Determination of Heavy Metals inDendrobium candidiumWall.ex Lindl.by lCP-MS
Long ZHENG,Chunliang YANG,Zipeng SU,Jianzhi YE
Agricultural Products Processing Research Institute,Chinese Academy of Tropical Agricultural Sciences,Zhanjiang 524001,China
Determination of Heavy Metals inDendrobium candidiumWall.ex Lindl.by lCP-MS
Long ZHENG*,Chunliang YANG,Zipeng SU,Jianzhi YE
Agricultural Products Processing Research Institute,Chinese Academy of Tropical Agricultural Sciences,Zhanjiang 524001,China
[Objective]This research aimed to establish an inductively coupled plasma mass spectrometry(ICP-MS)method for determination of five kinds of heavy metals(Pb,Cd,Cu,As and Hg)in Dendrobium candidium Wall.ex Lindl. [Method]The samples were digested in HNO3-H2O2acids system by closed vessel microwave digestion.At the same time,internal standard was added to avoid the matrix effect.[Result]The five kinds of heavy meals all showed good linear relationships,and the correlation coefficients ranged from 0.998 2 to 0.999 9.The detection limits of the five kinds of heavy metals were in the range of 0.008-0.750 μg/L,while the standard recovery rates were in the range of 90.4%-96.9%.The relative standard deviations ranged from 1.6%to 8.2%.The heavy metals in theGinsengandAstragalus national standard samples were determined by the established ICP-MS method,and the results were in conformity with the standard values.[Conclusion]The established method has simple and convenient operation and accurate and reliable results,and it meets the requirements by determination of heavy metals in Dendrobium candidium Wall.ex Lindl.
Dendrobium candidium Wall.ex Lindl.;Heavy metals;Microwave digestion;ICP-MS
Dendrobium candidium Wall.ex Lindl.(Orchidaceae:Dendrobium)is a perennial herbaceous plant,and it is named after its green skin.D.candidiumis a kind of rare Chinese medicine in China,and is described as"giant panda of medicine industry"by the international medicinal plant kingdom[1-3]. Studies have shown thatD.candidium is rich in polysaccharides,alkaloids,flavonoids,amino acids,stilbenoids,phenanthrenes and other chemical active ingredients[4-6],and it has a variety of pharmacological effects,such as fighting fatigue,promoting digestion,fighting aging,fighting rheumatism,reducing blood sugar,reducing blood lipid and fighting tumors.Currently,the researches on D.candidium mainly focus on the determination of active ingredients and pharmacological effects[7-11],instead of safety indices.With the continuous improvement of people’s consciousness of quality and safety and increasingly severe environmental pollution,heavy metal pollution has become of the focuses of attention.However,there have been no corresponding national standards in China for determination of heavy metals in D.candidium,and there have been also rare reports.Therefore,it is of great significance to establish a simple,convenient,efficient and accurate method for determination of heavy metals in D.candidium.
In China,the currently commonly used methods for determination of heavy metals include atomic absorption spectrophotometry(AAS)[11-13],atomic fluorescence spectrophotometry(AFS)[14-15],inductively coupled plasma optical emission spectrum (ICPOES)[16-17]and inductively coupled plasma mass spectrometry(ICP-MS)[18-20]. AAS and AFS can all determine only one heavy metal element at a time,and they are characterized by slow analysis speed and low efficiency. ICP-OES has faster analysis speed,and can determine simultaneously various elements.However,for the determination of trace elements in samples,the sensitivity of ECP-OES isdifficult to meet the analysis requirements.ICP-MS has advantages of high sensitivity,low detection limit and fast analysis speed,and it can also determine a variety of elements simultaneously.Therefore,in this study,ICP-MS was adopted to determine five kinds of heavy metals(Pb,Cd,Cu,As and Hg)in D.candidium,providing certain reference for researches on quality security of D.candidium.
