Laboratory Observations Regarding Different Instars of Cyclosainsulana (Costa, 1834) (Araneidae ) During Developmental Stages
Muhammad Saleem Khan, Muhammad Saleem Asghar, Iram Maqsood, Mohsin Bukhari*, Lemeng Han, Tang Li-jie, Li Yi-jing, Shahla A, and Khalil U R
1Department of Zoology, Wildlife and Fisheries GC University, Faisalabad, Pakistan
2College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
Laboratory Observations Regarding Different Instars of Cyclosainsulana (Costa, 1834) (Araneidae ) During Developmental Stages
Muhammad Saleem Khan1, Muhammad Saleem Asghar1, Iram Maqsood2, Mohsin Bukhari2*, Lemeng Han2, Tang Li-jie2, Li Yi-jing2, Shahla A2, and Khalil U R2
1Department of Zoology, Wildlife and Fisheries GC University, Faisalabad, Pakistan
2College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
The current experiment was conducted to find out the optimal conditions for mass rearing and developmental changes of Cyclosainsulana. The lab. conditions were maintained at (27±2)℃ and (65±5)% RH. The clear perplex cages and natural diet consisting of the aphids, larvae of the house fly and larvae of drosophila were used for rearing. C. insulana took (123.12±7.26) days to develop from eggs to adults passing through eight instars under prevailing vivo conditions. The eggs were greenish white in color with average size of 0.57 mm ±0.034. The eggs spent (7.52±1.64) days in emergence. Maximum number of spiderlings survived at the 5th instar (84%) and minimum at the 1st instar (34%). The measurements of different body parts including the total body length, cephalothorax and pedipalps of the both male and female C. insulana were done with the help of micrometer and presented as mean± SD. The measurements varied in the each developing instar. It was concluded that spiders were difficult to rear in the lab. conditions and each developing stage which was regarded as instars showed variations in size colors and body characteristics.
Cyclosainsulana, instars, vivo condition
Introduction
Due to the current race of pesticides, there is increasing interest towards Integrated Pest Management (IPM) for preventing the crop losses by this way to diminish reliance on chemical pest control and elevating the long term sustainability of agro-ecosystem. This approach uses multiple tactics to reduce or obstruct pest problems. So, IPM program needs to be encouraged (Biswas, 1996; Butt and Sherawat, 2012). Ecological diversity plays very important role in natural control (Huffaker, 1975; Khuhro et al., 2012). Through biological control, we can not only control insect pests, but also save a worth of foreign exchange used to purchase pesticides (Ghafoor and Ansar, 2011).
Spiders fall within the general area of the natural control forces which feed almost specifically on insects and form one of the most important groups of predatory organisms (Khuhro et al., 2012). Spiders dominantly inhibit the cotton fields and 3 935 genera and 44 906 spider species have been described ((Platnick, 2014; Sharma, 2014). They feed on insects kills, as much a 50 times the number of prey they actually consume (Kajak, 1978; Hooks et al., 2003). Spiders' prey searching capability, comprehensive predation range, and easy to multiply and polyphagy in their nature which make them as a plausible predatorin biological pest control (Rajeswaran et al., 2005). Being generalist cosmopolitans predator, spider act as bio-control in many natural systems (Nyffeler and Sutherland, 2003; Jeyaparvathi et al., 2013, Riaz and Naqvi, 2014) feeding on pest species including aphids, mites, cotton Jassid (Khuhro et al., 2012; Ghavami, 2008), leafhopper (Jeyaparvathi et al., 2013), lepidopteran larvae and their eggs (Hooks et al., 2003).
Among the spider families, Araneids are the most successful predatory family consisting 169 genera and 3 045 species in the natural ecosystems all over the world (Platnick, 2014). They predate all most all kinds of pests in the agro-ecosystems. Unfortunately, this spider family has never been studied for its potential predatory role and the development changes. So, the current study was constructed to study the life cycle of one predominate araneidae spider Cyclosainsulana (Costa) in the lab. condition.
