Gas chromatography mass spectrometry analysis and in vitro antibacterial activity of essential oil from Trigonella foenum-graecum
Moniruzzaman,Shahinuzzaman*,Ahsanul Haque,Rahima Khatun,Zahira YaakobDepartment of Chemical and Process Engineering,Universiti Kebangsaan Malaysia,43600 UKM,Bangi,Selangor,Malaysia
2Fuel Cell Institute,Universiti Kebangsaan Malaysia,43600 UKM,Bangi,Selangor,Malaysia
3Department of Applied Chemistry and Chemical Technology,Islamic University,Kushtia 7003,Bangladesh
Gas chromatography mass spectrometry analysis and in vitro antibacterial activity of essential oil from Trigonella foenum-graecum
Moniruzzaman1,Shahinuzzaman1*,Ahsanul Haque2,3,Rahima Khatun1,Zahira Yaakob1
1Department of Chemical and Process Engineering,Universiti Kebangsaan Malaysia,43600 UKM,Bangi,Selangor,Malaysia
2Fuel Cell Institute,Universiti Kebangsaan Malaysia,43600 UKM,Bangi,Selangor,Malaysia
3Department of Applied Chemistry and Chemical Technology,Islamic University,Kushtia 7003,Bangladesh
ARTICLE INFO
Article history:
in revised form 9 Jul 2015
Accepted 22 Jul 2015
Available online 17 Oct 2015
Essential oil
Antibacterial activity
Gas chromatography mass spectrometry analysis
Trigonella foenum-graecum
Objective:To evaluate the antibacterial activity of essential oil from Trigonella foenumgraecum seeds powder,and identify the compounds from the extracted oil.
Methods:The seeds powder of Trigonella foenum-graecum was subjected to Clevenger extractor.Seven strains of bacteria were used to test antibacterial activity of the extract. The activity against bacteria was tested by disk diffusion method using Whatman No.1 filter paper.Gas chromatography mass spectrometry analysis was performed with an Agilent7890/5975B-gas chromatography/mass selective detector.
Results:The hydrodistillation of seeds powder yielded 0.285%(v/w)of oil.Disk diffusion of the oil showed bactericidal activity against both Gram negative and Gram positive bacteria of tasted strains.The inhibition zone ranged from(8±0)mm to(15.0±0.7)mm depending on microbial strains.Gas chromatography mass spectrometry analysis showed 14 different compounds.The total compounds represented 80.96%of the oil.
Conclusions:The antibacterial activity is due to the effects of different biological active compounds present in the extract.Identification of the compounds may help to develop new effective antimicrobial agent(s).Further researches on purification,characterization and toxicology of the active compounds are needed.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2015.09.010
1.Introduction
Infectious diseases and drug resistance have been becoming a serious concern for successful treatment.Food safety,food packaging and storage shelf life are burning issues in food industry.Scientists are looking for new,effective,safe and environment friendly resources for preventing and curing of infection as well as flavoring and preserving food materials. Medicinal plants have been providing huge number of highly effective drugs.These plants are attractive for discovery of new molecular entities due to their largely untapped chemical diversity[1].For primary health care,about 80%of the world's population rely on traditional medicines(plant extracts).InIndia,almost 95%of the prescriptions were based on Unani,Ayurveda,homeopathy and Siddha,the traditional systems of treatment[2].Plants essential oils are natural compounds that have multi-purpose usages[3].In pharmaceutical industry,the oil has been used due to its anticancer,antinociceptive,antiphlogistic,antiviral,antibacterialandantioxidant properties[4].It has other uses in food and cosmetic industry[3,5,6].