Materials and Methods
Test materials
Main instruments and equipment
The used instruments and equipment in this study mainly included inductively coupled plasma mass spectroscopy(X seriesII,ThermoFisher,USA),high-throughput enclosed microwave digestion system (Mars,CEM,USA),ultrapure water generator(Milli-Q Element,Millipore,French)and digestion system(DV4000,Annan,China).
Reagents and samples The used reagents included HNO3(65%,excellent level of pure,Merck,Germany),H2O2(30%,excellent level of pure,Merck,Germany),ultra-purewater(resistivity of 18.2 MΩ·cm),Pb standard stock solution(1 000 mg/L,China National Center for Quality Supervision and Testing of Iron&Steel,CISRI),Cd standard stock solution(1 000 mg/L,China National Center for Quality Supervision and Testing of Iron& Steel,CISRI),Cu standard stock solution(1 000 mg/L,China National Center for Quality Supervision and Testing of Iron&Steel,CISRI),As standard stock solution(1 000 mg/L,China National Center for Quality Supervision and Testing of Iron&Steel,CISRI),Hg standard stock solution (1 000 mg/L,China National Center for Quality Supervision and Testing of Iron&Steel,CISRI),Ginseng standard (GBW 10027,Institute of Geophysical and Geochemical Exploration,CAGS),Astragalus standard(GBW 10028,Institute of Geophysical and Geochemical Exploration,CAGS)and ICP-MS tuning solution(10 μg/L,O2si,USA).The D.candidium was purchased from a local market.
Preparation of standard solutions The Pb,Cd,Cu,As and Hg standard stock solutions were all serially diluted with 2%HNO3for preparation of a series of mixed standard solutions.
Working conditions of lCP-MS
The working conditions of ICP-MS were shown in Table 1.
Test methods
A certain amount(0.5 g,accurate to 0.01 g)of ground D.candidium powderwas dissolved in certain amounts of HNO3(7 ml)and H2O2(1 ml).After stood for 30-60 min,the mixture was placed in a digestion furnace.According to the preset heating program,the sample was digested.After the digestion,the sample was cooled to room temperature and diluted to 50 ml with ultra-pure water.Similarly,the blank solutions were prepared.
Results and Analysis
Selection of microwave digestion conditions
Different type of food requires different temperature and time in microwave digestion.Therefore,the optimal conditions for microwave digestion of D.candidium were determined through several tests.Under the optimal digestion conditions, samples could be digested completely.The digestion process was shown in Table 2.
lsotope selection and interference correction
In the ICP-MS analysis,the mass spectral interference can be avoided to the greatest extent by adopting appropriate isotope for analyte,equation correction or CCT technology.The isotopes for various heavy metal elements were determined as follows:
208Pb,111Cd,65Cu,75As and202Hg.In order to overcome the matrix effects of samples,Ge-Rh-In-Re mixed internal standard was added online for result correction.
Linear range,linear equation,correlation coefficient and detection limit
The contents of heavy metals in the prepared standard solutions were determined.With the counting ratio between analyte and internal standard as the vertical axis,and the mass concentration as the horizontal axis,the standard curve was drawn.The heavy metals in each blank solution were determined 11 times,and the detection limit of each heavy metal element was calculated (3S/N).The linear range,linear equation,correlation coefficient and detection limit of each heavy metal element were shown in Table 3. As shown in Table 3,the correlation coefficients ranged from 0.998 2 to 0.999 9,and the detection limits ranged from 0.008 to 0.750 μg/L.
Accuracy and precision
The heavy metals in the Ginseng or Astragalus standard were determined 6 times by the established ICPMS method.As shown in Table 4 and Table 5,the determined results were all in the reference ranges.
Standard addition test
Standard addition test was conducted for the heavy metal contentknown samples.Each sample was determined six times by the established ICP-MS method.As shown in Table 6,the average recovery rates of the five kinds of heavy metal elements ranged from 90.4%to 96.9%,and the relative standard deviations ranged from 1.6% to 8.2%,meeting the analytical requirements by heavy metal elements in D.candidium.

