A comparative UV-HPLC analysis of ten brands of ibuprofen tablets
Sylvester Okhuelegbe Eraga,Mathew Ikhuoria Arhewoh,Rosemary Ngozi Chibuogwu,Magnus Amara Iwuagwu
Department of Pharmaceutics and Pharmaceutical Technology,Faculty of Pharmacy,University of Benin,Benin City,300001,Nigeria
A comparative UV-HPLC analysis of ten brands of ibuprofen tablets
Sylvester Okhuelegbe Eraga*,Mathew Ikhuoria Arhewoh,Rosemary Ngozi Chibuogwu,Magnus Amara Iwuagwu
Department of Pharmaceutics and Pharmaceutical Technology,Faculty of Pharmacy,University of Benin,Benin City,300001,Nigeria
ARTICLE INFO
Article history:
Accepted 9 Jun 2015
Available online 13 Aug 2015
HPLC
Analysis
Ibuprofen
Pharmaceutical equivalence
Label claim
Objective:To investigate the pharmaceutical equivalence of ten brands of ibuprofen tablets(400 mg)purchased from pharmacies in Benin City,Nigeria.
Methods:The drug samples were subjected to uniformity of weight,crushing strength,friability,melting point,disintegration and dissolution tests following acceptable and official protocols.The ibuprofen content was determined using UV and high performance liquid chromatography method.
Results:Crushing strength values of the drug samples ranged between 6 and 16 kp while the disintegration times were between 7.43 and 10.40 min(for uncoated tablets)and 3.25-37.32 min(for coated tablets).Friability values were less than 1%and the melting points of recrystallized ibuprofen from the samples ranged from 73.5 to 76.0°C.The amount of ibuprofen released within 1 h ranged between 18%and 102%and two brands failed the content of active ingredient in the UV method of assay while all the brands passed the test using HPLC.
Conclusions:Ibuprofen(400 mg)tablets marketed in Benin City,Nigeria vary in pharmaceutical quality.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2015.06.005
1.Introduction
Ibuprofen is formulated as tablets,caplets or capsules in 200 mg and 400 mg strengths.It is a nonsteroidal antiinflammatory drug with a half-life of 1.8-2 h.It is one of the safest drugs employed for treatment of inflammation,pain and fever[1,2].Its oral dose is 200-400 mg(5-10 mg/kg in children),every 4-6 h to a maximum of 1.2 g per day in adults.Although it is not the most potent of the nonsteroidal anti-inflammatory drugs,it represents a good balance of efficacy and safety over a wide dosage range when compared with others in the group and it is also readily available and cost-effective.
Ibuprofen has analgesic and anti-inflammatory properties.It is used in mild-to-moderate pain such as dysmenorrhea,headaches(including migraine),dental pain,postoperative pain and musculoskeletal/joint disorders including osteoarthritis,rheumatoid arthritis and ankylosing spondylitis[3,4].Reports havebeen made from various regions and quarters across the countryonthevariationsonthephysicochemicaland pharmaceutical equivalence of the various brands of ibuprofen tablets available in the Nigerian market[5,6].
Daily,health care givers are confronted with the problem of generic substitution,influenced by factors such as cost,efficacy,esthetic packaging and recently the assignment of National Agency of Food and Drug Control and Administration(NAFDAC)number on packaging[7].Thus,the community pharmacist faces the problem of making the right choice of selecting the most effective brand to dispense to the patient.Therefore,it is necessary to routinely assess the pharmaceutical quality of drugs in Nigerian market[8].Another salient aspect is in the instrumentation for content analysis which may be influenced by changes in environmental conditions.Hence it is necessary to compare UV and high performance liquid chromatography(HPLC)assay methods.
This work aims to establish if pharmaceutical equivalence exists among the various brands of ibuprofen tablets(400 mg)in the Nigeria market using Benin City as a reference point.Benin City is a large metropolitan city located in the south-south geopolitical zone of Nigeria.It has a projected population of about 1.2 million[9].The city has about 120 registeredcommunity pharmacies with about 50%of them located within the vicinity of the government hospitals(city centre),while the remaining ones are scattered over the rest of the city[10].