Trigonella foenum-graecum L.(T.foenum-graecum)is an annual,self pollinating,diploid legume plant underneath the family of Fabaceae.It is found in Eastern Mediterranean to Central Asia and Ethiopia,and produced in bulk in Pakistan,India and China[7].Trigonella seeds or Fenugreek is known generally as the dried ripe seeds of T.foenum-graecum.It has pungent aromatic properties and is often used to flavor homes[8,9].In India,Egypt and Yemen fenugreek seeds are used as condiment and food among the general population. Leaves are consumed widely in India as a green,leafy vegetable.They are rich source of calcium,iron,β-caroteneand other vitamins[10].Use as herbal medicine in many parts of the world is the traditional application of T.foenum-graecum. Alkaloid,yellow coloring matter,tannic acid,diosgenin,vitamin A,fixed and volatile oils are the components of fenugreek seeds[11].
This study aimed to extract essential oil,identify the compounds from the oil using gas chromatography mass spectrometry(GC-MS)analysis and evaluate the antibacterial activity of the oil.
2.Materials and methods
2.1.Essential oil extract preparation
The healthy and mature seeds of T.foenum-graecum were collected from the plants at Jessore District in Bangladesh in February 2014.Seeds were washed for 20 min with running tap water and finally rinsed two times with distilled water.Then the seeds were placed in oven at 40°C for 4 days for drying and avoiding daylight exposure to forestall loss of active components.Dried seeds were grinded and kept in air tight vials. Hydrodistillation of essential oil from seeds powder was conducted using Clevenger extractor[12].It yielded 0.285%(v/w)of the oil.Obtained essential oil was dried over anhydrous sodium sulfate(Na2SO4).Finally it was stored at-4°C for the test of antimicrobial activity and GC-MS analysis.
2.2.Test organisms
A total of seven organisms(bacteria)were tested for the antimicrobial activity.Among them Sarcina lutea(IFO 3232)(S.lutea)and Bacillus subtilis(IFO 3026)(B.subtilis)are Gram positive.The other organisms Xanthomonas campestris(IAM 1671)(X.campestris),Escherichia coli(IFO 3007)(E.coli),Klebsiella pneumonia(ATTC 10031)(K.pneumonia),Proteus vulgaris(MTCC 321)(P.vulgaris)and Pseudomonas denitrificans(KACC32026)(P.denitrificans)areGram negative.
2.3.Determination of antibacterial activity of essential oil
The antibacterial activity was tested out by disc diffusion method[13].Disks with 6 mm in diameter of Whatman No.1 filterpaperwereused.Briefly,150μLsuspensionof individual test microorganism was spread homogenously on each plate of mannitol salt agar media.Each disk was soaked with 150μL of essential oil and placed on the microbial lawns.Two disks were placed on each plate.The plates were incubated at 37°C for 24 h and the inhibition zones in mm were checked.Commercial antibiotic disks of gentamicin(10μg/disc),chloramphenicol(30μg/disc),ciprofloxacin(5μg/disc),erythromycin(15μg/disc),co-trimoxazole(25μg/disc),nalidixic acid(30μg/disc),vancomycin(30μg/ disc),azithromycin(30μg/disc),tetracycline(30μg/disc),cefuroxime(30μg/disc),cloxacillin(1μg/disc),ceftazidime(30μg/disc),ampicillin(25μg/disc),cefotaxime(30μg/disc)were also tested for their activity against these microbes.The tests were replicated three times and the data were presented in average.
2.4.GC-MS analysis
GC-MS analyses were carried out with an Agilent7890/ 5975B-GC/MSD(Palo Alto,CA,USA)equipped with a HP-5 MS capillary column(30 m×0.25 mm,i.d.0.25 mm)and a HP 5975B mass selective detector.The reaction was carried out according to Sun and coworkers[4].The sample was diluted as 1/10 in ether.One micro litter of diluted sample was injected manually with split ratio of 40:1.Then 70 eV was used for electron ionization for GC-MS detection.At first the oven temperature was kept at 50°C for 3 min.Then the temperature was gradually increased to 250°C at a 3°C/min rate and held at 250°C for 4 min.Temperature 220°C and 250°C were injector and MS transfer line temperatures respectively.Helium at flow rate of 1 mL/min was used as carrier gas.The components were identified based on the comparison of the retention time and the mass spectra with those in the NIST98 GC-MS library and those in the literature data[14].
