A saturated Keggin silicotungstate-based transition-metal derivative[Cu(biim)2]4[α-SiW12O40]2·4H2O
LUO Jie,CHANG Shenzhen,CHEN Lijuan(Henan Key Laboratory of Polyoxometalate Chemistry,College of Chemistry and Chemical Engineering,Henan University,Kaifeng 475004,Henan,China)
Polyoxometalates(POMs)constitute a unique class of inorganic metal-oxide clusters formed by early d-block elements in high oxidation states such as WVI,MoVIand VV[1].The use of POMs as basic building blocks to construct novel extended solid materials by means of different cationic linkers is of great interest and stems not only from their in-triguing variety of architectures and topologies,but also from their multiple latent applications in catalysis,magnetic materials,sensor-technology and nanotechnology[2-5].A brand-new advance in the field of POM chemistry is preparing POMbased hybrids with transition-metal complexes(TMCs)[6].Since the first Keggin POA-supported TMC[Ni(2,2′-bipy)3]1.5[PW12O40Ni(2,2′-bipy)2(H2O)]·0.5H2O[7]was reported,various high negative charge and oxygen-rich POMs were used as original materials,thus great efforts have been given to this field[8].However,the synthetic methods are primary focused on conventional aqueous solution approach,which prevents many unpredictable TMC containing POMs to be synthesized.Therefore,it is necessary that other synthetic assembly strategies should be adopted.As we know,the hydrothermal synthesis is an effective method for growing crystals of organic-inorganic hybrid POM materials since various POM precursors or organic ligands with the low solubility can be introduced to the reaction hydrothermal system.This method has led to some typical examples.For instance,in 2007,a 1Dcopper-4,4′-bipy containing ST[Cu(4,4′-bipy)]2[SiW12O40{Cu(4,4′-bipy)}4]·(OH)2·H2O was reported by NIU and co-workers[9].In 2008,YANG et al synthesized a series of octa-nuclear copper(II)cations substituted POMs[10].Very recently,a ST-based hybrid[SiW12O40][Ag4(2,2′-bpy)4(4,4′-bpy)3]·2H2O containing a discrete tetranuclear silver-2,2′-bpy-4,4′-bpy cluster was prepared[11].In 2009,we began to study the hydrothermal reaction of lacunary POM precursors with TMCs.Thereby,we have successfully obtained a series of TMC-including POMs[12-15]such as[enH2]2[Ni(H2O)4]2[Ni(en)2]2[Ni(en)]2{[(α-AsW6O26)Ni6(OH)2(H2O)3(en)(B-α-AsW9O34)]2[W4O16][Ni3(H2O)2(en)]2}·16H2O[14],[Cu(en)2(H2O)]2[Cu(en)2][Cu6(en)2(H2O)2(B-α-AsW9O34)2]·en·9H2O[15].As a part of our continuous exploration,a saturated copper-biim containing Keggintype ST[Cu(biim)2]4[α-SiW12O40]2·4H2O(1)was isolated by us.1 exhibits a 3Dsupramolecular structure formed by hydrogen bond interactions.It should be noted that 1 comprises two plenary[α-SiW12O40]4-POAs although the reaction was carried out by using the trilacunary POA as a staring material.The transformation of the trilacunary POA[α-SiW9O34]10-to the saturated Keggin-type POA[α-SiW12O40]4-had been reported by some groups[9].However,in structure of 1,bpy was not found although bpy was used as the starting material.Thus,the parallel experiments were carried out.When bpy was removed away from the reaction,1 was not obtained.This phenomenon suggests that bpy plays a certain synergistic action with other components in the preparation of 1(CCDC 1049082).Similar results have been previously encountered[15].
1 Experimental
1.1 Physical measurements
Na10[α-SiW9O34]·18H2O was prepared referring to the published method[16]and was identified by the IR spectra.Other chemicals were obtained from commercial resources and used without further purification.IR spectrum was recorded by the solid sample powder palletized with KBr on a Nicolet170SXFT-IR spectrometer over the range of 4 000-400cm-1.XPS analysis was performed on an Axis Ultra X-ray photoelectron spectrometer.
1.2 Preparation of 1
Na10[α-SiW9O34]·18H2O(0.130g,0.046 7 mmol),CuCl2·2H2O(0.460g,2.697mmol),2,2′-biim(0.050g,0.373mmol)and 4,4′-bpy(0.030g,0.192mmol)were successively dissolved in 6mL H2O and stirred for 3.0hat room temperature.The mixture was sealed in a Teflonlined stainless steel autoclave(25mL),kept 160℃for 8dand cooled to indoor temperature.Black cubic crystals were obtained by filtration,washed with distilled water and dried in air at ambient temperature.Yield:ca.35%based on Na10[α-SiW9O34]·18H2O.