2.Materials and methods
2.1.Sampling
The samples(Table 1)studied were purchased from pharmacies across the city centre.Brands available in each pharmacy were selected to make up the ten brands studied.A brand already selected from one pharmacy was no longer considered in any other one.Pure ibuprofen powder was gratefully received as a gift sample from Edo Pharmaceuticals Nigeria Limited,Benin City,Nigeria.All other reagents were of analytical grade and water was double distilled.
2.2.Uniformity of weight
Twenty tablets were randomly selected from each brand and weighed individually using the electronic weighing balance(College B154,Mettler Toledo,Switzerland).The weight(mean±SD)was calculated.
2.3.Hardness test
Thecrushingstrengthwasdeterminedbydiametric compression of each of ten tablets per sample using a motorized hardness tester(Campbell Electronics,Model HT-30/50,India). The mean±SD were calculated.
2.4.Friability test
The weight of ten tablets per sample was determined on the electronic balance.The tablets were then placed in the drum of a friabilator(Erweka GmbH,Germany)revolving at 25 r/min which exposed the tablets to rolling and repeated shock resulting from freefallwithintheapparatus.After4min,thetabletswereremoved fromthefriabilatoranddustedandreweighed.Theweightlosswas obtained from the differences between the initial weight and final weight.Thefriabilitywascalculated asthepercentageweightloss.
2.5.Melting point
A tablet randomly selected from each brand was used for the determination.The ibuprofen tablet was crushed to powderusing a mortar and pestle.The powder was added in 200 mL of n-hexane.The mixture was heated for 10 min with continuous stirring.The cooled mixture was filtered through a Whattman filter paper(No.1)and the filtrate evaporated to dryness in a hot water bath to give ibuprofen crystals.
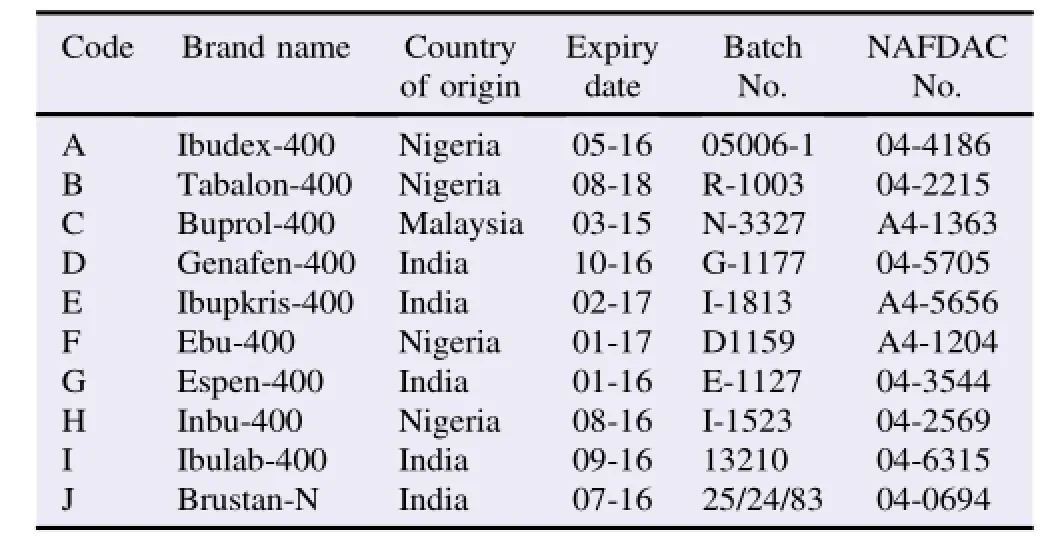
Table 1 Brands of ibuprofen tablets used in the study.
The crystals were packed into a capillary tube and tapped on a hard surface to form a column at the bottom of the capillary tube.The tube was inserted into a heating block of a Gallenkamp melting point apparatus and already maintained at a temperature of 70°C.The temperature of the heating block was raised at 0.5°C per min until the sample melts.The melting temperature was recorded as the melting point.Triple determinations were carried out in per brand.