Materials and Methods
Rearing of Cyclosainsulana
Female spiders with egg sac were collected from cotton plants, shrubs and bushes. All the spider species were identified up to species level using the keys formulated by Tikader and Biswas (1981), Tikadar and Malhota (1980), Barrion and Litsinger (1995) and other relevant literatures. After identification, Cyclosa spp. (Araneidae) was selected for the further studies. In the lab., each spider was transferred to opaque plastic containers (10 cm×7 cm×5 cm) containing crumpled paper towel and pieces of cotton plant leaves for shelter and moisture (Fig. 1). To prevent cannibalism, spiders were kept in separate rearing cages. Spiders were reared on natural diet consisted of the aphids, larvae of house fly and larvae of drosophila. Containers were covered with mesh cloth and maintained at room temperature (27-29℃) and humidity (65%-70%) and a light dark cycle of 12 : 12 L : D. For the mating of the adult spiders large chamber was used that contained the branches of the fresh tree for the development of the web (Fig. 2). The spiderlings were also fed on natural diet under same lab. conditions. Daily observations were made on each cage to study the complete picture of Cyclosa spp development.

Fig. 1 Rearing chamber for spiderlings

Fig. 2 Mating and incubation chamber of Cyclosainsulana
Measurements and statistical analyses
All the spiders and spiderlings body parts were measured with micrometer in respect of the total length, abdomen (length, width), padipalps, legs and cephalothorax, length and width. All the measurements were taken in millimeter (mm) for both male and female spiderlings as female of C. insulana was little larger than the male spiderand analyzed statically with SPSS (v.15). The data were represented as mean±SD.
Results
Diagnostic characteristics of Family Araneidae (Clerck, 1757)
Araneids were commonly known as orb web spidershaving three claws and eight eyes in two rows variable in size in different from genera to genera. The lateral eyes were somewhat distanced form the median eyes. The lateral and posterior median eyes had tapetum with canoe shape (Homann, 1952; Levi, 1994). Carapace variable in size and thoracic region apparently separated from cephalic region by a somewhat deep depression. Chelicerae were strong with boss and fang with two sets of strong teeth. Labium was mostly wider, free with distal edge swollen. Parallel maxillae were generally wider wide distally having scopulae. Sternum was triangular or heart shaped, feeling narrowing behind. Coxae IV nearly combined. Long strong legs little curved and covered with hair and spines. The legs were with three tarsal claws and foot claws, superior tarsal claws were similar and arranged in a single row, trichobotheria was absent on femora and tarsi, but presented on metatarsi and two rows on tibia. Female has pedipalp with single claw. Paracymbium of male palp attached to proximal end of cymbium. Abdomen overlaped the caraprace and globe in shape. Carapace had six spinnerets. Silk glands were aggregated in nature and produced viscous silk. Female epigynum contained copulatory structure located ventrally with a scape or lobe. The male had rotating palpuswithen the cymbium. Most araneidae spiders built viscid orb web.
Diagnostic characteristics of Cyclosainsulana
Cephalothorax had narrow cephalic region on anterior side. It had (1.83±0.047) mm length in the 8th molt female spider (Table 1, Fig. 2). Cephalic region was separated from thoracic region by oblique groove from "U" shape. Prosoma had dark brown pattern onwhite background. Sternum usually dark brown in color with light patches. Opisthosoma had silver and black parts. Anterior median eyes were larger than posterior median eyes. Patella of male palp had a long strong and curved spine. Femur was white, Tibia and metatarsus apically with annulations, Patella with a dark patch. Abdomen elongated with tubercles usually paired except median (Fig. 3). In female, it was generally (3.97 ±0.035) mm in length, whereas in male it was little smaller (2.56±0.018) mm in size (Tables 2 and 3). Epigyne usually small, weak and straight scape might be straight wrinkled bent, pointed or circular (Fig. 4).

Table 1 % age survival, mortality and duration among different instars at molting stage
Lab. observations
Cyclosainsulana was reared under standard laboratory conditions and found that it exhibited eight instars to develop from eggs to adults in (123.12±7.26) days. Spiders were very difficult to rear in the laboratory conditions, large number of mortality had seen in the current study, as the spiders needed natural conditions for growth. Most of the mortality occurred at the molting stage. After the emergence from the eggs66% mortality occurred during the 1st instar, 38%, 29%, 31%, 16%, 22% and 19% of mortality occurred among the 2nd, 3rd, 4th, 5th, 6th, 7th and 8th stages. The highest mortality was seen among the sipderlings might be due to starvations and unfavorable laboratory conditions.

