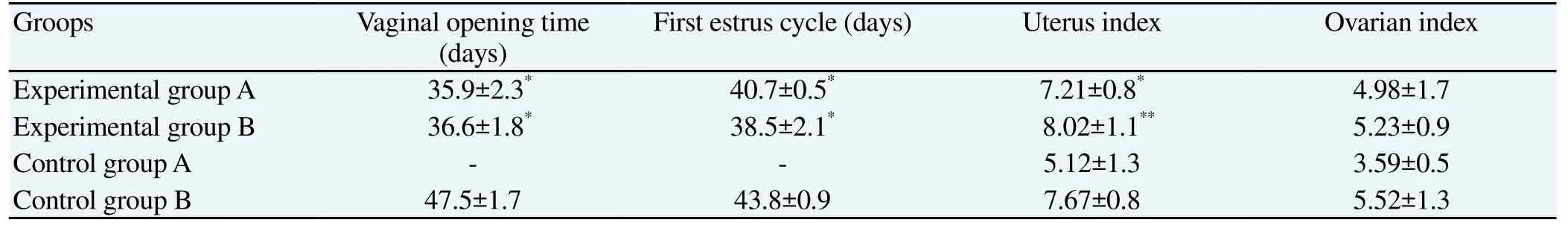
Table 2 Vaginal opening time, first estrus cycle, uterus index and ovarian index of rats in each group.
3.4. Co-immunoprecipitation and netrin-1 protein interaction analysis results
As shown in Figure 2, netrin-1 expression levels of experimental groups, specifically, the precocious puberty model group, were upregulated compared with control group A; while netrin-1 in the normal mid-puberty group (control group B) increased compared with control group A. In explaining puberty, netrin-1 in vivo is a regulated process. It also showed that the expression level of receptor UNUC5 with netrin-1 had consistent trend. Figure 3 showed bioinformatics analysis of netrin-1 interacting proteins.
4. Discussion
The startup process of puberty in mammals involves a neuromodulator and hormonal regulation that jointly participates in the complex network regulatory process. Hypothalamic-pituitarygonadal axis (HPGA) is coordinated by an integrated neuroendocrine regulation system. Prior to the onset of puberty, the system exists just out of a relatively inhibited state. On onset of puberty, hypothalamic GnRH secretion begins to be expressed, further causes expression and release of LH and FSH, thereby, regulates normal development and sexual cycle. Central precocious puberty is caused at the early start of HPGA in children, which causes endocrine system abnormalities. Thus, this enables serum LH, FSH and GnRH to be released at early stage and initiate Gamete formation[1]. It causes development and secretion of sex hormones. Eventually, this leads to genital development and emergence of secondary sexual characteristics. The biological mechanism of early HPGA has already become a hot topic in precocious puberty studies[6-8].
Axon guidance factor is a class of secreted proteins that plays an important role in the correct formation of nervous system and development of necessary axon guidance and cell migration. The growth pattern guide of axons is demonstrated by a specific form of movement after stimulating factors affect axons. At the same time, axon growth guidance is also the core of nervous system development. By way of attracting and repelling, axon guidance factor allows axons to properly reach the target site[9]. Recent studies have shown that the attraction or repulsion function may be because that they belong to the ligand-dependent receptor family. Currently identified axon guidance factors include netrins, ephrin, slit and semaphorins family[2]. Netrins is necessary for axon guidance factors during nervous system development of mammals. It is also the earliest identified kind of neurological guide factor that mainly depends on the growth cone by vario[10]. Dependence receptors can induce apoptosis with the lack of appropriate ligands. As such, DCC and UNC5H may not only transmit signals, but also cell survival factor; wherein, plays a role in cell survival and normal function.
In experiments, vaginal opening was first observed on rats from experimental groups A and B. Results showed significant differences in LH and FSH levels of rats between experimental groups A, B and control group A (P<0.01); but compared with the control group B, there was no significant difference (P>0.05). This result suggests that danazol successfully induced the central precocious puberty model. Results also showed that there was no significant difference in uterus index and ovarian index between experimental group A, experimental group B and control group B (P>0.05); however, compared with control group A, there was significant difference (P<0.05). The results showed that hypothalamic tissues in experimental group Bhad the highest expression of netrin-1 with the other three groups, which suggested that after puberty, netrin-1 guides the two levels, nerves and hormones in the hypothalamus; while plays an important role in regulating the expression and secretion of sex hormones. Netrin-1 expression level presented a gradual increase in the process, suggesting that netrin-1 may regulate nerve cell orientation in hypothalamic tissues and regulate apoptosis through binding to its receptor. Since many studies have shown that netrin-1 binds to its receptor, the dissociation process has an important regulatory role in tumorigenesis[15-17]. Co-immunoprecipitation experiments revealed that the complex of netrin-1 and its receptor UNC5C are positively correlated with netrin-1 expression levels.
