Experimental investigation of methyl tert-butyl ether dissolution in saturated porous media☆
Hong Li,Lei Zhao ,Xin Gao ,3,*,Xingang Li,3
1 School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 Collaborative Innovation Center of Chemical Science and Engineering,Tianjin 300072,China
3 National Engineering Research Center of Distillation Technology,Tianjin 300072,China
Keywords:MTBE Dissolution Mass transfer Porous media Organic compounds
ABSTRACT This study aims to investigate methyl tert-butyl ether(MTBE)dissolution in saturated porous media.A series of 1Dcolumn experiments were conducted in laboratory to obtain MTBE dissolution data with differentgroundwater velocity,initial MTBE saturation and grain size of porous medium,and in the presence of other nonaqueous liquids.Results indicate that higher groundwater velocity increases MTBE dissolution rate and higher initial MTBE saturation reduces effective permeability to slow MTBE dissolution rate.Smaller grain size medium gives higher MTBE dissolution rate because of higher permeability.The addition of trichloroethylene enhances MTBE dissolution,with an optimal mass ratio of 10:2,while the presence of p-xylene prolongs complete dissolution of MTBE.Mass transfer correlations are developed for MTBE dissolution rate based on the degree of MTBE saturation Sn.Mass transfer rate is characterized by Re′with a high exponent for 0.3000<Sn<0.5482,while it is related to medium grain size and Sn for Sn≤0.3000.
1.Introduction
Nonaqueous phase liquids(NAPLs),such as aromatic hydrocarbon,chlorinated solvents and methyl tert-butyl ether(MTBE),are the main contaminants in subsurface environments.The location,prediction,and remediation of NAPL contaminated zones are signi ficantly in fluenced by NAPL transport process,which mainly includes dissolution and diffusion of NAPL.It is important to understand NAPL dissolution in groundwater.MTBE is once an additive in gasoline,but its effect on groundwater has not received suf ficient attention.When MTBE leaks from an oil storage tank or pipeline,it will penetrate water surface quickly and then diffuse because of its high solubility in water[1],leading to wide-range and long-lasting pollution.Its half-life in groundwater is at least two years[2],so it will accumulate in the subsurface.MTBE has been reported[3-5]as a signi ficant pollutant in the aqueous environment.
Several investigations in laboratories and theories were carried out to better understand NAPL transport in porous media.Early experiments with homogenous short column indicated a dependence of dissolution rate on the distribution pattern of entrapped NAPLs and aqueous phase velocity[6],and volumetric NAPL content[7].Heterogeneous systems with a contaminated coarse sand region surrounded by fine sand were used to investigate the effect of porous medium on NAPL dissolution[8-10].Most of experiments have a common characteristic:ef fluent concentration approaches the NAPL equilibrium concentration at the beginning and gradually decreases to its detection limitation[8-12].It can be explained by local equilibrium or limited rate assumption of dissolution kinetics[13-16],based on which some Sherwood-Gilland empirical correlations have been established to evaluate the NAPL mass transfer process[6,7,17,18],which are dependent on the experimental system.
Recently,for the mass transfer of NAPL in aqueous phase,2D and 3D flow systems were used to investigate the effects of porous medium property and transverse dispersion on flow field heterogeneity[19-23].The flow in these systems is complicated and affects the NAPL dissolution process.Some mass transfer models were presented to describe the NAPL breakthrough curves with some controlling parameters,such as capillary and mass transfer kinetics in the media[24,25].Some experiments with multi-component NAPLs,such as BTEX(benzene,ethylbenzene,toluene and xylene)and gasoline mixture,were performed to explore the interactions and dissolution in porous media.These multi-component NAPLs were considered as ideal mixtures following Raoult's law,which is the driving mechanism of the mass transfer process[26-29].However,applying Raoult's law in the presence of non-ideal organic compounds such as chlorohydrocarbon and aromatic hydrocarbons will lead to appreciable errors in estimates of NAPL dissolution process in porous media.
These investigations provide valuable insights on NAPL dissolution in subsurface environments,but the study on MTBE is limited[30].A study has revealed that porous medium and fluid properties affect MTBE dissolution and its mass transfer in saturated and unsaturated porous media[31],so the data for MTBE are essential to improve our knowledge of its dissolution in porous media.Since a shortcolumn cannot provide a 1D steady flow,a column with length-diameter ratio above 10 is manufactured in this study to obtain MTBE dissolution data in saturated porous media.Some experiments focus on single MTBE to study its dissolution in saturated porous media and mass transfer coef ficient correlations dependent on experimental system are developed.Then multi-component NAPL experiments are conducted to explore how other NAPLs(trichloroethylene(TCE)and p-xylene)in fluence MTBE dissolution.MTBE dissolution data are correlated with Sherwood-Gilland empirical correlations.These data are critical to MTBE pollution treatment,especially in laboratory-scale or largerscale soil washing[32].According to the MTBE dissolution mechanism,different flushing flow rates can be used under differentconditions,and subsequent extraction and distillation can extract organic compounds from water.
2.Experimental
2.1.Materials
The physical property of MTBE,TCE,and p-xylene(chromatographic grade;Tianjin Guangfu Fine Chemical Research Institute)are presented in Table 1.