2.6.Disintegration time
The disintegration times of six tablets per brand were determined in distilled water at(37.0±0.5)°C using the British Pharmacopoeia(BP)disintegration tester(Mk IV,Manesty Machines,UK).The apparatus is an assembly of tubes covered at the lower end with a No.10 mesh of 2 mm diameter and opened at the upper end.The whole assembly of tubes is then immersed inside a 1 L beaker containing 900 mL of distilled water.The beaker was placed inside the water bath and maintained at a constant temperature of(37.0±0.5)°C.The tubes were made to oscillate at a constant rate,that at the upward stroke about 2.5 mL of the tubes were immersed in the medium and at the downward stroke,the tube went deep inside the medium leaving about 2.5 mL portion of the tube.The time taken for all the 6 tablets to break up into granules that were able to pass through the mesh was noted as the disintegration time. The test was repeated to obtain the average disintegration time. The SD were calculated.
2.7.Dissolution rate
Dissolution test was carried out on the tablets using the United States Pharmacopeia(USP)dissolution apparatus II(paddle method)(Dissolution Tester DIS 6000,Copley Scientific,UK).A dissolution medium of 900 mL of phosphate buffer pH 7.2 maintained at(37.0±0.5)°C with a basket revolution of 50 r/min was used.A 5 mL aliquots was withdrawn at various intervals and replaced with an equivalent volume maintained at same temperature(37.0±0.5)°C of the dissolution medium. The samples were diluted with an equal volume of phosphate buffer.This was continued for 60 min.The absorbances of the resulting solutions were measured spectrophotometrically at λmax of 221 nm(CE 7500,Cecil Instruments Ltd).The concentration and the percentage of drug released at each time interval was determined using the equation from the standard calibration plot obtained from the pure ibuprofen.A minimum of triplicate determinations were carried out for each brand and the results were reported as mean±SD.
2.8.Content of active UV assay
2.8.1.Standard preparation
A total of 10μg/mL standard solution was prepared by dissolving 100 mg of ibuprofen powder in a 100 mL volumetric flask with 0.1 mol/L NaOH solution and making up to volume. A 1 mL aliquot of the solution was further diluted to 100 mL togive the desired concentration.The absorbance of the resulting solution was read at 221 nm.
2.8.2.Sample preparation
The average tablet weight of 20 tablets from each brand was gotten.The tablets were crushed into powders using a tablet miller(IKA tablet miller).Powder quantity equivalent to 100 mg ibuprofen was dissolved in a 100 mL volumetric flask with 0.1 mol/L NaOH solution and made up to volume.The solution was filtered with a Whattman filter paper(No.1)and 1 mL aliquot of the solution was further diluted to 100 mL to give a 10μg/mL solution.The absorbance of the resulting solution was read at 221 nm and the percentage content was calculated.
2.9.HPLC assay
The HPLC system(Agilent Infinity 1260,Agilent Technologies Inc.,USA)has four gradient pumps incorporated with a solventdegasser,injector,columnovenandadiodearraydetector. An Agilent ZORBAX Eclipse Plus C18 100 mm×4.6 mm,3.5μm column was used as the stationary phase.
2.9.1.Standard preparation
Pure ibuprofen powder(160 mg)was weighed into a 100 mL volumetric flask containing about 70 mL of HPLC grade methanol and sonicated for 20 min.The resultant solution was allowed to settle and made up to volume.A 5 mL aliquot of the solution was diluted to 100 mL to get a concentration of 80μg/ mL.The standard solution was run on the HPLC.Six injections were run for the standard to determine the system suitability and were calibrated to see the correlations and relative standard deviation[11].