Fig. 3 Female cyclosainsulana body parts
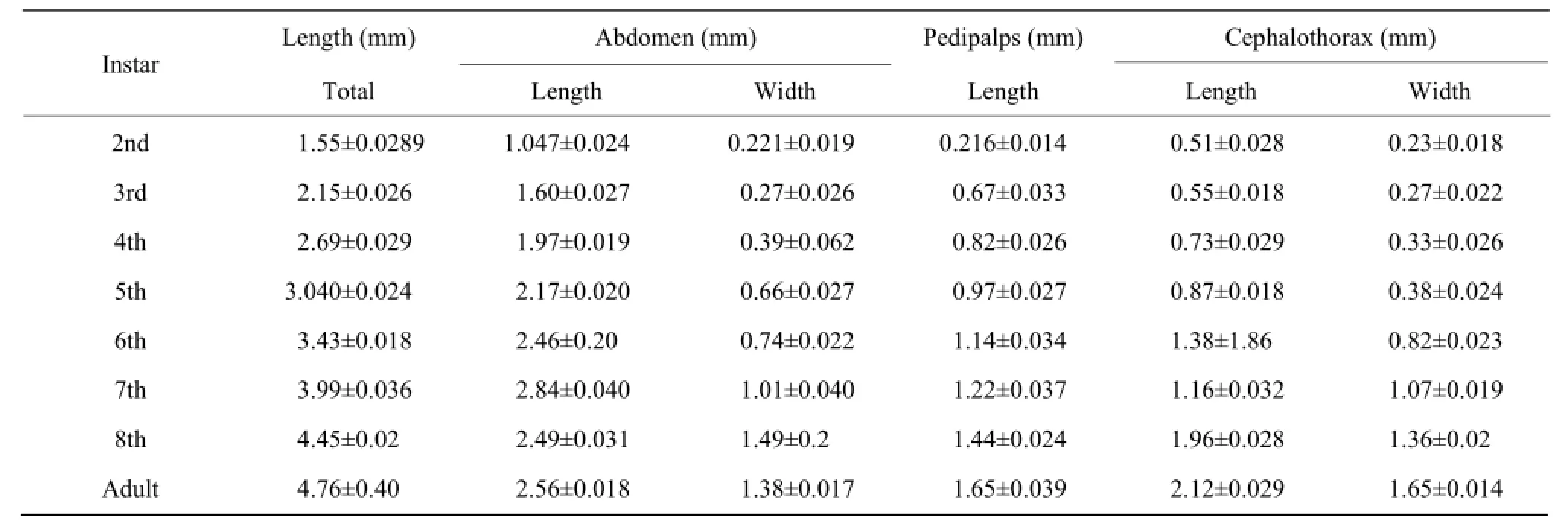
Table 2 Measurements of different body parts of male Cyclosa of each developing instar
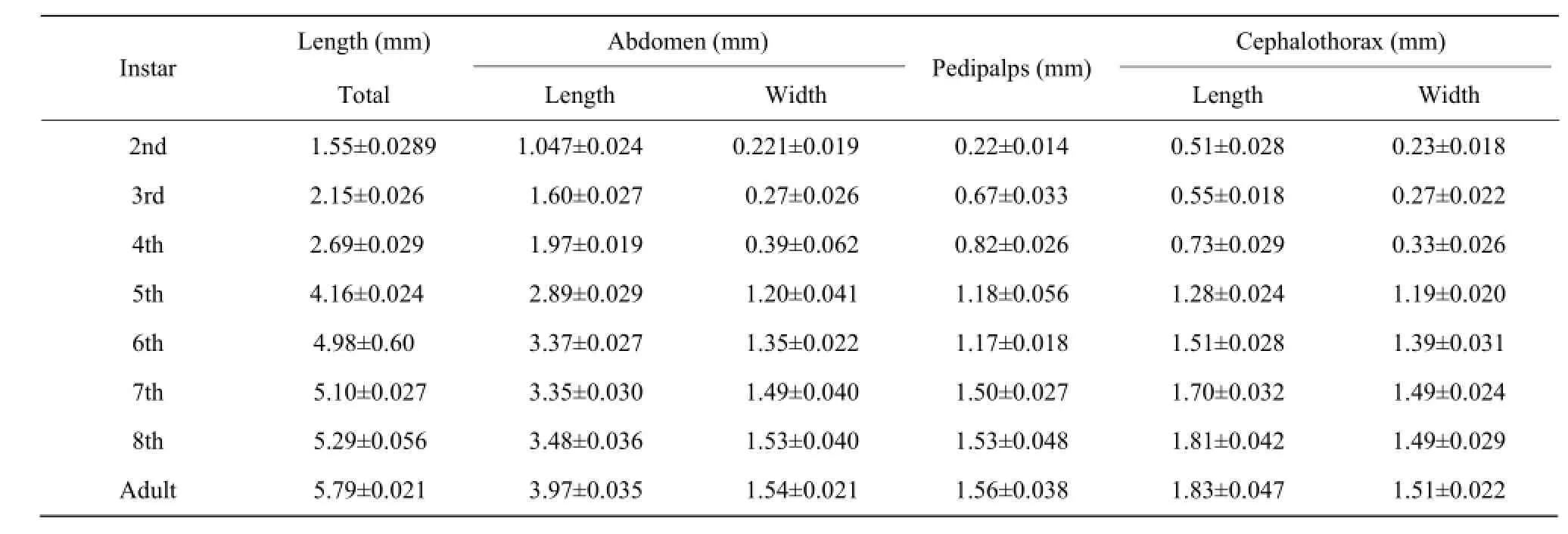
Table 3 Measurements of different body parts of female Cyclosainsulana of each developing instar
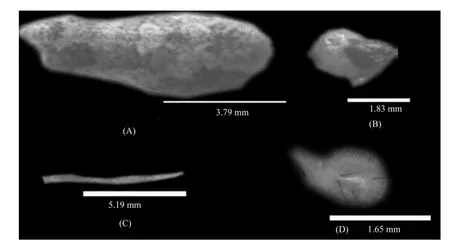
Fig. 4 Measurements of various body parts of adult female spider
Cocoon size was 3.58±0.23 with 22.4±2.60 number of the eggs. The eggs were greenish white in color with the average size of 0.57±0.034 and required (7.52± 1.64) days in emergence. Spiderlings were dependable on their mothers after emergence. They scratched their mothers' bodies as the early feeding. The mothers were observed very sluggish and feed very little on the pests provided. When the spiderlings started growing, they became independable and start preying the smaller insect larvae. The pattern of markings, structure and size of organs, the whole body color and feeding habit changed dramatically during the each instar (Fig. 5). The measurements are provided in Table 2 for male and Table 3 for female. Instars showed resemblance with the adult except body color, prey capturing techniques and sexual fecundity.

Fig. 5 Different instars
Great variations of the duration were observed among each instar of Cyclosainsulana. The 2nd instar required 3-7 days to change into the 3rd instar. The 3rd instar spent 6-12 days in the next molting stage. The 4th instar required another 13-22 days to change into the 5th instar. Spider stayed for 10-17 days in the 5th instar stage. The 6th instar changed into the 7th instar in 16-27 days. Cyclosainsulana spent 16-27 days in the 7th instar and lastly the 8th instar changed into adult in 12-24 days. Adult remained for 14-28 days up to the new molting (Table 4).