1.3 X-ray crystallography
The collection of diffraction data of 1 were carried out at 296(2)K on Bruker APEX-II CCD diffractometer using graphite monochromatized MoKαradiation(λ=0.071 073nm).Its structure was solved by direct methods and refined by fullmatrix least-squares techniques on F2using the SHELXTL-97 program package[17].All non-H atoms were refined anisotropically.In the difference Fourier map,no H atoms associated with the water molecules were added.The H atoms attached to C and N atoms were geometrically generated and all H atoms were refined isotropically as a riding mode using the default SHELXTL parameters.The crystallographic data and structural refinements for 1 are given in Table 1and the hydrogenbond data are provided in Table 2.
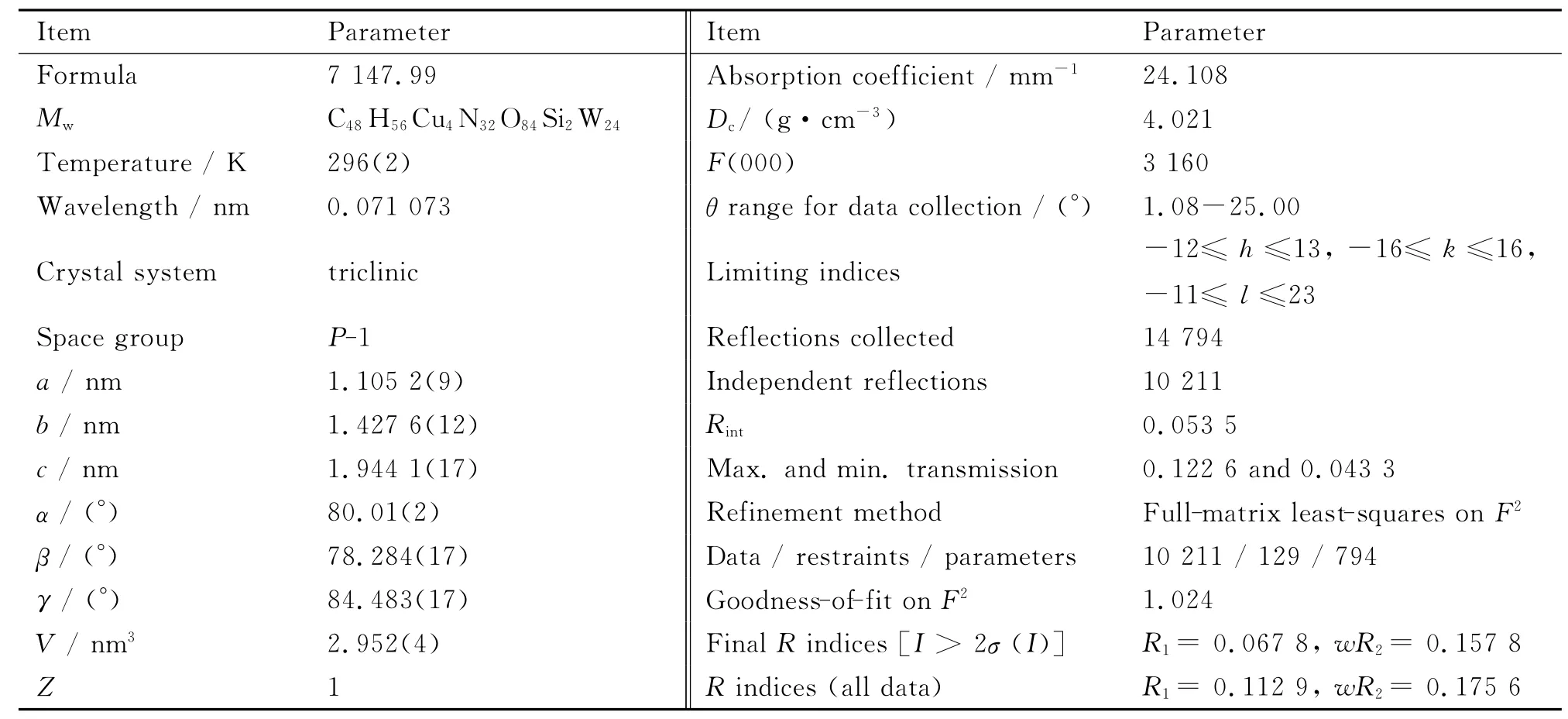
Table 1 Crystal data and structural parameters for 1
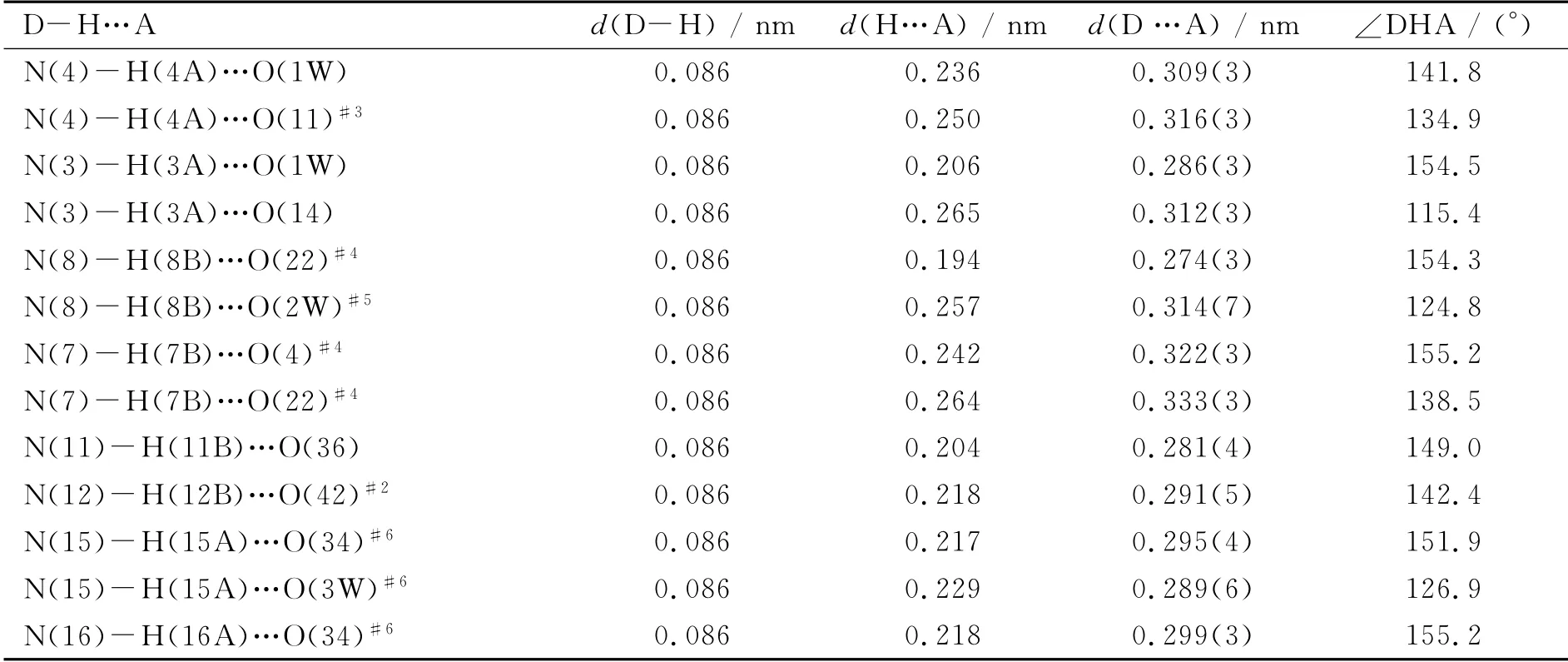
Table 2 Hydrogen bonds in 1
2 Results and discussion
2.1 Crystal structure
1 belongs to the triclinic space group P-1and consists of two neutral[Cu(biim)2]2[α-SiW12O40]units and four crystal water molecules(Fig.1a).The two[Cu(biim)2]2[α-SiW12O40]units that connected via hydrogen-bonds and each[Cu(biim)2]2[α-SiW12O40]unit is composed of two supporting[Cu(biim)2]2+coordination cations and one satu-rated Keggin-type[α-SiW12O40]4-POA.In each[Cu(biim)2]2[α-SiW12O40]unit,two mononuclear copper[Cu(biim)2]2+cations are symmetrically situated on both sides of the saturated[α-SiW12O40]4-POA and covalently link to the POA by two Cu-O-W bridges.Notably,in[α-SiW12O40]4-POA,the four oxygen atoms connected to the Si atom are all disordered