2.5.Statistical analysis
All the experiments were carried out in triplicates.Results were expressed as mean±SE of three independent experiments(n=3).
3.Results
3.1.Antibacterial activity of essential oil
The essential oil exhibited antibacterial activity against both Gram negative and Gram positive bacteria of tested strains. Disks(6 mm)containing 150μL essential oil were subjected to seven microbial strains individually.Among the tested microbial strains, stronginhibitioneffectwasfoundagainst P.denitrificans[(15.0±0.7)mm],P.vulgaris[(15±0)mm],E.coli[(15±0)mm]and B.subtilis[(14.0±2.8)mm]. X.campestris[(12.5±0.7)mm]is susceptible to the oil,whereas S.lutea[(9.0±1.4)mm]and K.pneumonia[(8±0)mm]are relatively less susceptible(Figure 1).The commercial antibiotic disks gentamicin,chloramphenicol,ciprofloxacin,erythromycin,co-trimoxazole,nalidixicacid,vancomycin,azithromycin,tetracycline,cefuroxime,cloxacillin,ceftazidime,ampicillin,cefotaxime were also tested against the microbes.It was found that B.subtilis is susceptible to all tested antibiotics.Cefuroxime,cloxacillin and ceftazidime were less effective to the tested organisms(Table 1).
3.2.Chemical composition of essential oil of T.foenumgraecum
The GC-MS analysis of the T.foenum-graecum essential(volatile)oil led to identification of 14 different compounds.The total compounds percentage was 80.96%.Decane,5,6-bis(2,2 dimethylpropylidene),hexadecanoic acid,methyl ester,dihydro methyl jasmonate,Pyrrolo[1,2-a]pyrazine-1,4-dione,hexahydro-3-(2-methylpropyl),5-Fluoro-1,1,3,3-tetramethyl-1,3-dihy droisobenzofuran,octadecanoicacid,methylester,oxiraneoctanoic acid,3-octyl-,methyl ester,cis-were major compounds(Table 2);and the minor compounds were hexadecanoicacid;ethyl ester;cis-calamenene;6-Octadecenoic acid;2-pentadecanone,6,10,14-trimethyl;1,2,3,4Tetrahydroisoquinolin-6-ol-1-carboxylicacidandMurolan-3,9(11)-diene-10-peroxy.

Table 1 Commercial antibiotics against the tested organisms.
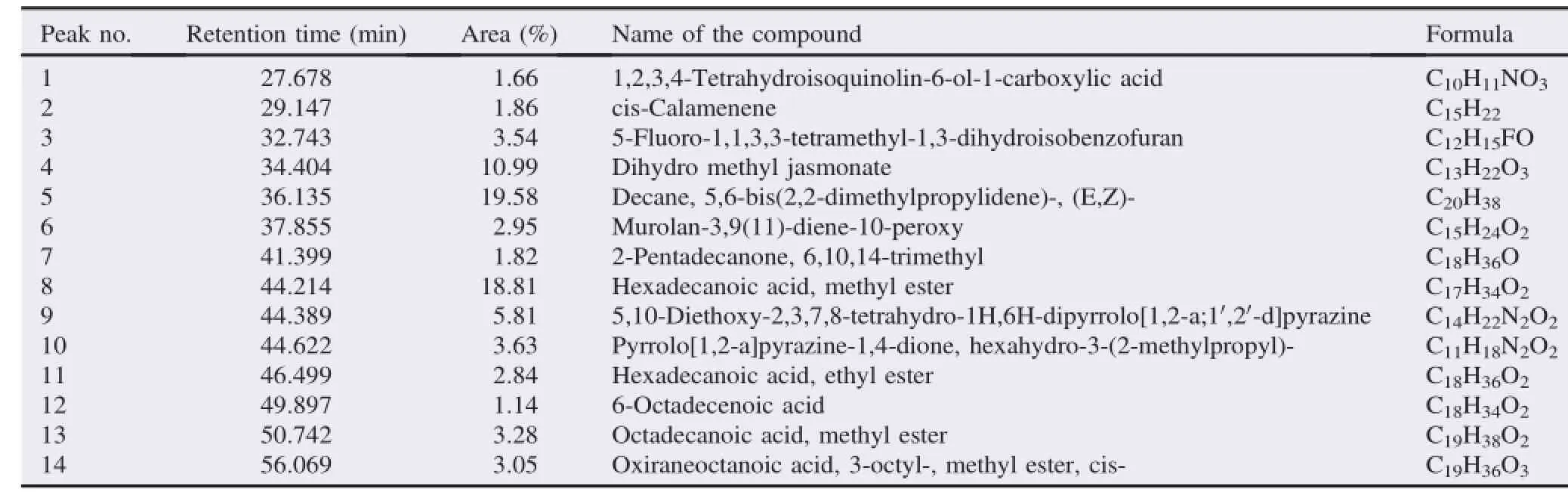
Table 2 Chemical composition of essential oil of T.foenum-graecum.