Table 1 Physical property of organic liquids
The porous media were 0.85-2.00 mm,0.42-0.85 mm,and 0.18-0.30 mm silica sand,sieved,washed and sterilized at high temperature.The basic characteristics are given in Table 2.

Table 2 Properties of experimental silica sand
2.2.Experimental setup
In order to provide horizontal one-dimensional flow,dissolution experiments were conducted with a thin column(ϕ38 mm × 400 mm)fabricated with normal transparent glass,which will not react with experimental materials.Schematic diagram of 1D dissolution experiments is given in Fig.1.Both ends of the column are bolted with flange connections,so it is convenient for loading and unloading silica sand.There are four glass ports with screw and screw cap on one side of the column,used for sample injection and sampling,and their distances to the left outlet 2 are 3,10,20,and 30 cm.NAPL injection port is in the middle of the 5 cm contaminant zone.

Fig.1.Schematic diagram of1Dcolumn dissolution experiments.1—NAPL injection port;2—outlet;3—NAPL contaminant zone;4— fine sand;5—coarse sand;6—cut off valve;7—peristaltic pump;8—in fluent reservoir;9—ef fluent reservoir.
2.3.Methods
All dissolution experiments were performed in a horizontal sand column.In order to reserve a 5.0 cm length NAPL contaminant zone,the column was filled with coarse sand in a lift of approximately 3.0 cm and 6.0 cm length was left.This layered and tightloading process ensured the soil in the column suf ficiently uniform to provide 1D flow.The 5.0 cm length contaminant zone was sandwiched by two layers of 0.5 cm fine sand(0.075 mm)as illustrated in Fig.1.Because of the difference in medium grain size,a capillary barrier could be produced by the fine sand so that the injected NAPL was retained in the 5.0 cm length contaminant zone.Stainless steel screens were placed inside the two ends of the column to prevent the loss of media.After compaction,the column was saturated by pumping distilled water ata low flow rate to eliminate air.Stable liquid flow was provided by a peristaltic pump(Master flex,Cole-Parmer Instrument Company).
Before experiments,all compounds were dyed with a small quantity of oil red O(0.05 g·L-1;Baihao Biological Technology Chemical)so that MTBE dissolution and transport could be visualized.The contaminant zone was prepared by injecting a known amount of dyed NAPLs.From the observation we could judge whether NAPL distribution in silica sand was uniform and whether NAPL was out of contaminant zone.According to the conservation of mass and silica sand properties,the initial NAPL saturation was estimated[18].A series of dissolution experiments were conducted under various conditions,i.e.,sand grain sizes with D50of 0.025,0.06,and 0.115 cm,initial NAPL saturations of 0.193,0.366,0.579,and 0.602,aqueous velocities of 2,3,and 4 ml·min-1,and different initial mass ratios of mixture.Aqueous samples were collected at the outlet of the column in certain time interval until NAPL concentration in the aqueous phase was reduced to below analytical detection limits.
2.4.Chemical analysis
Aqueous ef fluent samples were analyzed by Autosystem XL gas chromatograph(PerkinElmer,USA)equipped with a capillary column(FFAP,30 m × 0.25 mm i.d.×1.0 μm),Turbochrom4.1 workstation and Flame ionization detector.The chromatographic conditions for MTBE are as follows:injection temperature of180°C,oven temperature of105 °C,detectortemperature of210 °C,N2as the carrier gas with flow rate 0.8 ml·min-1,H2flow rate 45 ml·min-1,air flow rate 450 ml· min-1,and split ratio 10:1,injection volume of 1 μl aqueous sample.
3.Results and Discussion
3.1.MTBE dissolution curves in aqueous environment