over two positions with site occupancy of 50% for each.Similar disordered phenomena have been reported in the previous studies[18].This disordered phenomenon leads to the the cube geometry of the Si atom(Fig.1b).Simultaneously,the disorder of oxygen atoms also results in some W atoms no longer display the octahedral geometry(Fig.1c).According to the coordination numbers and types,all O atoms can be categorized to four types,Oa,Ob(c)and Ot.Oarefers to the O atoms that are bound to the central Si and three W atoms;Ob(c)represent the O atoms that shared by two W atoms(Obis shared by two W atoms from different W3O13triads and Ocis shared by two W atoms in a W3O13triad);Otstands for terminal O atoms linking to merely one W atom.From the crystallographic data,the WOa,W-O(b,c)and W-Otbond lengths respectively fall into 0.228(3)-0.250(3)nm,0.181 0(19)-0.196 6(17)nm and 0.162 3(18)-0.172(2)nm,which are in the normal ranges[19].In 1,each of[α-SiW12O40]4-POA serves as a bi-dentate inorganic ligand,providing two kinds of terminal O atoms(O(37),O(37A),O(13),O(13B))to connect with two[Cu(biim)2]2+complex cations.To keep under observation,we found that all[Cu(biim)2]2+complex cations exhibit a five-coordinate square pyramid geometry defined by four N atoms from two biim ligands[Cu-N:0.197 1(25)-0.198 9(22)nm]and one terminal O atom from the[α-SiW12O40]4-POA[Cu-O:0.249 6(18)nm].All copper atoms display“4+1”coordination geometry which is consistent with the fact that the CuIIion with a d9electron configuration tends to have the coordination mode because of a strong Jahn-Teller distortion effect.The two biim