4.Discussion
Fenugreek seeds are rich source of polyphenols(apigenin,kaempferol,quercetin glycosides)and flavonoids(vitexin,tricin, naringenin,quercetinandtricin7-O-b-D-glucopyranoside)[15,16].Alluri and Majumdar studied methanol extract of T.foenum-graecumseedspowder[17].Theyfound antimicrobial activity with inhibition zones against bacteria(Staphylococcus aureus,Bacillus cereus,methicillin resistant Staphylococcus aureus,Pseudomonas aeruginosa and E.coli)in range from(7.30±0.15)mm to(16.90±0.28)mm and fungus(Candidaalbicans, Trichophytonrubrumand Aspergillus flavus)in range from(8.10±0.21)mm to(19.60±0.28)mm depending on different concentration of extract[17].Omezzine and co-workers reported inhibitory activity of organic solvent extract from T.foenum-graecum against Fusarium oxysporum f.sp.radicis-lycopersici and Fusariumoxysporumf.sp.lycopersici[18].Ourstudy interprets similar result to that of previous work for common microbe E.coli.We found inhibitory zone ranging from(8±0)mm to(15.0±0.7)mm against P.denitrificans,P.vulgaris,E.coli,B.subtilis,X.campestris,S.lutea and K.pneumonia(Figure 1).The antimicrobial activity may be due to the combined effect of phenolic compounds,alkaloids,tannins,flavonoids and terpenoids present in oil.
We performed GC-MS analysis of essential oil obtained from hydrodistillation of seeds powder and obtained 14 peaks,each for individual compound(Table 2).Kenny et al.studied solid liquid sequential extraction(hexane,dichloromethane,methanol and water)of T.foenum-graecum seeds powder[19]. They quantified 18 phenolic compounds by using ultraperformance liquid-chromatography-mass spectrometry.
It is necessary to introduce new antimicrobial agent to resist antibiotic resistance and for effective control of infections.This study showed the antibacterial activity of essential oil of T.foenum-graecum with many biological active compounds.It may help to identify and develop new effective antimicrobial agent.Further study is needed to purify and characterize the active compounds.Toxicological study is also needed before using it as pharmaceutical ingredient.
Conflict of interest statement
We declare that we have no conflict of interest.
[1]Malherbe CJ,De Beer D,Joubert E.Development of on-line high performance liquid chromatography(HPLC)-biochemical detection methods as tools in the identification of bioactives.Int J Mol Sci 2012;13:3101-33.
[2]Savithramma N,Rao ML,Suhrulatha D.Screening of medicinal plants for secondary metabolites.Middle East J Sci Res 2011;8: 579-84.
[3]Riahi L,Elferchichi M,Ghazghazi H,Jebali J,Ziadi S,Aouadhi C,et al.Phytochemistry,antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L.in Tunisia.Ind Crops Prod 2013;49:883-9.
[4]Sun J,Wang X,Wang P,Li L,Qu W,Liang J.Antimicrobial,antioxidant and cytotoxic properties of essential oil from Dictamnus angustifolius.J Ethnopharmacol 2015;159:296-300.
