Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats
Mohamed Afifi,Aaser Mohamed AbdelazimDepartment of Biological Sciences,Faculty of Science,University of Jeddah,Jeddah,Saudi Arabia
2Department of Biochemistry,Faculty of Veterinary Medicine,Zagazig University,Zagazig,Egypt
Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats
Mohamed Afifi1,2*,Aaser Mohamed Abdelazim2
1Department of Biological Sciences,Faculty of Science,University of Jeddah,Jeddah,Saudi Arabia
2Department of Biochemistry,Faculty of Veterinary Medicine,Zagazig University,Zagazig,Egypt
ARTICLE INFO
Article history:
Accepted 20 Jun 2015
Available online 6 Aug 2015
Diabetic brain
Antioxidants
Zinc oxide nanoparticles
Silver nanoparticles
Objective:To test the ability of both zinc oxide nanoparticles(ZnONPs)and silver nanoparticles(SNPs)to ameliorate the oxidative stress resulted from diabetes in diabetic rats.
Methods:Fifty male albino rats were used;ten of them were served as control group and forty,as the experiment group,were injected with streptozotocin at the single intraperitoneal dose of 100 mg/kg.Then,the experiment group was subdivided into,diabetic,diabetic+ZnONPs,diabetic+SNPs and diabetic+insulin groups.The activities and mRNA expression levels of superoxide dismutase,catalase,glutathione peroxidase and glutathione reductase were determined in brain tissues.Malondialdehyde,total antioxidant capacity,zinc and silver concentrations were estimated in the brain tissues of all rats. Results:A significant increase in the activities and mRNA expression levels of superoxide dismutase,catalase,glutathione peroxidase and glutathione reductase was shown. Malondialdehyde levels were significantly decreased while there was a significant increase in the zinc,silver concentrations and total antioxidant capacity in brain of ZnONPs and SNPs treated rats,compared with diabetic or diabetic+insulin group and their control group.
Conclusions:ZnONPs and SNPs can be used to ameliorate the oxidative stress in brain resulted from diabetes mellitus.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2015.06.010
1.Introduction
Diabetes mellitus is referring to a group of metabolic impairments characterized by highly raised blood glucose levels[1].The most consequence of diabetes mellitus is the generation of reactive oxygen species(ROS)[2].The ROS can induceβ-cell failure and develop insulin resistance[3].Diabetes mellitus can directly affect brain cells,through the generation of an oxidative stress leading to apoptosis[4].The key mechanism is illustrated as an increase in glucose metabolism due to hyperglycemiathatpromotesmitochondrialrespiration,resulting in release of superoxide and other reactive oxygen or nitrogen species into the cytoplasm[5].Zinc has been reported to play a direct role in glucose homeostasis by enhancinghepaticglycogenesisthroughitsactionsontheinsulin signaling pathway and thus improves glucose utilization[6],inhibits intestinal glucose absorption[7],and increases glucose uptake in skeletal muscle and adipose tissue[6].Moreover,zinc is reported to inhibit glucagon secretion[8],thus reducing gluconeogenesis and glycogenolysis,and it also enhances the structural integrity of insulin[9].Decreased zinc in the pancreas may reduce the ability of the isletβ-cells to synthesize and lead insulin into blood[10].Furthermore,knowing zinc's antioxidant role,reduced zinc may exacerbate the oxidative stress-mediated complications of diabetes.The pivotal role of zinc in diabetes mellitus was discovered by supplementation studies in diabetic rats[11].In recent years,there is a great development of nanotechnology in the field of science and technology;and metallic nanoparticles,like gold,silvernanoparticles(SNPs), zincoxidenanoparticles(ZnONPs)and metal oxide nanoparticles,have shown great challenges in the field of medicine[12].In a previous study,we proved the antidiabetic effect of ZnONPs and SNPs as a novel agent to control diabetes mellitus in rats[13].The aim ofthe study was to test the ability of ZnONPs and SNPs to reduce the oxidative stress induced by diabetes mellitus in brain tissues of diabetic rats.