ligands in[Cu(biim)2]2+complex cation are not situated in the same plane but have a rotation angle which likes a fan that has two flabellums from the side view(Fig.1d).From the top and side view,we can find the[Cu(biim)2]2+complex cation exhibit the centrosymmetric structure with idealized D2symmetry relate to copper atoms(Fig.1e).In addition,the organic ligands play an important role in determining the packing arrangements of organic-inorganic assembly,in contrast to the inorganic component directing the ordered assembly of the organic moiety.
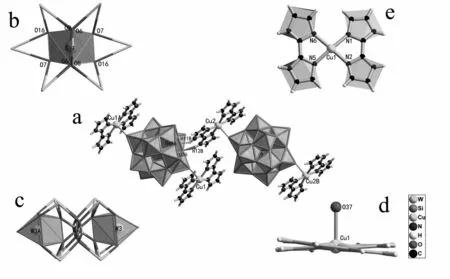
Fig.1 (a)View of the molecular structure of 1with the selected labeling scheme;(b)The central cube geometry of the central Si atom in the[α-SiW12O40]4-POA;(c)The distorted polyhedron of the tungsten atoms;(d)The side view of the[Cu(biim)2]2+cation;(e)The top view of the[Cu(biim)2]2+cation.The crystal water molecules are omitted for charity.The atoms with A and B are created by the symmetry operation:A:1x,2-y,1-z,B:1-x,1-y,-z
More interestingly,albeit 1 is an isolated structure,the 3D supramolecular architecture of 1 can be formed considering the hydrogen bonding interactions.Specifically,adjacent[Cu(biim)2]2[α-SiW12O40]units are connected together through hydrogen bond interaction(Table 2)giving rise to a 1D chain alignment,adjoining 1D chains are in-teracted with each other resulting in the 2D sheet.Finally,adjacent 2D sheets can further construct the 3D supramolecular architecture by means of hydrogen bonding interactions.In a word,the hydrogen-bonds are the main driving force that 1 can form the 3D structure(Fig.2).