[5]Ma T,Luo J,Tian C,Sun X,Quan M,Zheng C,et al.Influence of technical processing units on chemical composition and antimicrobial activity of carrot(Daucus carrot L.)juice essential oil. Food Chem 2015;170:394-400.
[6]Muriel-Galet V,Cran MJ,Bigger SW,Hern´andez-Muñoz P,GavaraR.Antioxidantandantimicrobialpropertiesofethylenevinyl alcohol copolymerfilmsbasedon thereleaseoforeganoessentialoil and green tea extract components.J Food Eng 2015;149:9-16.
[7]Morton JF.Mucilaginous plants and their uses in medicine. J Ethnopharmacol 1990;29:245-66.
[8]Perry LM.Medicinal plants of east and Southeast Asia:attributed properties and uses.Cambridge:The MIT Press;1980.
[9]Max B.This and that:the essential pharmacology of herbs and spices.Trends Pharmacol Sci 1992;13:15-20.
[10]Sharma RD.Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects.Nutr Res 1986;6: 1353-64.
[11]Jayaweera DMA.Medicinal plant.Part III.Peradeniya:Royal Botanic Gardens;1981.
[12]British pharmacopoeia.London:Department of Health;1999.
[13]Sheikhlar A,Alimon AR,Daud HM,Saad CR,Shanagi H. Screening of Morus alba,Citrus limon and Trigonella foenumgraecum extracts for antimicrobial properties and phytochemical compounds.Pak J Biol Sci 2013;http://dx.doi.org/10.3923/ pjbs.2013.
[14]Adams RP.Identification of essential oil components by gas chromatography/mass spectrometry.Carol Stream:Allured Publishing Corporation;2007.
[15]Chatterjee S,Variyar PS,Sharma A.Stability of lipid constituents in radiation processed fenugreek seeds and turmeric:role of phenolic antioxidants.J Agric Food Chem 2009;57:9226-33.
[16]Shang M,Cai S,Han J,Li J,Zhao Y,Zheng J,et al.[Studies on flavonoids from Fenugreek(Trigonella foenumgraecum L.)]. Zhongguo Zhong Yao Za Zhi 1998;23:614-6.39.
[17]Alluri N,Majumdar M.Phytochemical analysis and in vitro antimicrobial activity of Calotropis gigantea,Lawsonia inermis and Trigonella foecum-graecum.Int J Pharm Pharm Sci 2014;6:524-7.
[18]Omezzine F,Bouaziz M,Daami-Remadi M,Simmonds MSJ,Haouala R.Chemical composition and antifungal activity of Trigonella foenum-graecum L.varied with plant ploidy level and developmentalstage.ArabJChem2014;http://dx.doi.org/ 10.1016/j.arabjc.2014.03.013.
[19]Kenny O,Smyth TJ,Hewage CM,Brunton NP.Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek(Trigonella foenum-graecum)seeds and bitter melon(Momordica charantia)fruit.Food Chem 2013;141:4295-302.
20 May 2015
.Shahinuzzaman,Department of Chemical and Process Engineering,Universiti Kebangsaan Malaysia,43600 UKM,Bangi,Selangor,Malaysia.
Tel:+60 01112506445
E-mail:shahinchmiu@gmail.com
Peer review under responsibility of Hainan Medical University.
 Asian Pacific Journal of Tropical Biomedicine2015年12期
Asian Pacific Journal of Tropical Biomedicine2015年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cocktail of Theileria equi antigens for detecting infection in equines
- Antiplasmodialactivity oftraditionalpolyherbalremedyfrom Odisha,India: Their potential for prophylactic use
- Deletion of Salmonella enterica serovar typhimurium sipC gene
- Anticancer activity of Cyanothece sp.strain extracts from Egypt:First record
- Influence of CD133+expression on patients'survival and resistance of CD133+cells to anti-tumor reagents in gastric cancer
- Vascular endothelial growth factor before and after locoregional treatment and its relation to treatment response in hepatocelluar carcinoma patients