2.Materials and methods
2.1.Animals
Fifty male albino rats weighed(120±20)g at the beginning of the experiment.All the rats were first grouped into two groups:(1)control group(n=10)without any treatments;(2)experiment group,including the remaining forty animals,which were induced to be diabetic through intraperitoneal injection of single dose(100 mg/kg)of streptozotocin(Sigma-Aldrich,Catalog No.S0130 SIGMA,Seelze,Germany).Then,the experiment group was further divided into four groups:(1)diabetic group(n=10),which served as a positive control with no treatment;(2)diabetic+ZnONPs group(n=10),which was administered with daily ZnONPs per os(Sigma-Aldrich,Catalog No.721077,Seelze,Germany)at a dose of 10 mg/kg;(3)diabetic+SNPs group(n=10),which was administered with SNPs per os(Sigma-Aldrich,Catalog No.730793,Seelze,Germany)at a daily dose of 10 mg/kg;(4)diabetic+insulin group(n=10),which was injected with subcutaneous dose of insulin(0.6 units/50 g)for 30 constitutive days[13].
2.2.Ethical statement
All experimental procedures were performed in agreement with the Saudi Arabian laws and University Guidelines for the care and rights of experimental animals.
2.3.Sampling protocol
One gram of brain tissue was collected on liquid nitrogen and then divided into different aliquots which were preserved at-80°C until their use in biochemical and molecular biological investigations.
2.4.Biochemical assay
Brain zinc and silver concentrations were analyzed by using an inductively coupled plasma-atomic emission spectroscopy with an ULTIMA 2 apparatus(Horiba Jobin Yvon,France).
Brain homogenate was used for determination of catalase(CAT),glutathione peroxidase(GPx)and glutathione reductase(GRD)activity by using a kit(Catalog No.NWK-CAT01,NWKGPX01 and NWK-GR01)purchasedfrom Northwest Life Science Specialties,Vancouver,Canada.SOD activity was determined by using Cayman SOD diagnostic kit(Catalog No.706002,Cayman,USA).Malondialdehyde(MDA)was analyzed by measuring the production of thiobarbituric acid reactive substances by using TBARS assay kit(Catalog No.10009055,Cayman,USA).Total antioxidantcapacity(TAC)wasdeterminedbyusingakitsupplied by Bio-diagnostic(Catalog No.TA 25 12,Giza,Egypt).
2.5.Molecular analysis
Brain SOD,CAT,GPx,GRD gene expressions were quantified by using real-time PCR.Total RNA was isolated from tissue samples by using the Qiagen RNeasy Mini kit(Catalog No. 74104).About 0.5μg of total RNA,was used for production of cDNAbyusingQIAGENLongRange2StepRT-PCRkit(Catalog No.205920).About 5μL of total cDNA was mixed with 12.5μL of 2×SYBR®Green PCR mix with ROX from Bio-Rad and 10 pmol/μL of each forward and reverse primer for the measured genes.The house-keeping geneβ-actin was used as a constitutive control for normalization.Primers were designed by using Primer 3 software(http://bioinfo.ut.ee/primer3/)as per the published gene sequences of SOD,CAT,GPx,GRD andβ-actin in rats(Table1)fromNCBIdatabasewhereallprimerswereprovidedby Sigma Aldrich(Sigma-Aldrich Chemie GmbH,Steinheim,Germany).PCR reactions were carried out in an Abi Prism 7300(Applied Biosystems,USA).The RNA concentration in each sample was determined from the cycle threshold values.The mRNA expression levels were calculated relative toβ-actin gene mRNA levels by using the 2-DDCTmethod.
2.6.Statistical analysis
The obtained data were analyzed by using SPSS version 20(IBM 1 New Orchard Road Armonk,New York 10504-1722,United States).Data were presented as mean±SD(n=10). Student's t-test was used to calculate the differences between groups at P<0.05.
3.Results
3.1.Effect of ZnONPs and SNPs on zinc levels in brain tissue
Zinc levels in the brain tissue decreased significantly in diabetic group,compared with control group.Both SNPs and insulin treatment protected the brain tissue against the bad effect of diabetes.The ZnONPs carried the ability to increase zinc levels in brain tissue in ZnONPs treated rats,compared with the control group(Table 2).