Fig.2.Comparison of MTBE dissolution curves for different initial saturation and water velocity in 0.42-0.85 mm silica sand.
The dissolution curves at the outlet in the 0.42-0.85 mm silica sand are shown in Fig.2.The relative concentration undergoes a slight increase to the maximum solubility in approximately 30 min and then a long time drop to detection limit.The curve shape is similar to previous experimental results[9,10,17],but the maximum concentration is approximately 10%-20%of the equilibrium concentration,which is 48000 mg·L-1for MTBE.This indicates that MTBE dissolution in saturated silica sand is a rate-limiting process with signi ficant mass transfer resistance.For the two concentration curves with the same initial mass of 3 g,at 30 min,the peak relative concentration is 0.1775 and drops more quickly at 3 ml·min-1,while it is 0.1478 at 2 ml·min-1.Thus higher groundwater flow rate at the early stage of dissolution process could remarkably increase the MTBE dissolution rate so that the time needed for complete dissolution is less.This may be due to the increase in mass transfer at higher velocity.For different initial MTBE mass(3,6,and 9 g)at the same water velocity(3 ml·min-1),the time for maximum relative concentration is nearly the same,and the peak value for 3 g is the highest.Higher MTBE saturation corresponds to lower aqueous phase saturation,reducing effective permeability.In other word,the effective interface for mass transfer decreases as MTBE saturation increases.After short-time dissolution,the concentration remains constant from 100 min to 250 min for the initial mass of 6 and 9 g,indicating that effective permeability increases in the dissolution process.When the mass in the contaminant zone reduces to 3 g by dissolution from higher initial mass,the concentration curve differ from that with initial mass of 3 g.Thus,MTBE dissolution in the porous media is different in different processes.
The concentration curves from three grain sizes of silica sand are shown in Fig.3.In the first 120 min,the MTBE dissolution rate with 0.85-2.00 mm sand is the fastest,which presents the highest permeability in Table 2,while those with other two sizes are similar.Theoretically trapped large blobs could lower MTBE dissolution,but large silica particles promotes effective interface of mass transfer at low initial saturation.After 120 min,the concentration curve with 0.42-0.85 mm sand decreases faster than that with 0.18-0.30 mm sand.
3.2.Mass transfer correlation for MTBE dissolution


Fig.3.Comparison ofMTBEdissolution curves for different grain size with the same initial saturation at a water velocity of 4 ml·min-1.
The linear driving force model[28]based on the quasi-steady approximation of Fick's first law is adopted between for mass transfer of NAPL.where J is the net flux of species,Csand C represent the aqueous phase concentration of solute in thermodynamic equilibrium with the pure organic liquid and bulk aqueous phase concentration,respectively,and kfis a lumped mass transfer coef ficient,with mass transfer rate coef ficient multiplied by interfacial area of mass transfer.A mass transfer empirical correlation relate system variables as the modi fied Sherwood number,where lcis the characteristic length for mean particle diameter and D1is the molecular diffusion coef ficient of pure solute in aqueous phase.
The Sherwood-Gilland correlation presented in this study is considered as

Before the regression with Eq.(2),a group of 150 data sets from 8 experiments are processed.The lumped mass transfer coef ficient kfis calculated from a 1D steady state mass balance equation[6]:

where q is the Darcy or super ficial velocity estimated by averaging the flow over the cross-sectional area of the column.The solution of Eq.(3)in the contaminant zone is

where L is the length of contaminant zone.Meanwhile,Sn is estimated by a mass balance in the premise that the total mass of MTBE equals the sum of the mass in the ef fluent and entrapped in the column.The mass of MTBE in the ef fluent over the period is the integration of the ef fluent concentration as a function of pore volume.
Eq.(2)can be transformed to a linear form so that coef ficients α and βican be determined by multiple linear regression analysis.A stepwise regression is used with IBM SPSS Statistics.At MTBE saturation below 0.3000,the estimates of the coef ficients by a group of 110 data sets are shown in Table 3.