Netrin-1, in nervous system development and repair functions, is primarily dependent on its regulation of axon growth and oriented cell migration. When the central nervous system becomes impaired, netrin-1 mainly plays its anti-apoptotic role by regulating the direction of axon formation, inducing cell migration, and so on. The neurological guiding role of netrin-1 is mainly to guide neuroepithelial cells and axons; which can be extend along the ventral and dorsal, up to the lower levels of nerve cells and axons; forming the correct target organ transfer relationship[18-20]. In experiments, netrin-1 was highly expressed in the precocious puberty model of the hypothalamus. Netrin-1 is activated by dynamic hypothalamic tissues during nerve cells guiding, while generating neurological transmitters might directly act on HPGA. Thus, upregulating GnRH expression[21] and activating a downstream series of signaling molecules starts up puberty; while self-regulation of netrin-1 is inseparable from its receptor. As such, the disruption of expression and regulation of netrin-1 or its receptor could be an important cause for the occurrence of central precocious puberty.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Zhao L, Zhong Y. Effects of central precocious puberty on physical and sexual development in children. Zhongguo Dang Dai Er Ke Za Zhi 2014; 16(5): 555-559.
[2] Giger RJ, Hollis ER 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol 2010; 2(7): a001867.
[3] Karamikheirabad M, Behzadi G, Faghihi M, Raoofian R, Ejtemaei Mehr S, Zuure WA, et al. A role for endocannabinoids in acute stress-induced suppression of the hypothalamic-pituitary-gonadal axis in male rats. Clin Exp Reprod Med. 2013; 40(4): 155-162.
[4] Tian Z, Zhao H, Sun Y, Cai D, Chen B. Evaluation of the true precocious puberty rats induced by neonatal administration of Danazol: therapeutic effects of nourishing “Yin”-Removing “Fire” Chinese herb mixture. Reprod Biol Endocrinol 2005; 3: 38.
[5] K.J. Livak, T.D. Schmittgen. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-△△ Ct) Method. Methods 2001; 25(4): 402-408.
[6] Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update 2004; 10(1): 67-77.
[7] Kumar P, Sharma A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J Hum Reprod Sci 2014; 7(3): 170-174.
[8] Woller MJ, Tannenbaum PL, Schultz-Darken NJ, Eshelman BD, Abbott DH. Pulsatile gonadotropin-releasing hormone release from hypothalamic explants of male marmoset monkeys compared with male rats. Am J Physiol Regul Integr Comp Physiol 2010; 298(1): R70-R78.
[9] Tang X, Wadsworth WG. SAX-3 (Robo) and UNC-40 (DCC) regulate a directional bias for axon guidance in response to multiple extracellular cues. PLoS One 2014; 9(10): e110031.
[10] Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature 2004; 431(7004): 80-84.
[11] Auger ML, Schmidt ER, Manitt C, Dal-Bo G, Pasterkamp RJ, Flores C. unc5c haploinsufficient phenotype: striking similarities with the dcc haploinsufficiency model. Eur J Neurosci 2013; 38(6): 2853-2863.
[12] Goldman JS, Ashour MA, Magdesian MH, Tritsch NX, Harris SN, Christofi N, et al. Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. J Neurosci 2013; 33(44): 17278-17289.
[13] Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci 2011; 31(1): 15-25.
[14] Paradisi A1, Maisse C, Coissieux MM, Gadot N, Lépinasse F, Delloye-Bourgeois C, et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc Natl Acad Sci U S A 2009; 106(40): 17146-17151.
[15] Geisbrecht BV, Dowd KA, Barfield RW, Longo PA, Leahy DJ. Netrin binds discrete subdomains of DCC and UNC5 and mediates interactions between DCC and heparin. J Biol Chem 2003; 278(35): 32561-32568.
[16] Thiebault K, Mazelin L, Pays L, Llambi F, Joly MO, Scoazec JY, et al. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci U S A 2003; 100(7): 4173-4178.
[17] Küry S, Garrec C, Airaud F, Breheret F, Guibert V, Frenard C, et al. Evaluation of the colorectal cancer risk conferred by rare UNC5C alleles. World J Gastroenterol 2014; 20(1): 204-213.
[18] Madison RD, Zomorodi A, Robinson GA. Netrin-1 and peripheral nerve regeneration in the adult rat. Exp Neurol 2000; 161(2): 563-570.
[19] Qu C, Dwyer T, Shao Q, Yang T, Huang H, Liu G. Direct binding of TUBB3 with DCC couples netrin-1 signaling to intracellular microtubule dynamics in axon outgrowth and guidance. J Cell Sci 2013; 126(Pt 14): 3070-3081.
[20] Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/ neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell 2003; 4(3): 371-382.
[21] Low VF, Fiorini Z, Fisher L, Jasoni CL. Netrin-1 stimulates developing GnRH neurons to extend neurites to the median eminence in a calciumdependent manner. PLoS One 2012; 7(10): e46999.
ent heading
10.1016/S1995-7645(14)60322-9
*Corresponding author: Jie Zhang, Maternal and Children Health Hospital, Affiliated Hospital of Hebei United University, Tangshan, China.
Tel: 15931559599
E-mail: gb20566@sina.com
Project supported by the natural science foundation of Hebei province (No. H2013209314).
UNC5C
Central precocious puberty
 Asian Pacific Journal of Tropical Medicine2015年3期
Asian Pacific Journal of Tropical Medicine2015年3期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Afebrile presentation of 2014 Western Africa Ebolavirus infection: the thing that should not be forgotten
- Dengue in pregnancy: an under-reported illness, with special reference to other existing co-infections
- Relevance of EGFR gene mutation with pathological features and prognosis in patients with non-small-cell lung carcinoma
- Influence of artificial luminous environment and TCM intervention on development of myopia rabbits
- MicroRNA-126 inhibits the proliferation of lung cancer cell line A549
- Effects of antiarrhythmic peptide 10 on acute ventricular arrhythmia