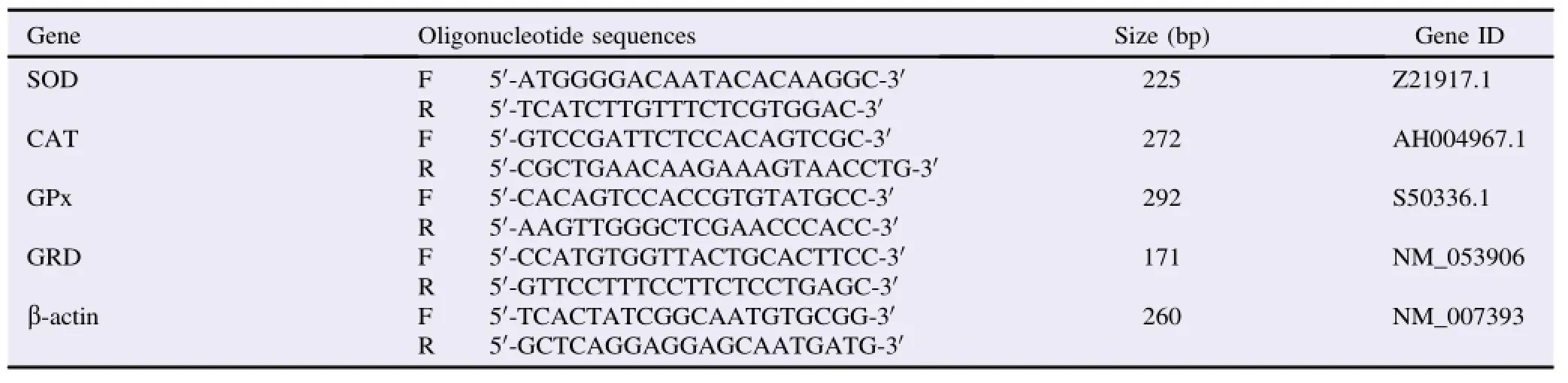
Table 1 Oligonucleotide primer sequences of SOD,CAT,GPx,GR andβ-actin genes.
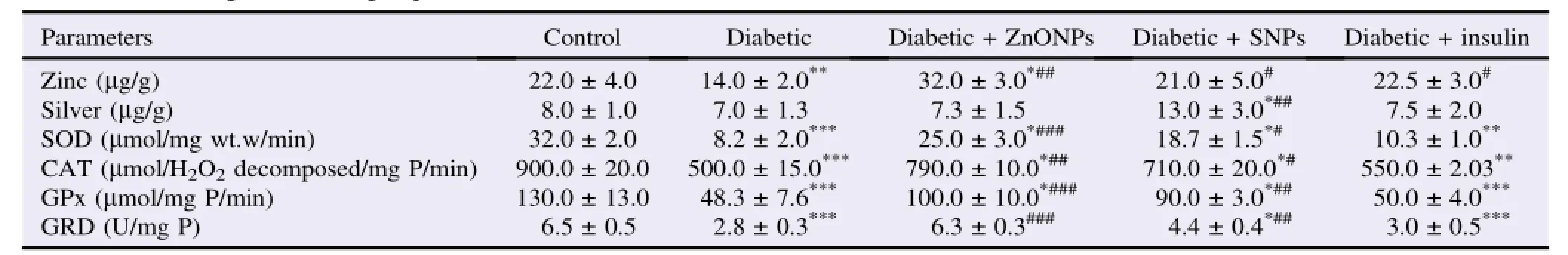
Table 2 Biochemical investigations in all groups.
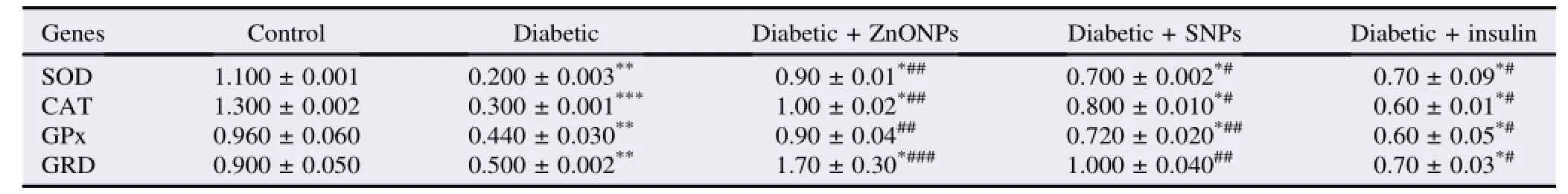
Table 3 Profile of mRNA expression(relative expression toβ-actin)of antioxidant genes in all groups.

Table 4 Biochemical effect of ZnONPs,SNPs on MDA and TAC in brain tissue of diabetic rats.
3.2.Effect of ZnONPs and SNPs on silver levels in brain tissue
The silver levels in the brain tissue were not changed in all treated groups,except that SNPs treated rats showed a significant increase,compared with the other groups(Table 2).
3.3.Effect of ZnONPs and SNPs on antioxidant enzyme activities in brain tissue
Both diabetic and diabetic+insulin treated rats showed a significant decrease in the activities of SOD,CAT,GPx and GRD,compared with the control group.Either ZnONPs or SNPs treatment had the ability to protect the antioxidant enzymes in brain tissue against the bad effect of diabetes(Tables 2 and 3).