After attaining the sexual maturity both the sexes observed involved in the social activities like construction of the characteristics type web (Table 4), building of the nest by female and collection of the leaves debris and mating searching (male). Individual spiders remained near to the hub of constructed web. After 3-5 days spiders observed involve in the destruction (at night) and formation of the new web. Both field and laboratory observations were recorded and found some differences in respect of the web diameter, number of the rings and radii perhaps due to different conditions (Table 4). These differences might be due to the fact that spiders liked the natural environment. Webs founded to use for courtship, mating and capturing the prey. Female remained near to the nest and move around the eggs clutch for safe guarding. Female spiders stopped eating during the incubation period.
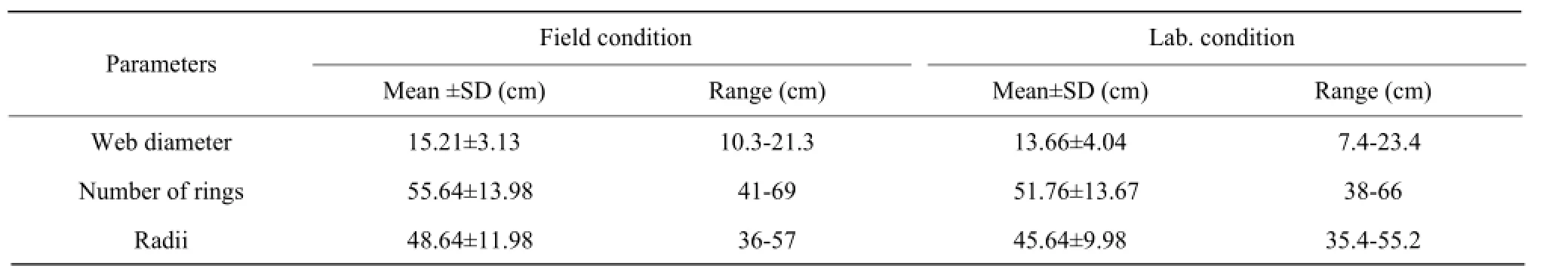
Table 4 Comparison of web properties of Cyclosainsulana in lab. and field conditions
Discussion
Spider families were extensively studied for their predatory roles in the agro-ecosystems. Most of the authors' work was based on the predatory potential of the spiders in different crops like wheat (Sherawat and Butt, 2014), cotton (Khuhro et al., 2012; Jeyaparvathi et al., 2013; Riaz and Naqvi, 2014) and in terrestrial ecosystems (Sharma, 2014). Unfortunately very little work was done on the life cycle and developmental changes in spiders' families previously. So, the current work was designed to study C. insulana.