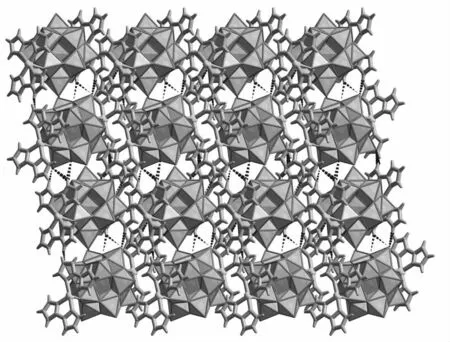
Fig.2 The 3Dsupramolecular architecture formed by hydrogen-bonding interactions viewed along b-axis
2.2 IR spectra
The IR spectrum of 1(Fig.3a)has been obtained through a KBr pellet from 4 000to 400 cm-1.We can see four characteristicν(Si-Oa),terminalν(W-Ot),corner-sharingν(W-Ob)and edge-sharingν(W-Oc)stretching vibration absorption bands for the plenary Keggin ST POA skeleton,which are observed at 922,978,877and 798cm-1,respectively,in accordance with those IR data of the saturated Keggin-type silicotungstate acid H4[α-SiW12O40]·xH2O(928,981,880,785cm-1)[20].To compare to the trivacant precursor Na10[α-SiW9O34]·18H2O(931,981,861,795cm-1),theν(W-Ot)vibration peak for 1 has a noticeable hypochromatic shift,the possible reason for which may be that the[Cu(biim)2]2+cations strongly interact with the O atoms of the POA,which weakens the W-Otbonding constants and thus results in the decline of W-Otvibration frequency.But theν(Si-Oa),ν(W-Ob)andν(W-Oc)vibration frequencies have batho-chromic shift,respectively,as compared to those of Na10[α-SiW9O34]·18H2O.The discrepancies between them reveal that the evolution of the trilacunary[α-SiW9O34]10-→the plenary[α-SiW12O40]4-.The signals at 3 382 and 3 136cm-1are attributable to theν(N-H)andν(C-H)stretching vibrations,respectively,whereas the peaks at 1 616 and 1 429cm-1are ascribed to the δ(N-H)and δ(C-H)bending vibrations,which affirms the occurrence of biim ligands in 1.O therwise,the broad vibration band at 3 502cm-1proves the presence of crystal water molecules.
2.3 XPS spectra
The bond valence sum calculations show that all the tungsten atoms are in+6oxidation state and all the copper atoms are in the+2oxidation state[21].To further verify the oxidation states of copper and tungstate atoms,the XPS investigation of crystallizing samples has been carried out to elucidate the electronic states for 1.The W4f7/2and 4f5/2peaks are observed at 33.7and 36.1eV,re-spectively(Fig.3b),which indicate that the tungstate atoms of 1 are in the+6oxidation state.The binding energy signal of 933and 952eV are attributed to Cu2p3/2and Cu2p1/2,which demonstrate the copper atoms are in the+2oxidation state(Fig.3c).These binding energy values coincide with the previous reported values[22].
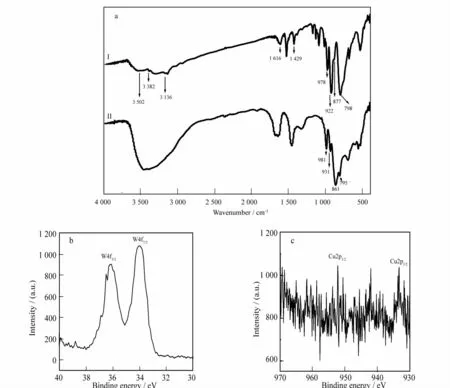
Fig.3 (a)The IR spectra of 1(I)and Na10[α-SiW9O34]·18H2O(II);(b)The XPS spectrum of 1for W4f7/2and W4f5/2;(c)The XPS spectrum of 1for Cu2p3/2and Cu2p1/2
3 Conclusion
A new organic-inorganic complicated saturated Keggin ST with Cu-biim copplexes 1 was synthesized by the hydrothermal technique and structurally characterized.1 exhibits the 3Dsupramolecular structure via the hydrogen-bond interactions between the N donors on[Cu(biim)2]2+cations and the O acceptors on[α-SiW12O40]4-POAs.Notably,the four oxygen atoms connected to the Si atom in the[α-SiW12O40]4-POA are disordered over two positions,which leads to the cubic coordination geometry of the Si atom and some W atoms no longer display the octahedral geometry.
[1]GLICK D C,BAKER L C W.Present general status of understanding of heteropoly electrolytes and a tracing of some major highlights in the history of their elucidation[J].Chem Rev,1998,98:3-49.
[2]COMPAIN J D,MIALANE P,DOLBECQ A,et al.Iron polyoxometalate single-molecule magnets[J].Angew Chem Int Ed,2009,48:3007-3081.
[3]VASYLYEV M V,NEUMANN R.New heterogeneous polyoxometalate based mesoporous catalysts for hydrogen peroxide mediated oxidation reactions[J].J Am Chem Soc,2004,126:884-890.
[4]LONG D L,TSUNASHIMA R,CRONIN L.Polyoxometalates:building blocks for functional nanoscale systems[J].Angew Chem Int Ed,2010,49:1736-1758.