Table 3 Results of stepwise regression
The best correlation is the last one,with the maximum R2and the strongest signi ficance of F and t statistics.Thus the mass transfer correlation for MTBE dissolution in the 1D column is

The con fidence intervals(95%)for the coef ficients are 0.031<α<0.043,0.638<β2<0.748,and 2.097<β3<2.367.This correlation is different from other correlations because parameters MTBE saturation and medium grain size,instead of aqueous phase velocity,which is characterized by Re′,are associated with mass transfer.This indicates thatsaturation and porous mediumplay a major role in the MTBE dissolution process,and the effect of aqueous phase velocity is not obvious for 0.0080<Sn<0.2913.The possible explanation for the high water solubility of MTBE is that low saturation result in the formation of small and discrete uniformly distributed NAPL blobs in porous media and the dissolution is mainly dependent on good contact between MTBE and water phase.
Another 40 data sets with a saturation of 0.3000<Sn<0.5482 are also analyzed by a stepwise regression.The correlation is

The con fidence intervals (95%) for the coef ficients are 0.709<α<2.123 and 1.711<β1<2.247.Thus MTBE dissolution with high saturation is highly dependent on aqueous velocity.It is a local equilibrium process,in which NAPL occupies the medium with a larger volume,and is mainly related to water velocity.
Based on the mass transfer correlation,the factors in fluencing MTBE dissolution in saturated porous media are quantitatively analyzed.This not only veri fies the experimental result in 3.1,but also provides theoretical guidance for selecting appropriate parameters to improve MTBE dissolution under different conditions.
3.3.Effect of TCE and p-xylene on MTBE dissolution
Another series of experiments were conducted to explore the effects of TCE and p-xylene on MTBE dissolution.Usually MTBE was considered to be main pollutants accompanied with other NAPLs in the pollution source area.TCE or p-xylene may have different effects on MTBE dissolution and could be removed in the subsequent study.Here different amounts of TCE or p-xylene were mixed with MTBE.
The concentration curves with MTBE:TCE at 10:0,10:1,10:2,10:3,10:4,and 10:5 in the 0.85-2.00 mm silica sand are shown in Fig.4.In the first 100 min,the concentration curves with TCE are above that without TCE,and then the situation is reversed.This indicates that the addition of TCE improves the MTBE dissolution,and the shortest time for complete MTBE dissolution is that with 10:2.More TCE does not accelerate the dissolution.In the experiment,it is found that some dyed TCE is entrapped in the porous media after MTBE is almost dissolved.One possible explanation is that TCE is more dif ficult to dissolve than MTBE.Thus TCE could occupy more void space in the sands.More MTBE molecules associate with the aqueous phase at the interface,enhancing the dissolution.However,more TCE molecules may surround some MTBE molecules to slow its dissolution.Another possible explanation is that MTBE and TCE are non-ideal compounds.Ideal compounds can apply Raoult's law[34]to express the equilibrium concentration of each individual constituent in the water phase.

Fig.4.MTBE dissolution curves with different ratios of MTBE:TCE in 0.85-2.00 mm silica sand with initial MTBE mass of 6 g at water velocity of 3 ml·min-1.

For p-xylene in the 0.85-2.00 mm silica sand,the result is shown in Fig.5.In the initial period,the existence of p-xylene enhances the dissolution of MTBE,and the optimal proportion is 10:1.The addition of p-xylene prolongs the time for complete dissolution of MTBE.This could be explained by that the interaction between MTBE and pxylene interfere MTBE dissolution as the relative content of MTBE decreases.