Cyclosainsulana was reared under standard lab. conditions and found to exhibit eight instars and developed in (123.12±7.26) days from eggs to adults. Egg eclosion, the formation of the post embryo and molting process was found to be similar as it was observed by Amalin and Pena (2003). There was difference in number of molts and days of maturity which was due to the difference in spider species. These results were also related to Punzo and Farmer (2006) and Punzo (2006) who reared and studied the life history of the lycosid spiders Pardosa sierra and Arctosalittoris. Number of instars, clutch size and gestation periods were found entirely different due to difference in species and standard conditions (27±2)℃, (65%-70% RH according to the local environment).
Observations regarding the maternal care were found to be similar as that of Amalin and Pena (2003) who found that female remained near to the egg mass during period of the spiderlings development. After the emergence, spiderlings were found depended on their mothers for feeding and scratched their mothers' body for their early feeding. Mother remained dormantduring the incubation period and parental care as observed by Punzo (2006) in his studies. It was found that adult female did not eat its own spiderlings during the period of maternal care. The female inhibited to eat during the maternal period to avoid the spiderlings being eaten by their mothers (Wagner, 1995; Nyffeler, 2000).
Being delicated, it was found difficult to measure the 1st instar. When the spiderlings passed the 1st molting stage, they became independent and transferred to the spiderlings rearing cage to prevent cannibalistic behavior. The measurements of the adult of both male female Cyclosainsulana spiders observed analogue to the measurements provided by Barrion and Litsinger (1995). Both the sexes attained the sexual maturity passing through eight instars, and then involved in the social activities like construction of characteristic type web, for mating capturing prey and preventing the predators attack. Both male and female participated in the courtship in newly built web. The contact might lead to the rejection or mating of the male by the desire of females. Male to male competition was also seen in C. insluana as observed by Mcclintock & Dodson (1999). Every instar has different body colors, structures and of organs and feeding behaviors. Each instar spent specific period of time and changed to the next instar with different survival rates.
It was concluded from the present study that C. insulana was very difficult to rear in the lab. conditions as large number of the mortality was recorded in the results. It was also concluded that the investigated spider was dominant spider among the Family Araneidae with specific web characteristics and survival rates. Being dominant predator spider as recorded by Sherma (2014) could be used in controlling the pest population in all the argo-ecosystems. So, mass rearing procedure and techniques must be well explained for this predatory spider. The present work has increased the knowledge of Araneid spider lifecycle. The question of the favorable lab. conditions for mass rearing of the predatory spiders like C. insulanaawaits need further studies.
References
Amalin D, Pena J E. 2003. Development of three sac spiders occurring on lime orchards at homestead, Florida. Proc Fla State Hort Soc, 116: 44-46.
Barrion A T, Litsinger J A. 1995. Riceland spiders of south and southeast Asia. CAB International, Wallingford UK. 701.
Biswas V. 1996. Spiders of genus Oxyopes LATREILLE ogica (Aranae: Oxyopidae) of Buxa tiger. Reserve W Beng. Acta Arachnol, 45(1): 53-61.
Butt A, Sherawat S M. 2012. Effect of different agricultural practices on spiders and their prey populations in small wheat fields. Acta Agric Scand Section B- Soil Pl Sc, 62: 374-382.
Ghafoor A, Ansar M. 2011. Population dynamics of the araneid fauna from district Gujranwala, Pakistan. The J Anim Plant Sci, 21(4): 812-816.
Ghavami S. 2008. The potential of predatory spiders as biological control agents of cotton pests in Tehran Province of Iran. Asian J Exp Sci, 22(3): 303-306.
Homann H. 1952. Die Nebenaugen der Araneen, Zweite Mitteilung. Zool Jb Anat, 72: 345-367 .