[5]MALIKA A.Polyoxometalates:formation,structures,principal properties,main deposition methods and application in sensing[J].J Mater Chem A,2013,1:6291-6312.
[6]SU Z M,SHA J Q,PEN J,et al.Keggin POMs modified by bonding to multitrack Cu(bipy)chains through linearly arrayed terminal and bridging oxygen atoms of the M3O3triad[J].Eur J Inorg Chem,2007:1268-1274.
[7]YOU X Z,ZHANG K L,ZHANG Y,et al.Keggin unit supported transition metal complexes:hydrothermal synthesis and characterization of[Ni(2,2′-bipy)3]1.5[PW12O40Ni(2,2′-bipy)2(H2O)]·0.5H2O and[Co(1,10′-phen)3]1.5[PMo12O40Co(1,10′-phen)2(H2O)]·0.5H2O[J].Chem Commun,2000:153-154.
[8]MIALANE P,DOLBECQ A,MARROT J,et al.A nonanuclear copper(II)polyoxometalate assembled around aμ-1,1,1,3,3,3-azido ligand and its parent tetranuclear complex[J].Chem Eur J,2005,11:1771-1778.
[9]NIU J Y,WANG J P,DU J,et al.Hydrothermal synthesis and crystal structure of a 1Dfourfold-chain poyoxometalate-based complex[Cu(4,4′-bipy)]2[SiW12O40{Cu(4,4′-bipy)}4]·(OH)2·H2O[J].Inorg Chem Commun,2007,10:1391-1393.
[10]YANG G Y,ZHAO J W,WANG C M,et al.Combination of lacunary polyoxometalates and high-nuclear transition metal clusters under hydrothermal conditions:IX.A series of novel polyoxotungstates sandwiched by octa-copper clusters[J].Chem Eur J,2008,14:9223-9239.
[11]WANG L M,CUI X B,XU J Q,et al.The design,syntheses and characterization of a series of hybrids base on polyoxometalates and metal complexes[J].CrystEngComm,2014,16:430-440.
[12]ZHAO J W,CHEN L J,NIU J Y,et al.A CdSO4-like 3-D framework[Cu(en)2]3[α-AsW11NaO39]·2H2O constructed from monosodium substituted Keggin arsenotungstates and copper(II)-ethylenediamine complexes[J].Inorg Chem Commun,2009,12:707-710.
[13]ZHAO J W,CHEN L J,NIU J Y,et al.An organicinorganic hybrid nickel-substituted arsenotungstate consisting of three types of polyoxotungstate units[J].Inorg Chem Commun,2010,13:20-53.
[14]ZHAO J W,CHEN L J,SHI D Y,et al.Two 1D multi-nickel substituted arsenotungstate aggregates[J].CrystEngComm,2011,13:3462-3469.
[15]ZHAO J W,CHEN L J,SHI D Y,et al.Two organic-inorganic hybrid 1-D and 3-D polyoxotungstates constructed from hexa-CuIIsubstituted sandwich-type arsenotungstate units[J].CrystEngComm,2012,14:2797-2806.
[16]HERVÉG,TÉZÉA.Study ofα-andβ-enneatungstosilicates and-germanates[J].Inorg Chem,1977,16:2115-2117.
[17]SHELDRICK G M.SHELXTL-97,Program for crystal structure solution[CP].Germany:University of Göttingen,1997.
[18]XU Y,ZHANG G G,NIE L B,et al.Hydrothermal synthesis,characterization and properties of bicapping pseudo-Keggn type tunstovanadophosphate compound:[Co4(HPO3)2(C12H8N2)8(H2O)2](H3O)[PW9VIV3IVO40(VIVO)2]·H2O[J].Inorg Chem Commun,2006,9:329-331.
[19]TIAN A X,PENG J,SU Z M,et al.Assemblies of copper bis(triazole)coordination polymers using the same Keggin polyoxometalate template[J].Inorg Chem,2009,48:100-110.
[20]FOURMIER M,FRANCK R,THOUVENOT R,et al.Vibrational investigations of polyoxometalates.2.Evidence for anion-anion interactions in molybdenum(VI)and tungsten(VI)compounds related to the Keggin structure[J].Inorg Chem,1983,22:207-216.
[21]ALTERMATT D,BROWN I D.Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database[J].Acta Cryst B,1985,41:244-247.
[22]ZHAO J W,CHEN L J,SHI D Y,et al.Novel polyoxometalate hybrids consisting of copper-lanthanide heterometallic/lanthanide germanotungstate fragments[J].Dalton Trans,2012,41:10740-10751.