Table 1 Working conditions of ICP-MS
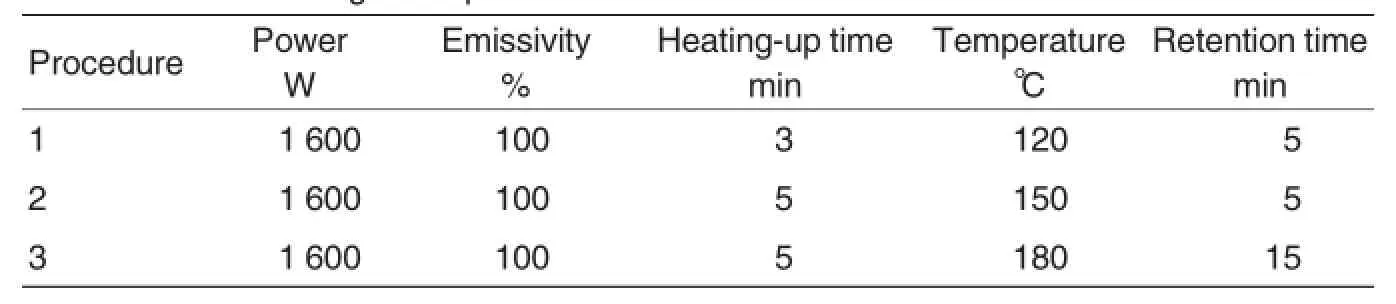
Table 2 Microwave digestion process
Sample determination
The contents of heavy metals in the five purchased D.candidium samples were determined by the established ICP-MS method(Table 7).According to the requirements by"the Import and Export Green-industry Criterion of the Drug Plant and Preparation",the Pb,Cd,Cu,As and Hg contents should not be higher than 5.0,0.3,20.0,2.0 and 0.2 mg/kg,respectively.As shown in Table 7,the Pb,Cd,Cu and As contents in the five D.candidium samples were all below the indexes.The Hg contents in four out the five samples were also below the index.
Conclusions and Discussion
Combining microwave digestion and ICP-MS methods,a new method was established in this study for determination of heavy metal content in D.candidium.Under the conditions of high pressure and closed environment,the digestion will be faster and complete.Thus,dozens of samples can be processed at a time,saving time and effort.ICP-MS can determine the contents of a variety of elements at a time,and it is characterized by fast analysis speed and high sensitivity,meeting the requirements by rapid analysis of heavy metal elements in D. candidium.
Based on the edition of"Pharmacopoeia of People’s Republic of China",the State Pharmacopoeia Commission of China intended to publish the limits of heavy metals and toxic elements in 2010.This study will not only provide a scientific method for determination of heavy metal elements in D.candidium,but also provide scientific data support for quality control,as well as preparation of heavy metal limit standards for D.candidium.
In this study,the heavy metal content differed greatly among different samples and differentelement,which was mainly related to heavy metal content in soil and selection,enrichment and accumulation ability of D.candidium.The correlation of heavy metal content between D.candidium and soil,as well as the absorption and enrichment characteristics of D.candidium for heavy metals,all require further studies.