Hooks C R R, Pandey R R, Johnson M W. 2003. Impact of avian and arthropod predation on lepidopteran caterpillar densities and plant productivity in an ephemeral agroecosystem. Ecol Entomol, 28: 522-532.
Huffaker C B. 1975. Biological control in the management of pest. Agro-ecosystem. Amsterdam, 2: 15-31.
Jeyaparvathi S, Baskaran S, Bakavathiappan G A. 2013. Biological control potential of spiders on the selected cotton Pests. International Journal of Pharmacy & Life Sciences, 4(4): 2568-2572.
Kajak A. 1978. Analysis of consumption by spiders under laboratory and field conditions. Ekol Pol, 26: 409-327.
Khuhro R, Ghafoor A, Mahmood A, et al. 2012. Assessment of potential of predatory spiders in controlling the cotton Jassid (Amrascadevastans) under laboratory conditions. The Journal of Animal & Plant Sciences, 22(3): 635-638.
Levi H W. 1994. New species of Bertranaand Amazonepeiraorbweaving spiders from the Neotropics (Araneae: Araneidae). Transactions of the American Microscopical Society, 113(3): 229-241.
McClintock W J, Dodson G N. 1999. Notes on Cyclosainsulana (Araneae, Araneidae) of Papua, New Guinea. J Arachnol, 27:685-688.
Nyffeler M. 2000. Do adult female lycosids feed during the period of maternal care? Bull Br arachnol Soc, 11(9): 388-390.
Nyffeler M, Sunderland S. 2003. Composition, abundance and pest control potential of spider communities in agriecosystems, a comparison of European and US studies. Agri Eco Environ, 95: 579-612.
Platnick. 2014. The World Spider Catalog. Version 15. American Museum of Natural History. http://research.amnh.org/iz/spiders/ catalog_15.0/COUNTS.html.
Punzo F. 2006. Life history ecology and behavior of the wolf spider. Arctosalittoralis (Araneae: Lycosidae) in Florida-orida Scientist, 69(2): 99–115.
Punzo F, Farmer C. 2006. Life history and ecology of the wolf spider Pardosa sierra banks (Araneae: Lycosidae) in southeastern arizona. The Southwestern Naturalist, 51(3): 310-319.
Rajeswaran J, Duraimurugan P, Shanmugam P S, et al. 2005. Role of spiders in agriculture and horticulture ecosystem. Food Agriculture & Environment (JFAE), 3(3/4): 147-152.
Riaz M, Naqvi S A H. 2014. Predation potential of foliage spiders and estimates of utilization curve, niche breadth and overlap in cotton filed from Punjab. Pakistan Journal of Biodiversity and Environmental Sciences, 5(3): 364-375.
Sharma S. 2014. A study on spiders as predators in the agro ecosystems. Munis Entomology & Zoology, 9(1): 80-83.
Sherawat S M, Butt A. 2014. Role of hunting spiders in suppression of wheat aphid. Pakistan J Zool, 46(2): 309-315.
Tikader B K, Biswas B. 1981. Spider fauna of Calcutta and vicinity. Part I, Rec. Zool Surv India Pub, 30: 1-148.
Tikader B K, Malhotra M S. 1980. The fauna of India. Araneae, Lycosidae. Zool Surv India Calculta, 1(2).
Wagner J D. 1995. Egg sac inhibits filial cannibalism in the wolf spider, Schizocosaocreata. Anim Behav, 50: 555-557.
Q96 Document code: A Article ID: 1006-8104(2015)-02-0052-08
11 December 2014
Muhammad Saleem Khan (1985-), male, Ph. D, engaged in the research of zoology. E-mail: samiikhan@yahoo.com
. Mohsin Bukhari, engaged in the research of veterinary medicine. E-mail: mohsinbukhari70@gmail.com
 Journal of Northeast Agricultural University(English Edition)2015年2期
Journal of Northeast Agricultural University(English Edition)2015年2期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Factors Hindering Pakistani Farmers' Choices Towards Adoption of Crop Insurance
- Agricultural Production Structure Adjustment Scheme Evaluation and Selection Based on DEA Model for Punjab (Pakistan)
- Where Pakistan Stands Among Top Rice Exporting Countries, an Analysis of Competitiveness
- Design and Experiment of Slave Computer Control System for Applying Variable-rate Liquid Fertilizer
- Rural Power System Load Forecast Based on Principal Component Analysis
- Cloning and Expression of Bile Salt Hydrolase Gene from Lactobacillus plantarum M1-UVS29