3.4.Effect of ZnONPs and SNPs on mRNA expression levels of antioxidant genes in brain tissue
A significant increase in mRNA expression levels of SOD,CAT,GPx and GRD was shown in rats treated with ZnONPs and SNPs,compared with the diabetic group(Tables 2 and 3).
3.5.Effect of ZnONPs and SNPs on MDA and TAC levels in brain tissue
MDA levels were significantly decreased while there was a significant increase in the TAC in the brain of ZnONPs and SNPstreatedratswhencomparedwithdiabeticor diabetic+insulin group and control group(Table 4).
4.Discussion
In the present study,we tended to examine the ameliorative effect of both ZnONPs and SNPs on the oxidative stress generated in brain cells of the diabetic rats.In the present study,zinc and silver particles were increased significantly in the brain tissues of diabetic rats subjected to treatment with ZnONPs and SNPs,with zinc levels of[(32±3)and(21±5)μg/g]and silver levels of[(7.3±1.5)and(13.0±3.0)μg/g],compared with control group containing zinc level of(22±4)μg/g and silver level of(8±1)μg/g(Table 2).Long circulation of nanoparticles leads to increase the chance of their passage to tissues,and hence higher cellular uptake[14].Once taken up in cells,particles encounter an increasingly acidic environment as they move from early to late endosomes and finally to lysosomes,resulting in their dissolution[15].Such phenomena may have contributed to the observed increases of zinc and silver levels in brain tissue in our study.ZnONPs and SNPs administration to diabetic rats resulted in prevention of the decrease in the activity and mRNA expression levels of SOD,CAT,GRD and GPx.We tended the high activities and expression levels of antioxidantenzymesinbrainsofZnONPsandSNPs administered rats to the effect of these nanoparticles to improve the insulin secretion and amelioration of the oxidative stress induced by impairment of glucose homeostasis[13]. These enzymes play a pivotal role in the elimination of free radicals from the tissues so their high activities and expression levels in brain cells protect them from the effect of oxidative stress[16].Diabetes mellitus is always associated with the generation of ROS[2],and this is clear in our study proved bya reduction in both activities and expression of the examined antioxidant enzymes in untreated diabetic rats.In the same line of our study,the overproductions of superoxide free radicalsareeliminatedbyoverexpressionofantioxidant enzymes[17].Many authors have cited that diabetes mellitus is usually associated with an increase in MDA production and thus,increases the MDA levels in tissues and blood in diabetic models[18].Our results showed a significant increase in MDA levels in the brain of diabetic rats.We tended this increase to the oxidative stress generated due to high glucose levels as mentioned before.In ZnONPs and SNPs groups,the levels of MDA were decreased in the brain tissues.The reduction of MDA levels in the brain of rats administered with nanoparticles may be due to the activity of these nanoparticles to improve the insulin secretion and to increase the SOD,CAT,GRD and GPx activities and mRNA expression[13].To demonstrate the TAC levels of the brain in diabetic rats and diabetic rats administered with ZnONPs and SNPs,the TAC was measured in the brains of all rats.The highest capacity was observed in diabetic rats administered with ZnONPs followed by diabetic rats administered with SNPs,compared to diabetic group and control group(Table 4).We contended that the high TAC in nanoparticles administered rats was due to the ability of these particles to improve the antioxidant power of the cells proved recently by Kunjiappan et al.[19].In all examined rats,the effect of ZnNPs and SNPs was more obvious than the insulin in diabetic rats.There are more improvements in total antioxidant power in the brain of diabetic rats administered with nanoparticles than those treated with insulin.Up till now,we have not a clear explanation about the action of these nanoparticles.Yet,the speed by which these nanoparticles were uptaken by the cells[14,15],and its ability to induce endogenous insulin secretion[13],may be the usual cause of their ability to improve the antioxidant capacity in the brain cells.ZnONPs and SNPs are potent agents for improvement of the antioxidant power of brain cells.They have the ability to protect brain cells from the oxidative stress generated in diabetes mellitus.
Conflict of interest statement
We declare that we have no conflict of interest.
[1]Lin Y,Sun Z.Current views on type 2 diabetes.J Endocrinol 2010;204(1):1-11.
[2]Bondeva T,Wolf G.Reactive oxygen species in diabetic nephropathy:friend or foe?Nephrol Dial Transplant 2014;29(11): 1998-2003.
[3]Stephens JW,Khanolkar MP,Bain SC.The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease.Atherosclerosis 2009;202(2):321-9.