2.9.2.Sample preparation
Twenty tablets randomly selected from each brand were weighed and pulverized.The weight of powder equivalent to 400 mg ibuprofen was transferred into a 100 mL volumetric flask.About 70 mL of methanol was added and sonicated for 20 min.After dissolving,the solution was allowed to settle down and made up to volume.The solution was filtered through a 0.45μm Sartorius nylon filter and 1 mL was taken and diluted to 50 mL with methanol in a volumetric flask to get a test solution of 80μg/mL.The sample was analysed using HPLC.Two injections were run on each brand and the area of the ibuprofen peaks were quantified with the area of the peak of the ibuprofen standard to get the amount of the ibuprofen in percentage present in each brand.The mobile phase was also run with the test samples to serve as the blank[11].Agilent ChemStation software was used to integrate and analyze HPLC peak responses for quantitation of the peaks by area percent.Chromatograms were shown in Figure 1.
3.Results
All the ibuprofen tablets investigated were all within their shelf lives and were immediate released dosage forms with label strength of 400 mg(Table 1).Brands A and B were uncoated tablets while the others were coated.Of the ten brands studied,five were formulated in India,four in Nigeria and one in Malaysia and they were all registered with the NAFDAC.
Table 2 shows some physicochemical parameters of the various brands of ibuprofen tablets.The weight uniformity test on the tablets indicated no significant differences(P>0.5)in the weights of tablets from the different brands,hence conformed to the BP specification,i.e.that not more than two of the individual weights should deviate from the average weight by more than ±5%and none should deviate by more than±10%[12].Despite the uniformity of weights within each brand,there were significant weight variations among the brands.Table 2 also shows that the mean tablet crushing strength for the samples ranged from 6.14 to 15.93 kp.Although friability is a nonofficial test,it is related to the hardness of the tablet and it is the tendency of tablets to powder,chip,or fragment.It can negatively affect the elegance,appearance and consumer acceptance of the tablet.All the samples showed friability values below 1%.The disintegration times of the samples did not meet the BP 2009 requirements of within 15 min for uncoated tablets and 60 min for coated tablets[12].Tablets of brand C,F,G and I which are uncoated did not meet the specifications.The in vitro drug release data showed that all the samples did not released up to 80%of their labeled contents within 1 h.The USP stated that not less than 80%of the labeled amount of ibuprofen should be released within 1 h[13].Brands D,E,G,I and J did not comply to this compendial requirement.The melting points of ibuprofen extracted from all the brands ranged from 73.5 to 76°C.The BP specifies ranged between 75 and 78°C.Two brands(B and G)did not meet this specification[14].
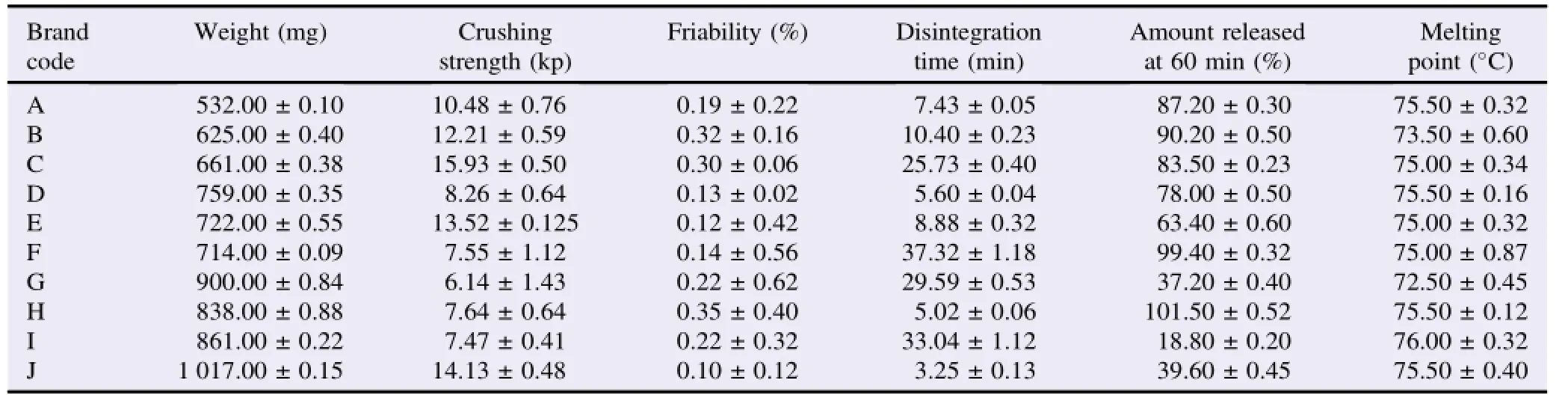
Table 2 Some physicochemical properties of ibuprofen tablets.