Table 3 Linear range,linear equation,correlation coefficient and detection limit of ICP-MS for each heavy metal element
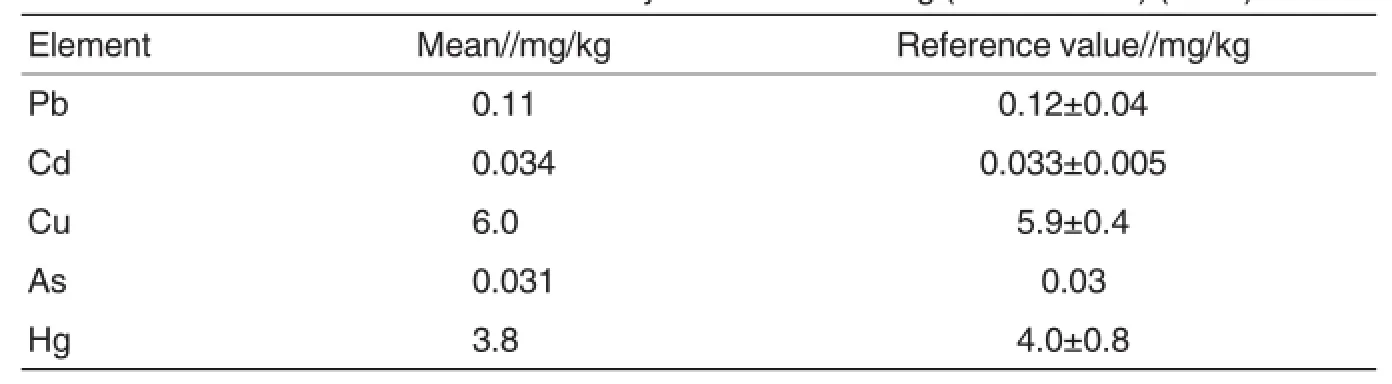
Table 4 Contents of the five kinds of heavy metals in Ginseng(GBW 10027)(n=6)
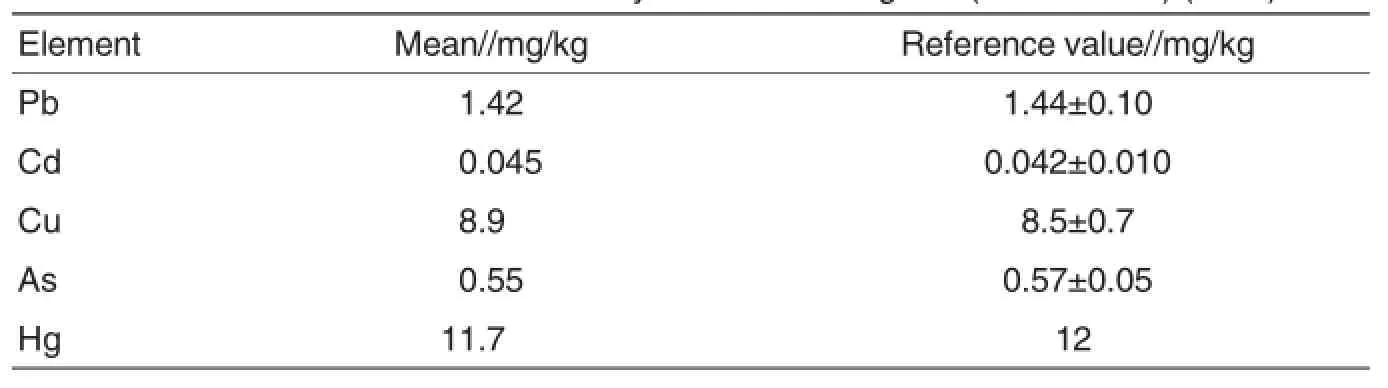
Table 5 Contents of the five kinds of heavy metals in Astragalus(GBW 10028)(n=6)

Table 6 Recovery rate of standard addition and relative standard deviation(n=6)

Table 7 Determination results of the samples(n=6)mg/kg
[1]SHAO H(邵华),ZHANG LQ(张玲琪),LI JM(李俊梅),et al.Advances in research of Dendrobium officinale(铁皮石斛研究进展)[J].Chinese Traditional and Herbal Drugs(中草药),2004,35(1):109-112.
[2]LI GF(李桂锋),LI JJ(李进进),XU JY(许继勇),et al.A review of Dendrobium candidium Wall.ex Lindl(铁皮石斛研究综述)[J].Journal of Chinese Medicinal Materials(中药材),2010,33(1):150-153.
[3]LI HY(李宏杨),LIU GM(刘国民),YANG ZJ(杨志娟),et al.Research and devel-opment review and prospects ofDendrobium officinale(铁皮石斛研究开发综述与展望)[J].Journal of Anhui Agricultural Sciences (安徽农业科学),2014,(34):12083-12086.