[4]May JM,Jayagopal A,Qu ZC,Parker WH.Ascorbic acid prevents high glucose-induced apoptosis in human brain pericytes.Biochem Biophys Res Commun 2014;452(1):112-7.
[5]Weidinger A,Kozlov AV.Biological activities of reactive oxygen and nitrogen species:oxidative stress versus signal transduction. Biomolecules 2015;5(2):472-84.
[6]Jansen J,Karges W,Rink L.Zinc and diabetes-clinical links and molecular mechanisms.J Nutr Biochem 2009;20(6):399-417.
[7]Ueda E,Yoshikawa Y,Sakurai H,Kojima Y,Kajiwara NM. In vitro alpha-glucosidase inhibitory effect of Zn(II)complex with 6-methyl-2-picolinmethylamide.Chem Pharm Bull(Tokyo)2005;53(4):451-2.
[8]Egefjord L,Petersen AB,Rungby J.Zinc,alpha cells and glucagon secretion.Curr Diabetes Rev 2010;6(1):52-7.
[9]Sun Q,van Dam RM,Willett WC,Hu FB.Prospective study of zinc intake and risk of type 2 diabetes in women.Diabetes Care 2009;32(4):629-34.
[10]Meyer JA,Spence DM.A perspective on the role of metals in diabetes:past findings and possible future directions.Metallomics 2009;1:32-41.
[11]Ukperoro JU,Offiah N,Idris T,Awogoke D.Antioxidant effect of zinc,selenium and their combination on the liver and kidney of alloxan-induced diabetes in rats.Mediterr J Nutr Metab 2010;3(1):25-30.
[12]Hirst SM,Karakoti A,Singh S,Self W,Tyler R,Seal S,et al.Biodistribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice.Environ Toxicol 2013;28(2):107-18.
[13]Alkaladi A,Abdelazim AM,Afifi M.Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats.Int J Mol Sci 2014;15(2):2015-23.
[14]Li SD,Huang L.Pharmacokinetics and biodistribution of nanoparticles.Mol Pharm 2008;5(4):496-504.
[15]Nel AE,M¨adler L,Velegol D,Xia T,Hoek EM,Somasundaran P,et al.Understanding biophysicochemical interactions at the nanobio interface.Nat Mater 2009;8(7):543-57.
[16]Tanaka M,Mokhtari GK,Terry RD,Balsam LB,Lee KH,Kofidis T,et al.Overexpression of human copper/zinc superoxide dismutase(SOD1)suppresses ischemia-reperfusion injury and subsequent development of graft coronary artery disease in murine cardiac grafts.Circulation 2004;110(11 Suppl 1):II200-6.
[17]Valko M,Leibfritz D,Moncol J,Cronin MT,Mazur M,Telser J. Free radicals and antioxidants in normal physiological functions and human disease.Int J Biochem Cell Biol 2007;39(1):44-84.
[18]Bas¸H,Kalender Y,Pandir D,Kalender S.Effects of lead nitrate and sodium selenite on DNA damage and oxidative stress in diabetic and non-diabetic rat erythrocytes and leucocytes.Environ Toxicol Pharmacol 2015;39(3):1019-26.
[19]Kunjiappan S,Bhattacharjee C,Chowdhury R.In vitro antioxidant and hepatoprotective potential of Azolla microphylla phytochemically synthesized gold nanoparticles on acetaminophen-induced hepatocyte damage in Cyprinus carpio L.In Vitro Cell Dev Biol Anim 2015;51(6):630-43.
22 May 2015
Mohamed Afifi,Department of Biological Sciences,Faculty of Science,University of Jeddah,Jeddah,Saudi Arabia.
Tel:+966 509562637
E-mails:mama200100@gmail.com,mafifi@kau.edu.sa
Peer review under responsibility of Hainan Medical University.
in revised form 9 Jun 2015
 Asian Pacific Journal of Tropical Biomedicine2015年10期
Asian Pacific Journal of Tropical Biomedicine2015年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Neurological symptoms in psoriasis patients under treatment with infliximab
- Review on herbal medicine on brain ischemia and reperfusion
- A comparative UV-HPLC analysis of ten brands of ibuprofen tablets
- Chemical constituents in the essential oil of the endemic plant Cotula cinerea(Del.)from the southwest of Algeria
- Searching for the best agarose candidate from genus Gracilaria,Eucheuma,Gelidium and local brands
- Identification of medicinal plants effective in infectious diseases in Urmia,northwest of Iran