Table 3 Assay result of the amount of ibuprofen obtained from UV and HPLC analysis.
The results of the assay of chemical content using UV and HPLC analysis to determine the amount of ibuprofen present in each brand were presented in Table 3.The BP stipulates a 95%-105%of active drug content[12].While brands C and D failed this requirement in the UV assay,all the brands did meet this specification in the HPLC assay method.
4.Discussion
The physicochemical and pharmaceutical equivalence of ten brands of ibuprofen tablets(400 mg)obtained from various pharmacies across Benin City has been evaluated.All the brands met compendial requirements with regards to uniformity of weight,crushing strength and friability.
The pharmacopeia compliance with regard to uniformity of weight of each brand studied is important since the uniformity of dosage unit can be demonstrated by either weight variation or content uniformity study[13].These either reflect indirectly or measure directly the amount of drug substance in the tablet[15].
Although there was compliance with each brand,the differences in tablet weight(a reflection of their sizes),among the brands may have some negative psychological effects on clinicians and their patients since they could raise some doubts on the general equivalence of the different brands of ibuprofen tablets(400 mg)available.The World Health Organization model formulary advises that a patient should be placed on a particular brand,probably due to pharmacokinetic and psychological reasons[16].In Nigeria where the availability of a particular brand for the patient concerned is never guaranteed at all times,it wouldbeadvisablethatmanufacturersofthisproduct formulate equivalent sizes of tablets in order to assuage patients'worry regarding the identity and efficacy of the different brands because of the wide differences in tablet sizes.
Sufficient tablet hardness is essential to ensure damage resistanceduringhandling,packagingandtransportation. Although BP recommends a crushing strength of 5-8 kp,an overly hard tablet would lower disintegration time significantly and in turn dissolution[12].The minimal friability values for all the tablet brands is an indication of the ability of the tablet to withstand stress due to abrasive forces,without crumbling during transportation,packaging,handling and dispensing. These values also reflect the hardness of the tablets.
Disintegration time is the rate determining step in drug absorption.The type and amount of excipients used by different manufacturers may influence disintegration and consequently the bioavailability of the drug.The disintegration times of the different brands could not be predicted from their tablet crushing strength values as brands C,F,G and I that failed the test and had satisfactory crushing strength values.The high crushing strength value of brand C could be responsible for its disintegration time.
Based on the USP specifications,brands A,B,C,F and H released over 80%of their drug content within 1 h while D,E,G,I and J released variable amounts of their active content[13]. Here,their disintegration time did not have a direct relationship with their drug release.Brands D,E and J had disintegration time below 10 min,hence a drug release above 80%would havebeenexpected.Thiscouldbeduetoimproper formulation technique leading to disintegration into coarse particles and preventing the drug from going into solution. The amount of drug released by brands G and I could be a direct consequence of their disintegration times that was greater than or equal to 30 min.Although brand F had a disintegration time of 37 min,its drug release still reached 99%in 1 h.
The chemical content assay using the UV spectroscopic method showed that brands C and D failed to meet the compendial requirements while the all the brands passed HPLC method.The chromatographic method is more sensitive and reliable assay.The HPLC technique is usually used to further support the results by the UV and it showed that all brands passed the assay test.
Itcanthereforebeconcludedthatibuprofentabletsmarketedin Benin City vary in their physicochemical qualities,though they may be chemically equivalent.Since there was much variation in their physicochemical properties,it is an indication that bioavailability will differ to a large extent.Thus,in clinical practice,brandsubstitution should not be encouraged as therapeutic outcome expected would not be achieved at the expected time.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors acknowledge the technical support received from the departmental laboratory staff.
[1]Rainsford KD.Ibuprofen:pharmacology,efficacy and safety. Inflammopharmacology 2009;17(6):275-342.