[4]LIU WJ(刘文杰),SUN ZR(孙志蓉),DU Y(杜远),et al.Main chemical compositions and fingerprints ofTiepishihu(Dendrobium candidium)produced from different areas(不同产地铁皮石斛主要化学成分及指纹图谱研究)[J].Journal of Beijing University of Chinese Medicine(北京中医药大学学报),2013,36(2):117-120.
[5]HU XC(胡贤春),GUO YB(郭永兵),XIANG JS(向劲松),et al.Advances in the study of polysaccharide from Dendrobium officinale(铁皮石斛多糖的研究进展)[J].Journal of Anhui Agricultural Sciences(安徽农业科学),2015,(15):78-80,84.
[6]LI J(李娟),LI SX(李顺祥),HUANG D(黄丹),et al.Advances in the research of resources,constituents and pharmacological effects ofDendrobium officinale(铁皮石斛资源、化学成分及药理作用研究进展)[J].Science&Technology Review(科技导报),2011,29(18):74-79.
[7]LIU LF(柳莲芳).Latest research advances in caulis dendrorbii(铁皮石斛的最新研究进展)[J].Journal of Anhui Agricultural Sciences(安徽农业科学),2012,40(11):6426-6428,6430.
[8]ZHOU J(周佳),ZHOU XL(周先丽),LIANG CQ (梁成钦),et al.Chemical constitutes ofDendrobium officinale(铁皮石斛化学成分研究)[J].Chinese Traditional and Herbal Drugs(中草药),2015,46(9):1292-1295.
[9]LI H(李昊),WU DH(吕鼎豪).Advances in studies on chemical constituents in Dendrobium officinale(铁皮石斛药用成分研究进展)[J].Chinese Journal of Spectroscopy Laboratory(光谱实验室),2013,30(4):1845-1849.
[10]LI L(李玲),DENG XL(邓晓兰),ZHAO XB(赵兴兵),et al.Advances in studies on chemical constituents inDendrobium candidiumand their pharmacological effects(铁皮石斛化学成分及药理作用研究进展)[J].Anti-tumor Pharmacy(肿瘤药学),2011,01(2):90-94.
[11]RASMUSSEN RR,QIAN Y,SLOTH JJ,et al.SPE HG-AAS method for the determination of inorganic arsenic in rice-Results from method validation studies and a survey on rice products[J].Analytical and Bioanalytical Chemistry,2013,405(24):7851-7857.
[12]JIN L(金烈).Atomic absorption spectrometry determination of terrace element in the oat(原子吸收分光光度法测定燕麦中微量元素)[J].Applied Chemical Industry(应用化工),2015,44(1):187-189.
[13]FAN YG (范亚刚),JI QL(季秋玲),FENG ZX (冯振兴),et al.Application of atomic absorption spectrophotometry on content determination of metallic elements in drugs(原子吸收分光光度法在药品金属元素含量测定中的应用)[J].China Modern Medicine(中国当代医药),2014,21(34):167-169.
[14]CHEN SY(陈思颖),LAN B(兰波),ZHU D (朱迪),et al.Determination of selenium in Gastrodia elata and Quantianma capsule by atomic fluorescence spectrometry with high pressure digestion(高压消解-原子荧光光谱法测定天麻药材及全天麻胶囊中硒的含量)[J].Chinese Journal of Experimental Traditional Medical Formulae(中国实验方剂学杂志),2013,19(12): 79-82.
[15]LI XF(李险峰),DENG BY(邓必阳). Determination of metals in Dendrobium officinale by microwave digestion with ICP-AES(微波消解-ICP-AES法测定铁皮石斛中多种金属元素)[J]. Hubei Agricultural Sciences(湖北农业科学),2012,51(24):5769-5770.