Fig.5.MTBE dissolution curves for different ratios of MTBE:p-xylene in 0.85-2.00 mm silica sand with initial MTBE mass of 6 g at a water velocity of 3 ml·min-1.
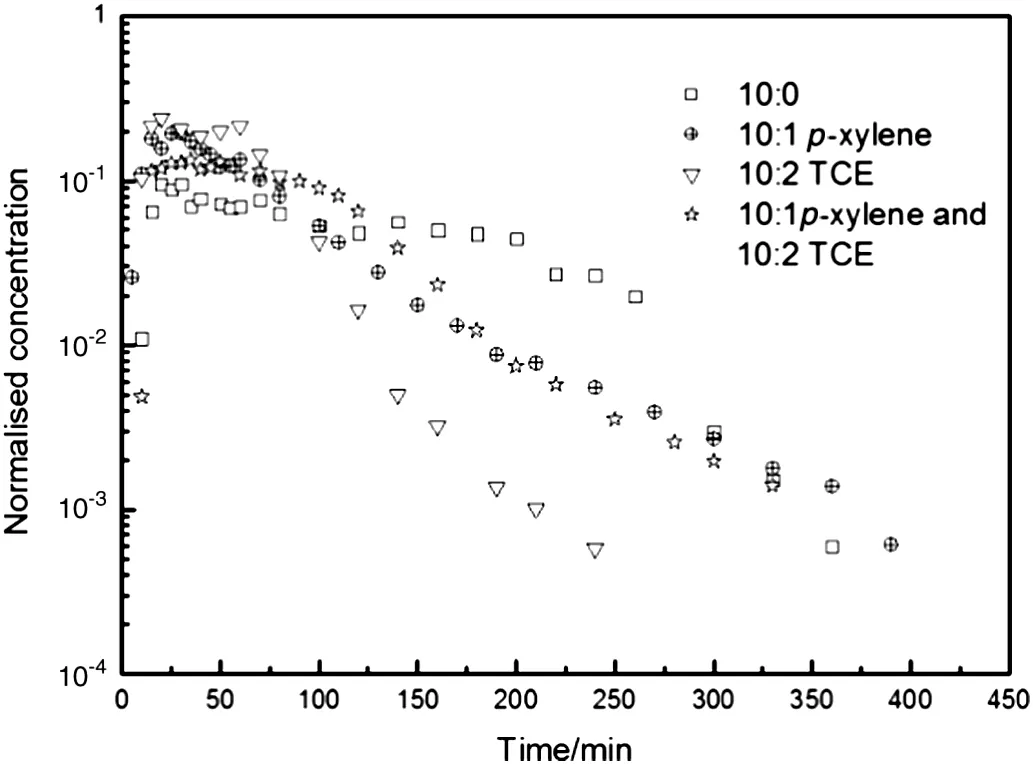
Fig.6.Comparison ofMTBE dissolution curves with the optimalamountof p-xylene or TCE in 0.85-2.00 mm silica sand with initial MTBE mass of 6 g at a water velocity of 3 ml·min-1.
The co-existence of 10:1 p-xylene and 10:2 TCE as shown in Fig.6 proves the abovementioned explanation.Before 100 min,TCE and pxylene enhance the dissolution of MTBE,but the enhancement effect is less than separate TCE or p-xylene because of low MTBE relative content.In approximately 100 min to 200 min,the dissolution concentration of co-existing TCE and p-xylene is higher than that with 10:1 p-xylene and lower than that with 10:2 TCE.This may be a consequence of TCE promotion and p-xylene inhibition,and TCE promotion is more signi ficant.The dissolution process then enters a long tail period,in which p-xylene plays an important role in prolonging complete MTBE dissolution.
4.Conclusions
MTBE dissolution in saturated porous media was investigated through a series of 1D column experiments.MTBE dissolution is related to initial MTBE mass,medium grain size,and water velocity.In the initial dissolution period,the MTBE dissolution rate increased with water velocity.However,with increasing initial MTBE mass and medium grain size,the dissolution process gradually slowed down.Future research will focus on establishing empirical correlations based on the experimental data.The factors in fluencing MTBE dissolution changed with MTBE saturation.The MTBE dissolution process mainly depends on water velocity with high saturation,and grain size and saturation play a major role in MTBE dissolution when saturation is less than 0.3000.As for MTBE dissolution with other organics,such as TCE,which is a low solubility material,can enhance MTBE dissolution and has an optimal mass ratio of that improves the dissolution rate.It also possesses the shortest time for complete MTBE dissolution.However,the presence of p-xylene may slow the dissolution rate because of the interaction between MTBE and p-xylene.
Nomenclature


 Chinese Journal of Chemical Engineering2015年10期
Chinese Journal of Chemical Engineering2015年10期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis and characterization of switchable ionic compound based on DBU,CH3OH,and CO2☆
- Comparison of numerical simulations and experiments in conical gas-solid spouted bed☆
- Molecular dynamics simulation of water transport through graphene-based nanopores:Flow behavior and structure characteristics☆
- Pore-scale study based on lattice Boltzmann method of density driven natural convection during CO2 injection project
- CFD gas-liquid simulation of oriented valve tray
- Biosorption of basic violet 10 onto activated Gossypium hirsutum seeds:Batch and fixed-bed column studies