[2]Mazaleuskaya LL,Theken KN,Gong L,Thorn CF,FitzGerald GA,Altman RB,et al.PharmGKB summary:ibuprofen pathways. Pharmacogenet Genomics 2015;25(2):96-106.
[3]KandreliMG,VadachkoriiaNR,GumberidzeNSh,MandzhavidzeNA.[Pain management in dentistry].Georgian Med News 2013;225:44-9. Russian.
[4]Gigante A,Tagarro I.Non-steroidal anti-inflammatory drugs and gastroprotection with proton pump inhibitors:a focus on ketoprofen/omeprazole.Clin Drug Investig 2012;32(4):221-33.
[5]Okunlola A,Adegoke OA,Odeku OA.Generic versus innovator: analysis of the pharmaceutical qualities of paracetamol and ibuprofen tablets in the Nigerian market.East Cent Afr J Pharm Sci 2009;12:55-64.
[6]Eichie FE,Arhewoh IM,Ezeobi OC.In-vitro evaluation of the pharmaceutical quality of some ibuprofen tablets dispensed in Nigeria.Afr J Pharm Pharmacol 2009;3(10):491-5.
[7]Auta A,Bala ET,Shalkur D.Generic medicine substitution:a cross-sectional survey of the perception of pharmacists in North-Central,Nigeria.Med Princ Pract 2014;23(1):53-8.
[8]Nnamdi JA,Arhewoh IM,Okhamafe AO,Enato EF.Evaluation of the pharmaceutical quality of some quinine preparations sold in Nigeria.Med Princ Pract 2009;18:193-7.
[9]Sahel and West African Club/Organization for Economic Cooperation and Development.West Africa Gateway.Nigeria. Paris:Sahel and West African Club/Organization for Economic Co-operation and Development;2012.[Online]Available from: http://www.oecd.org/swac/publications/Nigeria_e-version_en_ light.pdf[Accessed on 20th March,2015]
[10]Oparah CA,Iwuagwu MA.Public perceptions of community pharmacists in Benin City,Nigeria.Int J Pharm Pract 2001;9(3): 191-5.
[11]Umapathi P,Ayyappan J,Darlin Quine S.Quantitative determination of metformin hydrochloride in tablet formulation containing croscarmellose sodium as disintegrant by HPLC and UV spectrophotometry.Trop J Pharm Res 2012;11(1):107-16.
[12]British Pharmacopoeia Commission.British pharmacopoaeia Vol. III.London:The Stationery Office Limited;2009,p.6578-85.
[13]U.S.Pharmacopeial Convention.U.S.Pharmacopeia National Formulary 2011:USP 34 NF 29(United States Pharmacopeia/ National Formulary).Rockville:United States Pharmacopeial;2011.
[14]British Pharmacopoeia Commission.British pharmacopoaeia Vol. I.London:The Stationery Office Limited;2011,p.1110.
[15]AlderbornG.Tabletsandcompaction.In:AultonME,Taylor KMG,editors.Pharmaceutics-The design and manufacture of medicines.4th ed.Edinburgh:Churchill Livingstone;2013,p.505-49.
[16]Couper MR,Mehta DK.WHO model formulary.Geneva:World Health Organization;2002,p.236.
17 Apr 2015
Sylvester Okhuelegbe Eraga,Department of Pharmaceutics and Pharmaceutical Technology,Faculty of Pharmacy,University of Benin,Benin City,300001,Nigeria.
Tel:+2348030884928
E-mail:eragaso@uniben.edu
Peer review under responsibility of Hainan Medical University.
in revised form 1 Jun 2015
 Asian Pacific Journal of Tropical Biomedicine2015年10期
Asian Pacific Journal of Tropical Biomedicine2015年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Neurological symptoms in psoriasis patients under treatment with infliximab
- Review on herbal medicine on brain ischemia and reperfusion
- Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats
- Chemical constituents in the essential oil of the endemic plant Cotula cinerea(Del.)from the southwest of Algeria
- Searching for the best agarose candidate from genus Gracilaria,Eucheuma,Gelidium and local brands
- Identification of medicinal plants effective in infectious diseases in Urmia,northwest of Iran