[16]HU XL(胡小玲),CHEN JG (陈剑刚),ZHANG Y (张艳),et al.Detection of cadmium in rice by inductively coupled plasma optical emission spectrometry(ICP-OES测定大米中镉的方法研究)[J].Practical Preventive Medicine (实用预防医学),2015,22(8):930-932.
[17]WANG HF(王海凤),TANG XM(唐兴敏 ).Determination of calcium and strontium in beer by ICP-OES(电感耦合等离子体发射光谱法测定啤酒中的钙和锶)[J].Resources Environment& Engineering(资源环境与工程),2015,(3):351-353.
[18]LU MB(陆美斌),WANG BJ(王步军),LI JM (李静梅),et al.Research of heavy metals determination in cereals by inductively coupled plasma mass spectrometry(电感耦合等离子体质谱法测定谷物中重金属含量的方法研究)[J].Spectroscopy and Spectral Analysis(光谱学与光谱分析),2012,32(8): 2234-2237.
[19]YANG L(杨磊),AI XW (艾鑫卫),HUANG WY(黄文邺),et al.Determination of heavy metals in vegetables by inductively coupled plasma mass spectrometry(电感耦合等离子体质谱法同时测定市售蔬菜中8种重金属)[J].Journal of Anhui Agricultural Sciences(安徽农业科学),2015,(16): 254-255,267.
[20]FABIEN POINTURIER,AM LIE HUBERT,ANNE-CLAIRE POTTIN,et al. Performance of laser ablation: quadrupole-based ICP-MS coupling for the analysis of single micrometric uranium particles[J].Journal of Radioanalytical and Nuclear Chemistry:An international Journal Dealing with All Aspects and Applications of Nuclear Chemistry,2013,296(2):609-616.
Responsible editor:Tingting XU
Responsible proofreader:Xiaoyan WU
ICP-MS测定铁皮石斛中5种重金属元素的研究
郑龙*,杨春亮,苏子鹏,叶剑芝 (中国热带农业科学院农产品加工研究所,广东湛江524001)
[目的]建立电感耦合等离子体质谱(ICP-MS)法测定铁皮石斛中Pb、Cd、Cu、As、Hg等5种重金属元素的方法。[方法]样品采用硝酸、双氧水混合体系进行微波消解,电感耦合等离子体质谱(ICP-MS)进行测定。同时为避免基体效应等影响,加入内标进行校正。[结果]5种重金属元素线性关系良好,相关系数为0.998 2~0.999 9,检出限范围为0.008~0.750 μg/L。加标回收率为90.4%~96.9%,相对标准偏差为1.6%~8.2%。用该法测定国家标准物质人参和黄芪,测定结果均在标准参考值范围内。[结论]建立的方法操作简便快捷,结果准确可靠,满足铁皮石斛中重金属元素的测定要求。
铁皮石斛;重金属元素;微波消解;ICP-MS
郑龙(1981-),男,福建龙岩人,助理研究员,从事农产品质量与安全研究。*
2015-11-08
*Corresponding author.E-mail:zhenglong169@163.com
Received:November 8,2015 Accepted:December 10,2015
修回日期 2015-12-10
 Agricultural Science & Technology2015年12期
Agricultural Science & Technology2015年12期
- Agricultural Science & Technology的其它文章
- Control Effect of 35%Thiamethoxam-prochloraz FS to Plant Diseases and lnsect Pests at Rice Seedling Stage and Safety Evaluation
- Effects of Different Decolorants on Retention Rate of Total Triterpenes in Fruit and Rattan Stems of Schisandra chinensis(Turcz.)Baill
- Dynamic Variation in Sugar,Acid,and ASA Contents of‘Ganmi 6’Kiwifruit(Actinidia eriantha Benth)Fruits
- Construction and Development of GMS Agricultural lnformation Network Based on Stakeholder Analysis
- An lnnovative Strategy for Reciprocal Distant Hybridization between Spartina alterniflora and Rice
- Study on Absorptive Capacity to Formaldehyde and Physiological and Biochemical Changes of Scindapsus aureus Based on the Regulation of LaCl3
